The Midwest floods of 2008 added more than just water to the region's lakes, reservoirs, and rivers. Runoff from farms and towns carries a heavy load of silt, nutrients, and other pollutants. The nutrients trigger blooms of algae, which taint drinking water. Death and decay of the algae depletes oxygen, kills fish and bottom-dwelling animals, and thereby creates “dead zones” in the body of water. The syndrome of excessive nutrients, noxious algae, foul water, and dead zones—which ecologists call eutrophication—is depressingly familiar to those who depend on water from rich agricultural regions.
The cure sounds simple: decrease inputs of nutrients, especially nitrogen (N) and phosphorus (P). But which nutrient, and how deeply should the inputs be cut? In this issue of PNAS, Schindler et al. (1) present a remarkable 37-year experiment on nutrient management in Canadian lakes which shows that P inputs directly control algae blooms. Surprisingly, however, the authors also observed that algae blooms are made worse if N inputs are decreased without also decreasing P inputs. This finding is of critical importance for current programs aimed at mitigating eutrophication of both freshwaters and coastal oceans.
Human activity has greatly increased the inputs of reactive N and P to the biosphere. Reactive N (biologically active forms such as nitrate, ammonia, or organic N compounds, in contrast to N2 gas, which is not used by organisms except for a few nitrogen-fixing species) is supplied by natural sources, as well as by human activities such as industrial N2 fixation, combustion, and planting of soybeans and other N2-fixing crops. Global flux of reactive N to the biosphere from food production has increased from ≈15 Tg N year−1 in 1860 to ≈187 Tg N year−1 in 2005 (2). Additional reactive N is fixed for industrial or household use or is inadvertently created as a byproduct of fossil fuel combustion. Excess reactive N enters groundwater, surface water, or the atmosphere.
P enters the biosphere by natural weathering of rock, as well as through mining and other land disturbances by humans. Mined P is used in fertilizers and a host of other products. The global P flux to the biosphere increased from ≈10–15 Tg P year−1 in preindustrial times to 33–39 Tg P year−1 in 2000 (3). Excess P added to cropland accumulates in soil, which can be eroded to surface water. Global P production appears to be in decline (http://energybulletin.net/node/33164), suggesting that conservation and recycling of P could help sustain crop production and reduce pollution of surface waters.
N and P from farmland runoff or industrial and municipal discharges are associated with widespread and expanding eutrophication of freshwaters and coastal zones (4) (Fig. 1). Globally, over long time scales of centuries to millennia, P appears to be the nutrient that constrains biotic production of freshwater and ocean ecosystems (5–7). However, long-term global averages fail to express the enormous heterogeneity of reactive N and P supplies to particular sites over days to decades—the space and time scales of ecosystem management. Reactive N and P differ greatly in their mobility in the environment. Reactive N is transported rapidly in the atmosphere and hydrosphere. For example, nitrate is highly mobile in groundwater, and ammonia can move far through the atmosphere before entering aquatic ecosystems. In contrast, P tends to be bound to soil or sediment particles or tightly conserved by organisms. Atmospheric transport of P is limited, and erosion and transport of P in particles can be slow. Differences in mobility, combined with great spatial heterogeneity in abundance, lead to considerable variability among ecosystems in supply rates of reactive N and P (8). Therefore, it is difficult to infer drivers of eutrophication from global fluxes alone.
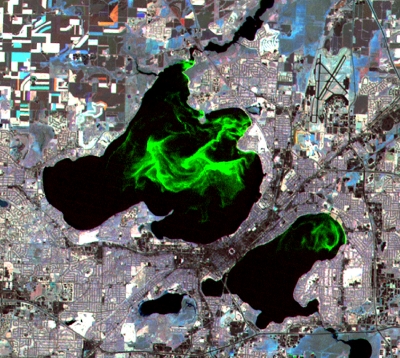
Fig. 1.
Surface blooms of cyanobacteria (Microcystis aeruginosa) in lakes Mendota and Monona, Madison, Wisconsin. Although the lakes can exhibit temporary symptoms of nitrogen limitation during summer blooms (14), eutrophication of these lakes is driven by phosphorus runoff from agricultural and urban lands. False-color LandSat image processed to highlight surface bloom. (Image courtesy North Temperate Lakes Long Term Ecological Research Program, http://lter.limnology.wisc.edu.)
Fifty years ago, no one knew for sure what caused eutrophication, even though the symptoms were well described in scientific literature. Nutrients were suspected, but the evidence was not definitive. Algal abundance was correlated with concentrations of many dissolved compounds, including N and P. Experiments in closed containers sometimes suggested that inorganic carbon (C) limited eutrophication. Other container experiments suggested that N and P were equally limiting for growth of algae and that both were needed to promote algae blooms. Physiological indicators also suggested that N and P were about equally limiting to algae, except during blooms, when cells showed signs of N shortage. The ratio of N to P in the environment, compared with that in algal cells, often suggested N limitation during algae blooms.
The confusion was resolved by a celebrated series of whole-lake experiments at Canada's Experimental Lakes Area (7). These experiments showed unequivocally that P, and not N or C, caused eutrophication. The manipulations produced massive growth of phytoplankton, clearly visible in air photos and from meticulously gathered lake data. Reference systems enriched with inorganic N and C showed no discernible changes. In P-rich lakes, diffusion of inorganic C from the atmosphere and N2 fixation by cyanobacteria are sufficient to meet the C and N demands of algae blooms. Thus, evidence of C or N control of eutrophication is an artifact of closed containers and short experimental durations. When P is abundant, algae appear to be limited by reactive N, but this does not mean that N mitigation will reduce algae blooms.
