Abstract
A large number of cytokines and growth factors support the development and subsequent maintenance of postnatal motor neurons. RegIIIβ, also known as Reg2 in rat and HIP/PAP1 in humans, is a member of a family of growth factors found in many areas of the body and previously shown to play an important role in both the development and regeneration of subsets of motor neurons. It has been suggested that RegIIIβ expressed by motor neurons is both an obligatory intermediate in the downstream signaling of the leukemia inhibitory factor/ciliary neurotrophic factor (CNTF) family of cytokines, maintaining the integrity of motor neurons during development, as well as a powerful influence on Schwann cell growth during regeneration of the peripheral nerve. Here we report that in mice with a deletion of the RegIIIβ gene, motor neuron survival was unaffected up to 28 weeks after birth. However, there was no CNTF-mediated rescue of neonatal facial motor neurons after axotomy in KO animals when compared with wild-type. In mice, RegIIIβ positive motor neurons are concentrated in cranial motor nuclei that are involved in the patterning of swallowing and suckling. We found that suckling was impaired in RegIIIβ KO mice and correlated this with a significant delay in myelination of the hypoglossal nerve. In summary, we propose that RegIIIβ has an important role to play in the developmental fine-tuning of neonatal motor behaviors mediating the response to peripherally derived cytokines and growth factors and regulating the myelination of motor axons.
Keywords: Schwann cells, suckling, hypoglossal nerve, LIF
A large number of neurotrophic factors and cytokines have been shown to sustain developing motor neurons and to encourage the survival of postnatal motor neurons after damage (1–6). Rat Reg2 [also known as RegIIIβ in mouse and HIP/PAP1 in humans (7)] has been reported to play an important role in both the support of motor neurons during development and in the process of axon regeneration through axon–Schwann cell signaling in the adult peripheral nervous system (8, 9). Reg2 is a member of a large family of over 17 related genes divided into four subtypes (types I, II, III, and IV) based on the primary structures of the encoded proteins of the genes (10–13). Reg2 is the equivalent of mouse RegIIIβ gene, which share 90% homology at both the nucleotide and protein level. First identified as a transcript up-regulated in pancreatitis, Reg2, a secreted protein (relative Mr 16,000) found in many sites throughout the body, was shown to have an anti-apoptotic action on pancreatic cell lines (14). Reg2 also promotes the growth of epithelial intestinal cells, whereas loss of Reg2/RegIIIβ delayed liver regeneration (10, 15–18).
Two roles have been proposed for Reg2 in the nervous system. First, in vivo studies have suggested that Reg2 is released from damaged motor and sensory neurons and has a proregenerative function (9, 19, 20). Secondly, in vitro data have cast Reg2 as a neurotrophic factor for motor neurons acting as an obligatory intermediate for the ciliary neurotrophic factor (CNTF)/leukemia inhibitory factor (LIF) family of cytokines (8). In the adult rat, Reg2 is massively up-regulated in motor neurons and some sensory neurons after nerve crush (19) and is rapidly transported to the lesion site, where it is thought to be secreted and act on Schwann cells. In vitro, Reg2 has a mitogenic effect on Schwann cells and inhibition of Reg2 with a neutralizing antibody retards the progress of regeneration. Taken together, it seemed likely that the Schwann cell response at the point of axotomy was potentiated by release of Reg2 from damaged axons (9).
Here, we describe the effects of targeted ablation of the RegIIIβ gene in mice and show a developmental role of RegIIIβ in axon–Schwann cell signaling. We also report that RegIIIβ is required to promote the response of subsets of motor neurons to CNTF but not for motor neuron survival.
Results
Generation of RegIIIβ-Deficient Mice.
KO mice were homozygous for a targeted disruption of the RegIIIβ gene locus created in ES cells, in which a region from exons 2 to 5 was deleted and replaced by an IRES-Tau-LacZ-loxP/MC1neopA/loxP reporter/selection cassette, thus resulting in a null allele. Mice homozygous for the RegIIIβ-null allele were phenotypically indistinguishable from wild-type or heterozygous littermates. No embryonic lethality or significant developmental defects were observed in RegIIIβ−/− animals. Adult RegIIIβ−/− mice of both sexes were fertile, and litter size was normal. We compared the expression of RegIIIβ between wild-type and KO animals by using quantitative RT-PCR (RT-qPCR) and found that, as expected, the expression of RegIIIβ mRNA dropped from 100 ± 25.2% (wild type) to 0.08 ± 0.02% (KO) (n = 6 per group). Immunohistochemistry also showed a complete lack of RegIIIβ protein-like immunoreactivity in postnatal KO mice (Fig. 1). To check for potential compensatory up-regulation, we also measured the expression of RegIIIα and RegIIIγ: There was no difference in RegIIIγ expression (100 ± 67% in wild type vs. 62 ± 43% in KO), but we found a substantial elevation of RegIIIα in the brainstem of KO mice (100 ± 26% in wild type vs. 667 ± 181% in KO).
