Abstract
Genetic studies have shown that ubiquitination and endocytosis of the Drosophila ligand Delta in signal-sending cells are required for activation of Notch signaling, but how these events promote Notch activation remains poorly understood. Here, we show that an ubiquitination-defective mutant of the murine Delta-homologue Dll1 is endocytosed but, in contrast to the wild-type Dll1, is unable to subsequently recycle back to the cell surface or to bind Notch1 efficiently. These results demonstrate that ubiquitination, although not required for endocytosis, is essential for Dll1 recycling and that recycling is required to acquire affinity for the receptor. On the other hand, a chimeric molecule encompassing the extracellular domain of Dll1 and the transmembrane/intracellular domain of Dll3, which contains no lysine, is endocytosed, recycled, and interacts with Notch1 but is unable to induce transendocytosis of the extracellular region of Notch1 or to signal. These observations suggest that the chimera uses an ubiquitination-independent signal to recycle, allowing it to acquire affinity for Notch1. Our results support the idea that ligand recycling determines its competence to bind efficiently to the receptor but that this is insufficient to allow it to perform transendocytosis, an event required for activation of Notch signaling. Finally, the present study indicates that Dll1 partially localizes to lipid microdomains, whereas both ubiquitination-defective Dll1 and the Dll1–3 chimera are excluded from these compartments, suggesting that these microdomains provide the environment necessary for Dll1 to activate Notch signaling.
Keywords: ubiquitination, recycling, transendocytosis, membrane microdomains
Notch signaling is an evolutionary conserved pathway, which ensures correct specification of multiple cell types through local cell–cell interactions (1). Ligands of the DSL (Delta/Serrate/Lag2) family expressed at the cell surface interact with heterodimeric Notch molecules on adjacent cells and activate a signaling cascade, which relies on consecutive Notch cleavages. The linear nature of this signaling pathway apparently leaves little space for fine tuning and/or amplification, but data suggest that specific events affecting receptors and ligands contribute to regulate Notch signaling (2). In particular, endocytosis has been shown to play a key role in Notch signaling. In Drosophila, Seugnet et al. (3) have shown that dynamin-dependent endocytosis is required for Notch activation in both signal-receiving and signal-sending cells. Two main models have been proposed to explain this requirement in signal-sending cells (for review, see refs. 4–6). The first one postulates that endocytosis is required after ligand/Notch interaction to generate a sufficient “pulling” strength to dissociate the heterodimeric Notch receptor or to modify its conformation to allow Notch cleavages. The second one postulates that endocytosis followed by recycling back to the plasma membrane is necessary to generate an “active” ligand, although how the ligand becomes active is currently unknown. Incidentally, the two models are not mutually exclusive, and therefore, endocytosis might be required both to generate an active ligand and to induce Notch cleavages. Genetic studies in Drosophila have identified two E3 ubiquitin ligases, Neuralized (Neur) and Mind bomb (Mib), as regulators of ligand activity. Neur and Mib have been shown to promote ligand ubiquitination and internalization (5).
To establish a link between ligand ubiquitination, trafficking, and activation of the Notch signaling cascade, we examined trafficking and signaling activity of three Dll1 derivatives that differ essentially in their intracellular domain: wild-type (wt) murine Delta-like 1 (Dll1), a ubiquitination-defective Dll1 mutant that contains no acceptor lysine residue in its intracellular domain, and a chimeric molecule encompassing the extracellular region of Dll1 and the transmembrane and the intracellular region of murine Delta-like 3 (Dll3), which is a Delta homologue naturally devoid of lysine in its intracellular domain. The ability of the three molecules to interact with Notch1, induce transendocytosis of the extracellular region of Notch1 (NECD), and activate the subsequent signaling cascade was assayed. Our results demonstrate that Dll1 ubiquitination is not required for its internalization but is necessary for its recycling back to the plasma membrane and efficient interaction with Notch1. However, although it allows recycling and interaction with Notch of the chimeric molecule, the intracellular region of Dll3 does not allow it to induce NECD transendocytosis or to activate signaling. Finally, we investigated whether wt and mutant Dll1 reside in membrane microdomains. By providing an ordered membrane microenvironment, lipid rafts (7) or other domains may contribute to the clustering of Dll1 molecules or to the interaction with specific cofactors (4). Interestingly, a genetic screen performed in Caenorhabditis elegans identified BRE-5/Brainiac as a positive regulator of Notch signaling that acts before the ligand-induced cleavage of Lin-12 and may target the ligands (8). Brainiac is an enzyme that participates in the biosynthesis of glycosphingolipid, a component of lipid rafts. Indeed, we observed that wt Dll1 preferentially localizes to lipid microdomains, whereas the ubiquitin-defective Dll1 mutant and the Dll1–3 chimera were almost excluded from these fractions.