Now Schindler et al. (1) present the results of a long-term experiment on the mitigation of eutrophication. For 5 years, a lake was eutrophied by adding excess N and P. Then, N inputs were decreased for 16 years, so the N:P supply ratio was below that of algal cells. N fixation by cyanobacteria made up the N deficit. For the final 16 years of the experiment, no N was added to the lake and P fertilization continued. If N can control eutrophication, this treatment should have mitigated algae blooms. However, the lake remained highly eutrophic, with abundant cyanobacteria, even though physiological indicators were consistent with N limitation. Blooms of cyanobacteria are among the most severe consequences of eutrophication, coating shorelines and boat hulls with foul-smelling scum and causing taste and odor problems in drinking water. In addition, some strains are highly toxic. From a water quality perspective, decreasing N inputs alone made eutrophication worse.
It is now generally accepted that P inputs must be decreased to mitigate eutrophication of lakes and reservoirs. However, reactive N is often thought to be the key controlling factor for eutrophication of estuaries or coastal oceans (9). Evidence for N control comes from multiple sources, including N:P ratios, physiological studies of phytoplankton, cross-ecosystem correlations of nutrients and algal productivity, experiments in various-sized containers (some large and open to the atmosphere), long-term observations offshore of sewage treatment plants that have perturbed N and P somewhat independently over time, and mechanistic arguments about biogeochemical differences in lakes and estuaries (10). The evidence resembles that from freshwater science in the 1960s, before whole-ecosystem experiments clarified the roles of P and N in eutrophication. Because nearshore marine ecosystems are large, open, and rapidly flushed by rivers or currents, it has been impossible to perform massive, whole-ecosystem experiments with undisturbed reference ecosystems. In the absence of such experiments, arguments about the roles of N and P in coastal eutrophication are likely to remain unresolved.
Nutrient regulation of coastal marine ecosystems is a critical research topic because of the global expansion of dead zones in these environments. By 2005, 146 coastal marine dead zones had been documented globally, 43 of them in the U.S. (11). In the northern Gulf of Mexico, nutrient discharge from the Mississippi and Atchafalaya rivers creates an extensive dead zone during summer (12). The area of this dead zone was ≈20,000 km2 in 2001, roughly the size of New Jersey. It is expected to become larger as a result of the floods and nutrient inputs of 2008. The dead zone has depleted animal production, with severe impacts on fisheries for shrimp and finfish. There is widespread agreement that the Gulf of Mexico's dead zone could be controlled by reducing flows of nutrients from the two rivers.
Scientific uncertainty about the effects of decreased N vs. P inputs may not matter if management practices control both nutrients at the same time. Control measures for runoff of both N and P include decreased use of fertilizers, containment and treatment of manure, tillage practices that conserve soil, vegetated buffers along shorelines, and maintenance or restoration of wetlands (13). Croplands sensitive to erosion can be converted to other uses that do not pollute waterways. If human diets were less rich in meat, fewer fertilizers would be needed to grow grain for meat production and less manure would be produced. These and other practices mitigate both N and P release to the environment. Nonetheless, in some cases it may be important to know which nutrient is more limiting. Sewage treatment plants, for example, can be engineered to adjust the N:P ratio of discharges to aquatic ecosystems. Information about the relative impacts of N and P could be used to design plants that minimize the cost of eutrophication mitigation.
There are many reasons besides eutrophication to decrease reactive N pollution of the environment. Reactive N emissions are involved in greenhouse warming, smog, growth of weedy terrestrial plants, and human health impacts from air and groundwater pollution (4). These would be important reasons for concern about reactive N emissions, even if eutrophication was not considered.
Yet, the results presented by Schindler et al. (1) show that a single-minded focus on control of reactive N would have disastrous consequences for aquatic resources. To decrease eutrophication, control of reactive N alone is not sufficient—P control is essential and must be included in management programs designed to decrease eutrophication of freshwaters and coastal zones.
Footnotes
The author declares no conflict of interest.
See companion article on page 11254.
References
- 1.Schindler DW, et al. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci USA. 2008;105:11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions and potential solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 3.Bennett EM, Carpenter SR, Caraco NF. Human impact on erodable phosphorus and eutrophication: A global perspective. BioScience. 2001;51:227–234. [Google Scholar]
- 4.Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Status and Trends. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 5.MacKenzie FT, Ver LM, Lerman A. Century-scale nitrogen and phosphorus controls of the carbon cycle. Chem Geol. 2002;190:13–32. [Google Scholar]
- 6.Tyrrell T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature. 1999;400:525–531. [Google Scholar]
- 7.Schindler DW. Evolution of phosphorus limitation in lakes. Science. 1977;195:260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- 8.Vitousek PM, Howarth RW. Nitrogen limitation on the land and in the sea: How can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 9.National Research Council. Clean Coastal Waters: Understanding and Reducing the Effects of Nutrient Pollution. Washington, DC: Natl Acad Press; 2000. [Google Scholar]
- 10.Howarth RW, Marino R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol Oceanogr. 2006;51:364–376. [Google Scholar]
- 11.Dybas CL. Dead zones spreading in world's oceans. BioScience. 2005;55:552–557. [Google Scholar]
- 12.Rabalais NN, Turner RE, Wiseman WJ. Gulf of Mexico hypoxia, a. k.a. the “Dead Zone.”. Annu Rev Ecol Syst. 2002;33:235–263. [Google Scholar]
- 13.Carpenter SR, et al. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl. 1998;8:559–568. [Google Scholar]
- 14.Lathrop RC. Perspectives on the eutrophication of the Yahara lakes. J Lake Reserv Manage. 2007;23:345–365. [Google Scholar]