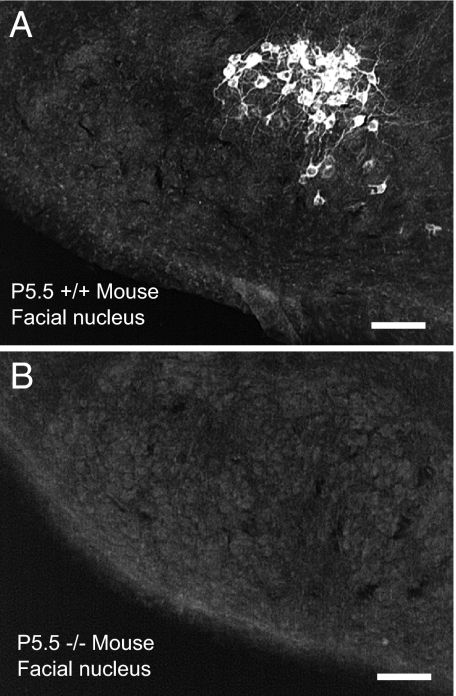
Fig. 1.
RegIIIβ immunoreactivity is found in subsets of facial motor neurons in P5 wild-type mice but was not found in the postnatal KO mouse central nervous system. (A) Facial motor nucleus (P5) of a wild-type mouse. (B) Absence of staining in KO facial nucleus. (Scale bar, 120 μm.)
RegIIIβ Labeling of Neuronal Populations Is Restricted and Developmentally Regulated.
In wild-type embryos, RegIIIβ proteins are first detected in subsets of motor neurons at embryonic day (E)13–E14 in cervical spinal cord, as previously described in rat (8), with expression peaking at E18.5 (2). Within the brainstem at postnatal day (P)1–P5, subsets of neurons along the medial half of the motor nucleus of the trigeminal and in the dorsal and medial regions of the facial nucleus were immunoreactive for RegIIIβ (Fig. 2). The heaviest concentrations of RegIIIβ positive neurons (up to 50% at P1) were found throughout the caudal half of the hypoglossal nucleus (XII) and nucleus ambiguus notably at day 1, but staining intensity reached maximal levels by P4. By P11, RegIIIβ staining had been lost from all areas of the brain and spinal cord.
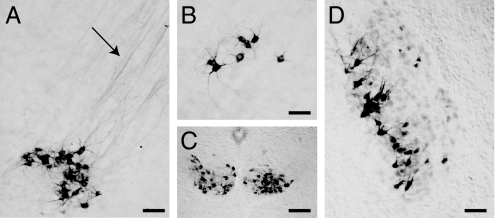
Fig. 2.
RegIIIβ immunoreactivity is found in subsets of cranial and spinal motor nuclei up to P12. (A) Facial motor nucleus with labeled axons (arrow) streaming into the facial nerve. (B–D) RegIIIβ-positive neurons within the nucleus ambiguus (B), the hypoglossal nuclei (C), and the motor nucleus of the trigeminal (D). All specimens were from P5 mice. [Scale bars: 100 μm (A, B, and D); 150 μm (C).]
Motor Neurons Are Not Lost After RegIIIβ Deletion.
Two lines of evidence imply that motor neuron survival is not compromised by loss of RegIIIβ. First, in KO mice, β-galactosidase staining is still visible in motor neurons similar in position to those that show RegIIIβ expression in wild-type mice (Fig. 3). The tau–lac-Z strategy designed to transport β-galactosidase into axons met with limited success because the reaction product was only obviously visualized in motor neuron cell bodies and dendrites. These motor neurons included the spinal motor neurons and trigeminal, facial, hypoglossal, and nucleus ambiguus motor neurons up to P10. Postnatally, sacral motor neurons stained positively for β-galactosidase (Fig. 3B), but brainstem expression was restricted to small foci within the cytoplasm of motor neurons (Fig. 3C) or the occasional heavily stained motor neuron (Fig. 3A). In the spinal cord, β-galactosidase reaction product was found in only the medial column of neurons comparable in position to RegIIIβ immunoreactive neurons seen in wild-type mice (Fig. 3B). β-Galactosidase expression was not present beyond P10. Second, the total number of motor neurones in the facial motor nucleus of P5.5–P10.5 mice or mice 24 weeks old were not significantly different between wild-type and KO mice implying that no motor neuron cell death had resulted from the loss of RegIIIβ [see supporting information (SI) Fig. S1].