Results
Ubiquitination and Endocytosis of wt and Mutant Dll1.
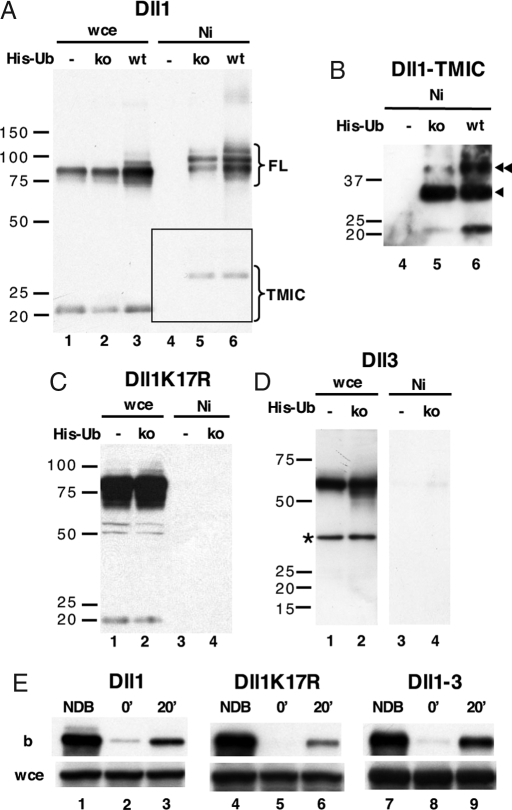
To define the importance of ubiquitination in the regulation of wt Dll1 trafficking, we generated a Dll1 mutant, Dll1K17R, by replacing the 17 intracellular lysine residues with arginine. HEK 293T cells were transiently cotransfected with Dll1 and wt His-tagged ubiquitin (wt His-Ub) and lysed, and then the ubiquitinated proteins were analyzed. Fig. 1A (lane 6) indicates that two major species of Dll1 are detected and undergo ubiquitination, the full-length (FL) and the metalloprotease-mediated cleavage product [TMIC for TransMembrane and IntraCellular domains (9)]. The apparent molecular weight difference between the TMIC or the FL forms present in the crude extract (Fig. 1A, lanes 1–3) and those bound to nickel beads (Fig. 1A, lanes 5–6) suggest that the latter represent poly- or multiubiquitinated species. To clarify this issue, we used a His-tagged ubiquitin construct in which all lysine residues were mutated to arginine (ko His-Ub). Nickel bead pull-down experiments using the ko His-Ub confirmed that Dll1 was monoubiquitinated at several positions (Fig. 1A, lane 5). A longer exposure revealed that Dll1-TMIC was also monoubiquitinated on one or two lysines (Fig. 1B, lanes 5–6). We then analyzed Dll1K17R and Dll3, which do not contain any lysine in their intracellular domain. As expected, no ubiquitinated species of either protein could be isolated on nickel beads (Fig. 1 C and D, respectively). To compare the role of the intracellular sequence of Dll1 and Dll3, we generated a Dll1–3 chimera encompassing the extracellular domain of Dll1 and the transmembrane and intracellular domain of Dll3. No ubiquitinated species of Dll1–3 were detected by nickel bead pull-down experiments (data not shown).
Fig. 1.
Ubiquitination and endocytosis. (A–D) Ubiquitination of wt Dll1 (A and B; B is a longer exposure of the area boxed in A), Dll1K17R (C) and Dll3 (D). HEK 293T cells were transfected with ligands, without (−) or with wt or lysine-less (ko) His-Ub. Whole-cell extracts (wce) and fractions retained on nickel beads (Ni) were analyzed by immunoblotting with anti-Dll1CT (A–C) or anti-Dll3ic antibody (D). (E) Surface proteins of OP9 stromal cell lines stably expressing Dll1, Dll1K17R, or Dll1–3 were labeled with NHS-SS-biotin and incubated for 20 min at 37 °C (20′) or left at 4 °C (0′). Cell surface biotin was then stripped with MesNa. Whole-cell extracts (wce) and fractions retained on streptavidin-agarose (b) were analyzed by immunoblotting with anti-Dll1CT or anti-Dll3ic antibody. Arrowheads in B indicate ubiquitinated forms of Dll1-TMIC. The star (D) shows a band arising from a nonspecific reaction with the anti-Dll3ic antibody. FL, full-length Dll1 species; TMIC, Dll1 TMIC species; NDB, not debiotinylated cells.