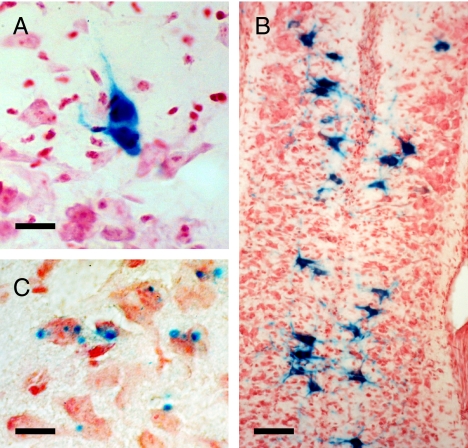
Fig. 3.
β-Galactosidase activity is present postnatally in subsets of motor neurons in the RegIIIβ KO mouse. (A) Motor neuron in the facial motor nucleus at P3. (B) Sacral motor neurons at P1. (C) Punctate β-galactosidase staining in the facial motor nucleus at P5. [Scale bars: 20 μm (A); 60 μm (B); 40 μm (C).]
Lack of Effect of CNTF on Facial Motor Neuron Survival in RegIIIβ KO Mice.
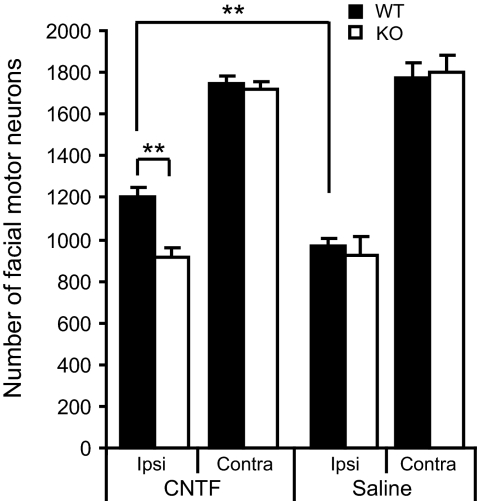
CNTF applied to the cut end of the facial nerve delays the axotomy provoked death of neonatal rat facial motor neurons (21). To examine the effects of CNTF in RegIIIβ KO mice, we cut the facial motor nerve unilaterally at P3.5 and then immediately applied CNTF or saline to the cut end of the nerve. Mice were then allowed to survive for 3 days. The loss of facial motor neurons after facial nerve lesion (FNL) was comparable between wild-type and KO mice (45% and 49% loss when compared with contralateral side in wild-type and KO, respectively) (Fig. 4). There was a significant effect of CNTF on wild-type mice when compared with both CNTF-treated KO and saline-treated wild-type mice (24% and 32% savings respectively, P < 0.001 for both) (Fig. 4). When mice were allowed to survive for four days after FNL, cell loss was considerable but CNTF produced significant savings in the numbers of dying neurons in wild-type mice when compared with KO mice (Fig. S2).
Fig. 4.
Application of CNTF to the cut facial nerve results in increased survival of facial motor neurons in wild-type but not KO mice counted 3 days after nerve section. The data show means ± SEM (n = 4-5 in each group). **, P < 0.001.
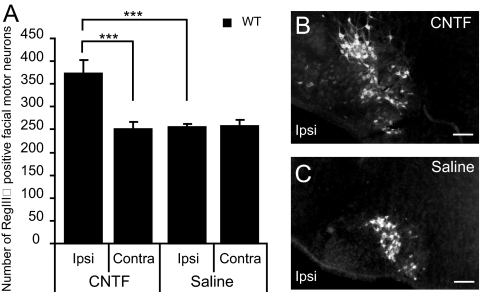
We have previously shown (9) that section of the sciatic nerve results in down-regulation of Reg2/RegIIIβ in motor neurons in rat pups and up-regulation in adult rats. However, in mouse pups with facial nerve axotomy at P3.5 and perfused 2 days later, we did not detect any change in numbers of RegIIIβ-positive neurons in the facial motor nucleus. However, application of CNTF after facial nerve section at P3.5 in mouse pups significantly increased the number of RegIIIβ-positive neurons seen 2 days later to 148% compared with the contralateral side (P < 0.0001) (Fig. 5 and Fig. S3). Finally, whereas in adult rats, Reg2 is expressed by all facial motor neurons within 24h of axotomy, in adult mice, RegIIIβ is not reexpressed in facial motor neurons 1–7 days after axotomy (Fig. S4).
Fig. 5.