To investigate the physiological significance of Dll1 monoubiquitination, we compared the efficiency and kinetics of internalization of Dll1, Dll1K17R, and Dll1–3 stably transfected into OP9 stromal cell lines by using a reversible biotinylation strategy involving surface biotinylation at 4°C followed by endocytosis at 37°C. Preliminary experiments showed that maximal internalization took place 20 min after the temperature switch (data not shown). When cells were kept at 4°C, therefore preventing internalization, treatment with the membrane-impermeable reducing agent MesNa abrogated detection of the ligands, indicating that it successfully removed 90–100% of surface biotin (Fig. 1E, lanes 2, 5, and 8). After 20 min at 37°C, biotinylated species of the three Dll1 derivatives could be detected, indicating that they had been internalized (Fig. 1E, lanes 3, 6, and 9). The extent of Dll1-3 internalization was similar to that of wt Dll1. Dll1K17R was also internalized, although not as efficiently as wt Dll1. These results indicate that Dll1 ubiquitination is not essential for its internalization.
Recycling of wt and Mutant Dll1.
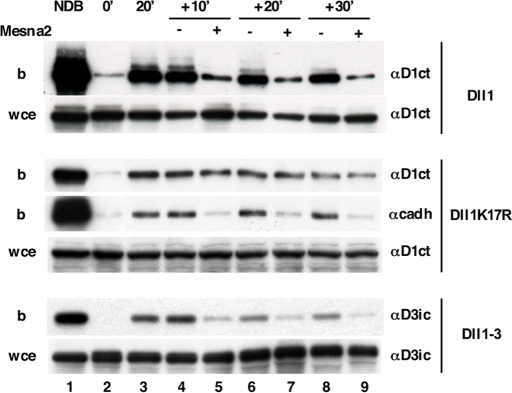
As direct evidence of recycling of Dll1 or other Notch ligands has not been provided so far, we assayed this event through an extension of the endocytosis assay used above. After 20 min of endocytosis and a first MesNa treatment, cells were incubated at 37°C to allow transport through recycling endosomes for various periods of time (10, 20, or 30 min). At each time point, some cells were reexposed to MesNa to strip biotin from ligands that had reached the cell surface. Immunoblots were quantified as described in supporting information (SI) Text. At least 60–70% of Dll1 and Dll1–3 had recycled to the cell surface after 10 min (Fig. 2). This rate was constant during 30 min. The amount of Dll1K17R was identical whether the cells were treated with MesNa or not, indicating that the mutant Dll1K17R was not able to recycle. As an internal control, we used cadherin, whose recycling has been largely documented (10). Fig. S1 indicates that the half-lives of surface-expressed and endocytosed Dll1 and Dll1K17R are different. Dll1K17R is degraded 2.5-fold more rapidly than the wt ligand, most likely as a consequence of its inability to recycle, which implicated feedback into the biosynthetic and degradation rates. All together, our data suggest that Dll1 recycling depends on its ubiquitination but that the intracellular domain of Dll3 allows recycling in the absence of ubiquitination.
Fig. 2.
Dll1 and Dll1–3, but not Dll1K17R, are recycled back to the plasma membrane. After internalization of biotinylated proteins for 20 min as described in Fig. 1E, cells were reincubated at 37°C for 10′, 20′, and 30′ and then submitted (+) or not (−) to a second MesNa treatment (Mesna2). Cadherin was used as an internal positive recycling control. Whole-cell extracts (wce) and fractions retained on streptavidin-agarose (b) were analyzed by immunoblotting with anti-Dll1CT (αD1ct), anti-Dll3ic (αD3ic), or anti-pan cadherin (αcadh) antibody.
Binding of Notch to Cells Expressing wt or Mutant Dll1.