CNTF increases the number of RegIIIβ positive neurons. (A) Application of CNTF to the cut facial nerve results in increased numbers of RegIIIβ-positive facial motor neurons in wild-type mice 2 days after nerve section. The data show means ± SEM (n = 7 in each group). ***, P < 0.0001. (B and C) RegIIIβ-positive neurons in wild-type mice after facial nerve cut and CNTF (B) or saline (C) application. The arrow indicates the increased number of RegIIIβ-positive neurons induced by CNTF. [Scale bar, 150 μm (B and C).]
Loss of RegIIIβ Impairs Suckling Behavior.
In newborn mouse pups, we noted that neurons immunoreactive for RegIIIβ were found to be concentrated in cranial motor neurons that have been associated with suckling and swallowing, such as the hypoglossal nucleus and nucleus ambiguus. We therefore examined the ability of RegIIIβ KO mice to ingest milk during a 1-h period of suckling after a 2-h period of isolation from the mother. Compared with wild-type pups, we found a significant reduction in milk/colostrum ingestion: percentage weight increase in wild-type mice, 3.1 ± 0.2%; and in KO mice, 0.45 ± 0.2 (P < 0.01).
Loss of RegIIIβ Delays Myelination of the Hypoglossal Nerve.
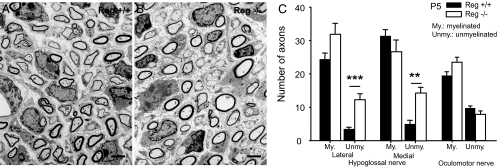
Because of the influence of Reg2 on Schwann cells (9) and the reduced efficiency of suckling described above, we used electron microscopy to analyze myelination in the hypoglossal nerve at P5 in wild-type and KO mice. Oculomotor neurons never express RegIIIβ, and we, therefore, used the P5 oculomotor nerve as a control. We found a significant increase in the numbers of unmyelinated nerve fibres in both the medial and lateral hypoglossal nerves in KO mice at P5 (Fig. 6 A–C). One-way ANOVA revealed a significant effect of genotype on axon type in both the medial (F1,14 = 24.8; P < 0.01) and lateral hypoglossal nerves (F1,13 = 21.5; P < 0.01) There were no differences in the numbers of unmyelinated axons in the oculomotor nerves in P5 wild-type and KO mice. In the lateral hypoglossal nerve, the total numbers of axons in the RegIIIβ KO mice exceeded that in the wild-type animals (Fig. 6C). We therefore counted the total number of myelinated axons in the lateral and medial hypoglossal nerves at P21. We found that there were no differences between the two groups (wild type: lateral, 352.3 ± 25.8; medial, 980.3 ± 9.5; KO: lateral, 360.0 ± 16.5; medial, 950.3 ± 41.9 KO; n = 3 in each group). We concluded that excess collateralization of hypoglossal axons occurs because of delayed myelination during the early postnatal period in KO mice and is lost later by selective pruning of excess axonal branches (22, 23).
Fig. 6.
Increased number of unmyelinated axons in RegIIIβ KO mice. Electron micrograph of the medial hypoglossal nerve of RegIIIβ wild-type (A) or RegIIIβ KO (B) mice. There is an increase in the number of unmyelinated fibers in the lateral and medial hypoglossal motor nerve of RegIIIβ KO mice (n = 8 in each group). (C) No differences were observed in the oculomotor nerve (n = 3 in each group). The data show means ± SEM. **, P < 0.001; ***, P < 0.0001. All nerves were studied at P5. [Scale bar, 2 μm (A and B).]
The impaired suckling phenotype, thus, may result from a disruption of myelination in mutant mice. We also examined the hypoglossal innervation of tongue musculature in wild-type and KO mice by using protein gene product (PGP) as a marker for nerve fibers and α-bungarotoxin for muscle end plates. We found no evidence of changes in innervation density or size of muscle end plates (data not shown).
Discussion
Previous research has suggested that in vitro rat Reg2 is a motor neuron survival factor essential for the actions of CNTF-like cytokines and a powerful Schwann cell mitogen that potentiates axonal repair and regeneration in vivo (8, 9). However, we show here that in mice with a genetic deletion of the RegIIIβ gene (the equivalent gene to Reg2 in rats), there is no increased motor neuron cell death during development. Nevertheless, we report that the efficacy of CNTF in reducing neuronal cell death was diminished in RegIIIβ KO mice indicating that RegIIIβ is important for the survival functions of CNTF. Finally, we found that myelination was delayed in subsets of hypoglossal motor neurones in KO animals and the efficiency of milk ingestion reduced.
RegIIIβ Is an Intermediate in the CNTF Survival Pathway.
The suggestion that Reg2/RegIIIβ was a motor neuron neurotrophic factor and a signaling intermediary in the CNTF survival pathway stems from elegant in vitro studies demonstrating that purified Reg2 can act as a paracrine/autocrine neurotrophic factor for a subset of motor neurons (8). Furthermore, it has been shown that CNTF, as well as the related factors LIF, cardiotrophin 1, and oncostatin-M, rapidly induces Reg2 mRNA in some motor neurons and that released Reg2 acts in a paracrine/autocrine fashion to support motor neurons (8, 9). The CNTF/LIF family of cytokines signal through a receptor complex that includes the LIF receptor (LIFR) subunit, and it has been shown previously that in LIFR KO mice, there is no expression of Reg2/RegIIIβ during development (9). Thus, Reg2/RegIIIβ expression in some developing motor (and sensory) neurons depends on cytokines of the LIF family acting through a receptor complex containing LIFR.
The absence of motor neuron cell death in KO mice, therefore, is puzzling, especially given that RegIIIβ is essential for the in vitro neuronal survival effects of CNTF to be manifest. The evidence for a lack of effect on motor neuron survival comes from the presence of β-galactosidase reaction product in postnatal neurons up to P10 and the normal numbers of facial motor neurons in KO mice. However, in CNTF KO mice, there is similarly no increased loss of motor neurons during the first weeks of life (24, 25). This, perhaps, is not surprising, given that levels of CNTF are low during development and, although Schwann cells are the richest source of CNTF in the adult peripheral nervous system, levels only rise during the first postnatal week (26–28). Knockout of the CNTF gene did result in motor neuron loss 28 weeks after birth (24); however, RegIIIβ mice did not show motor neuron loss in later adult stages.
Previous studies suggest that CNTF itself is not the key ligand acting at the CNTF/LIF receptor complex. Nishimune et al. (8) found that RegIIIβ expression was unimpaired or delayed in a range of KOs including cntf, ct1, and cntf/lif double mutants, leading them to suggest that a factor as yet unknown was key to driving the expression of RegIIIβ in motor neurons.
That CNTF-like factors are as important in vivo as in vitro and require ReIIIβ expression is implied by the observation that RegIIIβ expression increases in axotomized neurons only when CNTF is applied to the nerve stump and that CNTF has no survival effect on motor neurons in RegIIIβ KO mice. We also show that the number of RegIIIβ neurons increased in wild-type mice when CNTF was applied to the cut nerve. This implies that many motor neurons have the capacity to express RegIIIβ, but this number is restricted by availability of CNTF-like factors during development. Nevertheless, RegIIIβ may not be the only intermediary involved in CNTF-like factor signaling as only ≈15–20% of facial motor neurons express RegIIIβ, and CNTF has previously been shown to rescue ≈75% of rat facial motor neurons from cell death when axotomized soon after birth (24). However, the substantially larger concentration of CNTF used in these experiments (5 μg vs. 250 ng used in the present study) may well have driven the expression of RegIIIβ in many more axotomized facial motor neurons than seen here and resulted in greater levels of survival (24). Also the dynamics of Reg2 expression in rat are different from that of RegIIIβ in mice. RegIIIβ never reappeared in the adult mouse after section of the facial nerve (Fig. S4) or sciatic nerve (data not shown) and did not noticeably decrease 24 h after axotomy, as was observed after sciatic transection in neonatal rats. Presumably other factors may play a similar role to RegIIIβ in adult mouse motor neurons, although it is unlikely to be RegIIIα, which although up-regulated in the KO mouse, did not potentiate CNTF-mediated survival.
Growth Factors and Myelination.
We show here that myelination of a subset of hypoglossal motor neuron axons is delayed in RegIIIβ KO mice at P5.5 but normal by P21. This seems likely to be attributable to the loss of RegIIIβ because myelination of oculomotor axons in KO mice was normal and oculomotor motor neurons did not express RegIIIβ at any stages of development. This delayed myelination would be expected to disrupt the transmission of signals along the axon and may account for the reduced efficiency of suckling behavior. Delayed myelination did not, however, result in any reduction in the size or number of motor neuron end plates in target muscles of the hypoglossal such as the glossohyoid (unpublished data). However, how then does RegIIIβ promote myelination in discrete populations of motor neuron axons, and why is there such a restricted pattern of RegIIIβ expression?