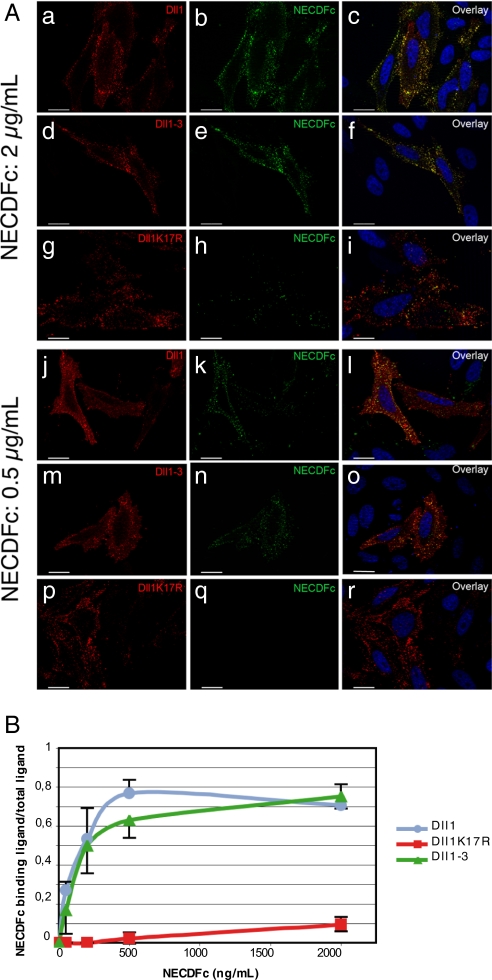
We next asked whether the three different Dll1 derivatives were able to interact with the extracellular domain of Notch1 fused to the Fc domain of human Ig (NECDFc). NECDFc preclustered with an anti-IgG-Fc has previously been shown to interact with surface-expressed Dll1 (11). We transiently transfected HeLa cells with either wt Dll1, Dll1K17R, or Dll1–3 tagged with an extracellular VSV tag and incubated them with NECDFc preclustered with a FITC-conjugated anti-IgG-Fc. Cells were then fixed and immunolabeled with Cy3-conjugated anti-VSV to detect the ligands. Fig. 3A shows that, under conditions of similar level of surface expression of the ligands, wt Dll1 (Fig. 3 Aa–c and j–l) as well as Dll1–3 (Fig. 3 Ad–f and m–o) interact with NECDFc. Notch binding to Dll1-expressing cells was detected at low (Fig. 3 Aj–l) and high (Fig. 3 Aa–c) Notch concentrations, whereas binding to Dll1K17R was only weakly detectable at a high Notch concentration (Fig. 3 Ag–i), indicating that Dll1K17R interacts with the receptor with a much lower affinity than wt Dll1 or Dll1–3. To confirm our observations, these experiments were repeated by using four different NECDFc concentrations. The quantification of this experiment (Fig. 3B) shows that Dll1 and Dll1–3 exhibit a similar affinity for NECDFc, whereas the affinity of Dll1K17R is much lower. All together, these results demonstrate that ligand recycling is necessary to acquire a strong affinity for the receptor.
Fig. 3.
Preclustered NECDFc binding to Dll1, Dll1–3 or Dll1K17R-expressing cells. (A) HeLa cells were transiently transfected with VSV-tagged Dll1 (a–c and j–l), Dll1–3 (d–f and m–o), or Dll1K17R (g–i and p–r) construct and incubated with 2 μg/ml or 0.05 μg/ml NECDFc preclustered with a FITC-conjugated anti-Fc antibody (b,e,h,k,n, and q). Cells were stained with a Cy3-conjugated anti-VSV antibody (a,d,g,j,m, and p). Hoechst staining is shown. (Scale bar, 20 μm.) (B) Same experiment as in A but with four different concentrations of NECDFc. The amount of surface ligand colocalizing with NECDFc versus the total amount of surface ligand was quantified by using AxioVision 4.6.3 (n = 10) and plotted as a function of NECDFc concentration.
Signaling Activity of wt or Mutant Dll1.