Axons regulate Schwann cells during development of the peripheral nervous system (29–33). The axon signals for regulating Schwann cell differentiation are known to include both proteins encoded by the neuregulin gene (NRG) signaling through the erbB family of receptors, adhesion molecules such as L1 and N-cadherin and neurotrophic factors such as brain-derived neurotrophic factor (BDNF) (34–37). It has been suggested that cell adhesion molecules on the axon surface as well as signals from the extracellular matrix and neurotrophins are also required for myelination to proceed efficiently and accurately. For example, BDNF supports postnatal facial motor neurones after axotomy and acts through p75 NTR to inhibit Schwann cell migration and promote myelination (35, 36, 38). It seems likely that RegIIIβ released from subsets of motor neuron axons is playing much the same role as BDNF in driving the process of myelination. As with NRG1 type III, it has been shown that RegIIIβ signals through the PI3-kinase pathway (8, 17), and this may represent a common pathway for axonally released RegIIIβ to influence Schwann cells. Why RegIIIβ expression is restricted to motor neurons concerned with the suckling and swallowing is unclear, but we suggest that the patterning of this critical function in neonatal mice may be plastic and regulated by release of factors from target musculature concerned with suckling in an activity-dependent fashion (8).
Materials and Methods
All procedures complied with the United Kingdom Animals (Scientific Procedures) Act 1986.
Generation of the RegIIIβ-Deficient Mice by Gene Targeting.
The generation of these mice has been described elsewhere (18). Complete knockout of the gene was confirmed in nervous tissue with immunocytochemistry by using antibodies generated against rat Reg2 protein, and absence of RegIIIβ mRNA was confirmed by using real-time PCR in brain, pancreas, and regenerating liver (16, 18).
Real-Time RT-qPCR Assay.
Tissue preparation and RNA extraction were as described previously (39). RNA samples were treated with DNase I (Qiagen). Equal amounts (3 μg) of total RNA were reversed transcribed by using random nonamers (Sigma) and SuperScript TM III RT (Invitrogen) for 1 h at 50°C in a total reaction volume of 20 μl. cDNAs were immediately quantified by real-time PCR or kept at −20°C until further experiments. Real-time PCRs were performed with a DNA Engine (Bio-Rad) by using SYBR Green Jump Start RT-PCR master mix (Sigma) with each gene-specific primer (RegIIIβ forward, 5′-AAGAATATACCCTCCGCACGC-3′; RegIIIβ reverse, 5′-CAGACATAGGGCAACTTCACC-3′; RegIIIα forward, 5′-GAAGTGCCCTCTCCACGTACC-3′; RegIIIα reverse, 5′-ACAAATGGTAATGTCCCATCG-3′; RegIIIγ forward, 5′- GCTCCTATTGCTATGCCTTGTTTAG-3′; RegIIIγ reverse 5′-CATGGAGGACAGGAAGGAAGC-3′; β-actin forward, 5′-CAACGAGCGGTTCCGATG-3′; β-actin reverse, 5′-GCCACAGGATTCCATACCCA-3′). One microliter of cDNA was amplified in a three-step cycling program in a final reaction volume of 25 μl. Control cDNA samples (obtained without transcriptase) were always included, as well as samples without any cDNA template. Reactions were performed in triplicate for five to six biological replicates, and threshold cycle values were normalized to β-actin gene expression. The specificity of the products was determined by melting curve analysis. The ratio of the relative expression of target genes to β-actin was calculated by using the 2ΔCT formula.
Facial Nerve Section.
Pups were cooled on wet ice (4°C), and the extent of anesthesia was determined by assessing reflex responses to tail pinch. Adult mice were anesthetized with Fluothane. The right facial nerve was transected at the stylomastoid foramen. One to 10 days later, mice were deeply anesthetized with Euthatal i.p. and transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer (PB) (pH 7.4) preceded by a brief wash with heparinized saline. After a 2 h postfix in PFA, tissue was moved to sucrose (30%) PB solution in preparation for freeze sectioning. In some pups, 5 μl of CNTF (250 ng) applied to a piece of Gelfoam in 5 μl saline, or saline alone, was applied to the cut end of the facial nerve at the time of section. Animals received only one treatment: either CNTF or saline.
Detection of β-Galactosidase Staining by X-Gal Staining.
PFA-fixed tissue was washed in PBT (PBS/0.1% Tween-20) several times. After a rinse in X-Gal staining solution [5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Triton plus X-Gal (4-chloro,5-bromo,3-indolyl-β-galactosidase) at 1 mg/ml, suspended in PBS (pH 7.2)], tissue was transferred to fresh staining solution. Tissue was incubated at 37°C in the dark overnight. Sections were washed several times with PBT.
Cell Counting.
Sections (40 μm) were cut through the facial nucleus and all sections (the facial nucleus is ≈700 μm in length in the anterior–posterior direction) were mounted and stained with neutral red. All neuronal profiles in every section were counted and total counts corrected by using the Abercrombie correction (40).
Immunohistochemistry.
Mice were deeply anesthetized with pentobarbitone (60 mg/kg, i.p.) and transcardially perfused with 4% PFA in 0.1 M sodium phosphate buffer (PB) (pH 7.4) preceded by a brief wash with heparinized saline. Tissue was dissected and postfixed for 2 h at 4°C and then transferred to 30% (wt/vol) sucrose in 0.1 M PB containing 0.02% (wt/vol) sodium azide. 40 μm free-floating tissue sections were processed as previously described but for detection of RegIIIβ (9, 39). Hypoglossal innervation of tongue musculature in wild-type and KO mice was assessed with PGP, a marker for nerve fibres, and α-bungarotoxin, a marker for muscle end plates.
Electron Microscopy.
Mouse pups or adult mice (P21) were deeply anesthetized with Nembutal and perfused with 10 ml heparinized saline followed by 20 ml 4% PFA plus 0.5% gluteraldehyde. Tissue was fixed overnight before dissection of the caudal tongue and attached hypoglossal nerves and the oculomotor nerve still attached to the optic nerve and eyecup. After being osmicated (30 min in 1% OsO4 in 0.1 M PB), the sections were stained for 15 min in 0.1% uranyl acetate in sodium acetate buffer at 4°C, dehydrated in ethanols, cleared in propylene oxide, and embedded in Araldite. Semithin sections were cut with glass knives and stained with toluidine blue adjacent to thin sections cut with a diamond knife on an Ultracut ultramicrotome (Reichert). The sections were collected on mesh grids coated with a thin Formavar film, counterstained with lead citrate, and viewed in a JEOL 1010 electron microscope. Counts were made from four photographs of the lateral and medial nerves for each animal taken at ×4,000 magnification. Total counts of myelinated fibers at P21 were made from photomicrographs of the lateral and medial hypoglossal nerves at ×1,000 magnification.
Measurement of Milk/Colostrum Intake.
The procedure described by Fujita and colleagues (41, 42) was followed. Briefly, pups were separated from the dam for 2 h and kept in a warm, dark chamber. The pups were then weighed and placed back with the mothers for 1 h before reweighing. Thirty to 40 pups of each genotype were used for the measurement of milk intake.
Statistical Analysis.
The data are expressed as means ± SEM. The data were analyzed by general linear model univariate or multivariate test, as appropriate, followed by Bonferroni post hoc tests or Student's t test, as appropriate. For all statistical analysis, statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments.
We thank Austin Smith for support and advice during the early stages of this project and Rhona Mirsky for discussion. This work was supported by the Motor Neuron Disease Society (United Kingdom) and the Medical Research Council (United Kingdom).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711978105/DCSupplemental.
References
- 1.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 2.Henderson CE, et al. Role of neurotrophic factors in motoneuron development. J Physiol Paris. 1998;92:279–281. doi: 10.1016/s0928-4257(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheim RW. Neurotrophic survival molecules for motoneurons: An embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 4.Sendtner M, Holtmann B, Hughes RA. The response of motoneurons to neurotrophins. Neurochem Res. 1996;21:831–841. doi: 10.1007/BF02532307. [DOI] [PubMed] [Google Scholar]
- 5.Thoenen H, Hughes RA, Sendtner M. Trophic support of motoneurons: Physiological, pathophysiological, and therapeutic implications. Exp Neurol. 1993;124:47–55. doi: 10.1006/exnr.1993.1173. [DOI] [PubMed] [Google Scholar]
- 6.Wiese S, Beck M, Karch C, Sendtner M. Signalling mechanisms for survival of lesioned motoneurons. Acta Neurochir Suppl. 2004;89:21–35. doi: 10.1007/978-3-7091-0603-7_4. [DOI] [PubMed] [Google Scholar]
- 7.Narushima Y, et al. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII alpha, RegIII beta, RegIII gamma. Gene. 1997;185:159–168. doi: 10.1016/s0378-1119(96)00589-6. [DOI] [PubMed] [Google Scholar]
- 8.Nishimune H, et al. Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat Cell Biol. 2000;2:906–914. doi: 10.1038/35046558. [DOI] [PubMed] [Google Scholar]
- 9.Livesey FJ, et al. A Schwann cell mitogen accompanying regeneration of motor neurons. Nature. 1997;390:614–618. doi: 10.1038/37615. [DOI] [PubMed] [Google Scholar]
- 10.Iovanna JL, Dagorn JC. The multifunctional family of secreted proteins containing a C-type lectin-like domain linked to a short N-terminal peptide. Biochim Biophys Acta. 2005;1723:8–18. doi: 10.1016/j.bbagen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto H. The Reg gene family and Reg proteins: With special attention to the regeneration of pancreatic beta-cells. J Hepatobiliary Pancreat Surg. 1999;6:254–262. doi: 10.1007/s005340050115. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto H, Takasawa S. Recent advances in the Okamoto model: The CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in beta-cells. Diabetes 51 Suppl. 2002;3:S462–S473. doi: 10.2337/diabetes.51.2007.s462. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YW, Ding LS, Lai MD. Reg gene family and human diseases. World J Gastroenterol. 2003;9:2635–2641. doi: 10.3748/wjg.v9.i12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malka D, et al. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology. 2000;119:816–828. doi: 10.1053/gast.2000.16491. [DOI] [PubMed] [Google Scholar]