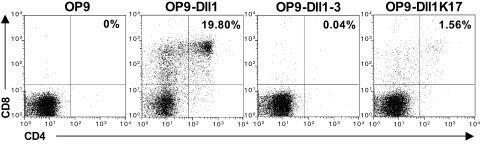
To test the signaling activity of the Dll1 derivatives, we took advantage of the OP9-Dll1 coculture system that enables in vitro T cell differentiation of hematopoietic precursor cells through Notch signaling (12). Murine ScaI+ precursor cells were cocultured with OP9 cell lines expressing one of the three Dll1 derivatives (at similar levels). After 21 days, flow cytometric analysis showed that OP9 cells expressing wt Dll1 induced differentiation of precursor cells to double-positive CD4+ CD8+ T cells (Fig. 4). OP9 cells expressing Dll1K17R only activated Notch at a very low level–giving rise to 12-fold less double-positive T cells than wt Dll1. Interestingly, despite its strong physical interaction with the receptor, Dll1–3 did not induce any T cell differentiation in this cellular context, indicating its inability to activate Notch signaling.
Fig. 4.
Dll1K17R and Dll1–3 cannot efficiently induce Notch activation. Bone marrow-derived hematopoietic stem cells were cocultured with Dll1-, Dll1K17R-, or Dll1–3-expressing OP9 cells. After 3 weeks, cocultures were analyzed by flow cytometry using CD4 and CD8 T cell markers. The percentages of double-positive T cell population are indicated.
Transendocytosis of Notch1 Extracellular Domain in Cells Expressing wt or Mutant Dll1.
In 2000, Parks et al. (13) showed that endocytosis of Drosophila Delta is required to transendocytose the extracellular region of Notch, and more recently, Nichols et al. (14) demonstrated that these events correlate with Notch activation in mammalian cells. To characterize the endocytosis of Notch1 into Dll1-expressing cells in our cellular system, immunofluorescent staining was performed on OP9 cells stably expressing VSV-tagged Dll1 cocultured with MEF cells expressing a human HA-tagged Notch1 receptor. After fixation, cells were either permeabilized or not to distinguish between surface expressed and intracellular molecules. In permeabilized cells, positive vesicles costained with anti-HA and anti-VSV antibodies were detected (Fig. S2A). In contrast, in nonpermeabilized cells, no such positive vesicles were detected, even when cells expressing Notch1 and Dll1 were clearly in contact (Fig. S2B). To further specify the endocytic nature of the Notch1-Dll1 containing vesicles, cells were incubated with fluorescent dextran, a fluid phase tracer, for 4 h before processing. Several of the Notch1- and Dll1-positive vesicles colocalized with the fluorescent dextran (Fig. S3) indicating they were of endocytic origin.
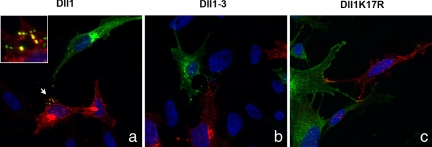
These data prompted us to investigate whether uptake of NECD could occur in cells expressing Dll1–3 or Dll1K17R compared with cells expressing wt Dll1. We cocultured MEF cells stably expressing a HA-tagged Notch1 receptor with HeLa cells transiently transfected with VSV-tagged ligands. HA-positive intracellular vesicles appeared only in wt Dll1-expressing cells, where they colocalized with VSV staining (Fig. 5a), indicating that NECD was transendocytosed into the wt Dll1-expressing cells. On the contrary, we could not detect any HA staining in cells expressing Dll1–3 (Fig. 5b) or Dll1K17R (Fig. 5c).
Fig. 5.
Notch1 is transendocytosed into Dll1-expressing cells but not Dll1K17R- or Dll1–3-expressing cells. HeLa cells transiently transfected with VSV-tagged Dll1 (a), Dll1–3 (b), or Dll1K17R (c) constructs were cocultured with MEFs that stably express HA-tagged Notch1 receptor. Cells were stained with Cy3-conjugated anti-VSV and Alexa488-conjugated anti-HA antibodies. Hoechst staining is shown.
Distribution of wt and Mutant Dll1 in Membrane Microdomains.