- 15.Christa L, et al. Hepatocarcinoma-intestine-pancreas/pancreatic associated protein (HIP/PAP) is expressed and secreted by proliferating ductules as well as by hepatocarcinoma and cholangiocarcinoma cells. Am J Pathol. 1999;155:1525–1533. doi: 10.1016/S0002-9440(10)65468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gironella M, et al. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56:1091–1097. doi: 10.1136/gut.2006.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieu HT, et al. HIP/PAP accelerates liver regeneration and protects against acetaminophen injury in mice. Hepatology. 2005;42:618–626. doi: 10.1002/hep.20845. [DOI] [PubMed] [Google Scholar]
- 18.Lieu HT, et al. Reg2 inactivation increases sensitivity to Fas hepatotoxicity and delays liver regeneration post-hepatectomy in mice. Hepatology. 2006;44:1452–1464. doi: 10.1002/hep.21434. [DOI] [PubMed] [Google Scholar]
- 19.Averill S, Davis DR, Shortland PJ, Priestley JV, Hunt SP. Dynamic pattern of reg-2 expression in rat sensory neurons after peripheral nerve injury. J Neurosci. 2002;22:7493–7501. doi: 10.1523/JNEUROSCI.22-17-07493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam J, Rosenberg L, Maysinger D. INGAP peptide improves nerve function and enhances regeneration in streptozotocin-induced diabetic C57BL/6 mice. FASEB J. 2004;18:1767–1769. doi: 10.1096/fj.04-1894fje. [DOI] [PubMed] [Google Scholar]
- 21.Sendtner M, et al. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992;358:502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- 22.Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Ueda R, Takahashi K, Saigo K, Uemura T. Control of axonal sprouting and dendrite branching by the Nrg-Ank complex at the neuron-glia interface. Curr Biol. 2006;16:1678–1683. doi: 10.1016/j.cub.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 24.Masu Y, et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 25.Sendtner M, Arakawa Y, Stockli KA, Kreutzberg GW, Thoenen H. Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J Cell Sci Suppl. 1991;15:103–109. doi: 10.1242/jcs.1991.supplement_15.14. [DOI] [PubMed] [Google Scholar]
- 26.Holtmann B, et al. Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J Neurosci. 2005;25:1778–1787. doi: 10.1523/JNEUROSCI.4249-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweizer U, et al. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol. 2002;156:287–297. doi: 10.1083/jcb.200107009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockli KA, et al. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J Cell Biol. 1991;115:447–459. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ffrench-Constant C, Colognato H, Franklin RJ. Neuroscience. The mysteries of myelin unwrapped. Science. 2004;304:688–689. doi: 10.1126/science.1097851. [DOI] [PubMed] [Google Scholar]
- 30.Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–376. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 32.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taveggia C, Salzer JL. PARsing the events of myelination. Nat Neurosci. 2007;10:17–18. doi: 10.1038/nn0107-17. [DOI] [PubMed] [Google Scholar]
- 34.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 35.Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci USA. 2001;98:14661–14668. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolwani RJ, et al. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J Neurosci Res. 2004;77:662–669. doi: 10.1002/jnr.20181. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12:4171–4180. [PubMed] [Google Scholar]
- 38.Yan Q, Elliott J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360:753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 39.Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogelezang MG, et al. Alpha4 integrin is expressed during peripheral nerve regeneration and enhances neurite outgrowth. J Neurosci. 2001;21:6732–6744. doi: 10.1523/JNEUROSCI.21-17-06732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita K, et al. Effects of hypoglossal and facial nerve injuries on milk-suckling. Int J Dev Neurosci. 2006;24:29–34. doi: 10.1016/j.ijdevneu.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Fukuyama T, et al. Differential effects of hypoglossal and facial nerve injuries on survival and growth of rats at different developmental stages. Int J Dev Neurosci. 2006;24:307–317. doi: 10.1016/j.ijdevneu.2006.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.