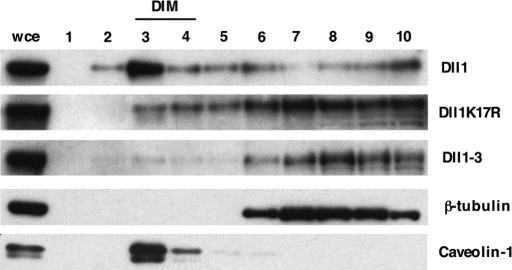
Lipid rafts are examples of membrane microdomains that have been suggested to function as signaling platforms. To test this hypothesis in the context of Notch signaling, we isolated membrane lipid microdomains of ligand-expressing OP9 cells on the basis of their relative insolubility in nonionic detergents and their ability to float in density gradients (7). As expected, caveolin-1 was detected in detergent-insoluble membrane fractions (DIM) (Fig. 6, lane 3), and β-tubulin was found in soluble fractions (Fig. 6, lanes 6–10). Interestingly, wt Dll1 was specifically enriched in the buoyant fractions (DIM) where caveolin-1 segregates (Fig. 6, lane 3), suggesting an association with lipid microdomains. Dll1K17R was found throughout the gradient but showed an enrichment in the fractions corresponding to soluble material (Fig. 6, lanes 6–10), whereas Dll1–3 was only found in the soluble fractions (Fig. 6, lanes 6–10), copurifying with β-tubulin.
Fig. 6.
Dll1, but neither Dll1K17R nor Dll1–3, localizes to lipid microdomains. OP9 stromal cell lines stably expressing Dll1, Dll1K17R, or Dll1–3 were lysed in 1% Brij98 at 4°C and subjected to ultracentrifugation on sucrose gradients. Fractions were collected from top (fraction 1, low density) to bottom (fraction 10, high density) and analyzed by immunoblotting with anti-Dll1CT and anti-Dll3ic antibodies. Caveolin-1 (24 kDa) was used as a marker of lipid microdomains, whereas β-tubulin (55 kDa) was used as a marker of detergent soluble subcellular fractions.
Discussion
Several authors have reported that ubiquitination and endocytosis of Notch ligands in signal-sending cells are required for Notch activation in signal-receiving cells (for review, see ref. 5). To gain some insight into the role of these various events in the “activation” of Notch ligands, we assayed endocytosis, recycling, localization in membrane microdomains, NECD binding, transendocytosis, and Notch signaling activation by using three Dll1 derivatives that differ in their intracellular domain.
The first conclusion we reached was that ubiquitination is not necessary for Dll1 internalization. Indeed, the ubiquitination-defective Dll1K17R and the lysine-less Dll1–3 molecules are effectively internalized, although the Dll1K17R is reproducibly less efficiently endocytosed than the wt molecule. Many yeast surface proteins have been demonstrated to require ubiquitination for their internalization (15), but the situation is less clear in mammals. In the case of the EGF receptor, mutation of multiple intracellular lysines within the kinase domain of the protein leads to an almost complete loss of EGF receptor ubiquitination but did not result in the decrease of its internalization rate (16). Regarding Notch ligands, it has been shown that the E3 ligase Mind Bomb 1 promotes ubiquitination and internalization of mammalian Dll1 (17). It has also been reported that to be active, Drosophila Delta has to traffic through a Neur/Mib- and epsin-dependent pathway, although the majority of Delta molecules traffic in a nonproductive manner through a different pathway, which is ubiquitination-dependent but epsin-independent and may lead to degradation (18). Here, we demonstrate that Dll1K17R is endocytosed but is unable to signal, and these results can be interpreted in two ways: (i) bulk endocytosis of Dll1 is mildly affected by the lack of ubiquitination, whereas the small amount of ligand that follows the activating pathway can no longer be endocytosed in the absence of ubiquitination or (ii) ubiquitination is required for a subsequent step of Dll1 trafficking. Our results suggest that the latter explanation is correct and that ubiquitination is necessary for Dll1 recycling. It has been suggested that trafficking through Rab11-positive recycling endosomes ensures proper Delta activity (19). Sec15, an effector of Rab11 (20), has also been shown to be required for Delta recycling and signaling activity in signal-sending cells; however, direct evidence of ligand recycling has not been provided. Our experiments demonstrate that wt Dll1 is effectively endocytosed and recycled back to the cell surface, whereas Dll1K17R is endocytosed but not recycled. These results are in agreement with a model proposed by Wang and Struhl that suggests that epsin/lqf directs ubiquitinated ligands into a recycling pathway that is necessary to produce active ligands (21). In mammalian cells, internalization of cell surface proteins occurs through both clathrin-dependent and clathrin-independent pathways; the latter generally depend on cholesterol-rich membrane domains, which have been postulated to play an important role in cell signaling (22). We demonstrate here that the Dll1K17R mutant is unable to enter the membrane microdomains as well as to recycle, two apparent requirements for activation of the signaling pathway. The epistatic relationship between localization in membrane microdomains and recycling, however, remains to be characterized.
In contrast to Dll1K17R, the chimeric ligand Dll1–3, although not ubiquitinated, is able to recycle to the cell surface, indicating that an unknown signal present in the intracellular domain of Dll3 can bypass the requirement for ubiquitination. However, this molecule is unable to signal. Geffers et al. (23) generated a similar Dll1–3 chimeric molecule found that this molecule did not activate Notch signaling; however, the details of its trafficking were not analyzed.
When binding of clustered NECD to cells expressing either wt or mutant Dll1 molecules was tested, we observed that, whereas Dll1 and the Dll1–3 chimera were able to bind NECD with a similar efficiency, binding by Dll1K17R was much less efficient, suggesting that recycling is somehow required for Dll1 to acquire a strong affinity for the receptor. However, when we assayed NECD transendocytosis by the wt and mutant Dll1 molecules, we observed that wt Dll1 was the only one to be able to perform this task. Similarly, when using the hematopoietic coculture system, a sensitive and physiological readout for Dll1-mediated activation of Notch1, we observed that only wt Dll1 was able to markedly induce T cell differentiation. Thus, if ligand recycling is necessary to generate a molecule able to interact with a soluble clustered form of the receptor, this recycling is not sufficient to activate Notch signaling in a T cell differentiation assay. A specific event may take place either during endocytosis or recycling of Dll1 and Dll1–3 (or during both) and cause their differential behavior. Because membrane microdomains have been postulated to be involved in the assembly of signaling complexes, we tested the localization of the three Dll1 derivatives and observed that Dll1, but not Dll1K17R or Dll1–3, was present in these microdomains. The inability of the Dll1–3 chimera to transendocytose NECD may be due to the fact that this molecule is excluded from these microdomains.
In conclusion, our results support a model whereby specific intracellular endocytosis/recycling events of the Notch ligands are necessary both before contact with Notch, to somehow “activate” the ligand, and after the interaction, to allow NECD transendocytosis and the subsequent cleavages necessary for Notch activation. Lipid microdomains may play an important role in endowing Dll1 with the ability to transendocytose the extracellular region of Notch. The challenge is now to identify the different adaptor proteins implicated in these processes and to identify more precisely the sequential events that allow intracellular “activation” of the ligands.
Materials and Methods
Constructs.
Dll1- and VSV-Dll1-coding constructs in pcDNA6 or in MSCV-IRES-GFP (MIG) vector have been described previously (9). To generate a Dll1K17R-expressing construct, the sequence coding for the extracellular and the transmembrane domain of Dll1 was amplified by PCR and was inserted into ICK17R-pcDNA3 (ATG:biosynthetics), which encodes a Dll1 intracellular domain in which all intracellular lysines were replaced with arginines (mutations are at the following amino acid positions: 572, 575, 600, 613, 617, 618, 628, 629, 633, 648, 660, 664, 675, 689, 699, 702, and 713). To generate a Dll1–3-expressing construct, the sequence coding for the extracellular region of Dll1 and the sequence coding for the transmembrane and the intracellular domain of Dll3 were amplified by PCR and inserted into pcDNA3. 6× His-ubiquitin wt and ko plasmids were a gift from M. Treier and D. Bohmann (European Molecular Biology Laboratory, Heidelberg).
Antibodies.
The antibody directed against the C-terminal part of Dll1 (anti-Dll1CT) has been previously described (9). The antibody directed against Dll3 (anti-Dll3ic) was generated in rabbits against the intracellular peptide from amino acids 551–566 and 573–585 derived from the intracellular domain of the molecule (Eurogentec).
Cell Culture, Transfection, and Cell Line Establishment.
HEK 293T, HeLa, Plat-E, and stromal bone marrow-derived OP9 cell lines were cultured and transfected as described previously (12). MEF cells stably expressing human HA-Notch1 were generated by retroviral infection (C. Brou and P. Chastagner, personal communication).
Ubiquitination Assay.
HEK 293T transfected with a ligand-expressing construct and with a plasmid expressing a His-Ub variant (24) were lysed in 8 M urea buffer. Cell extracts were centrifuged at 186,000 × g. Ubiquitinated proteins were isolated on nickel-agarose columns according to the manufacturer's instructions (GE Healthcare) and analyzed by immunoblotting with anti-Dll1CT or anti-Dll3ic antibody.
Endocytosis and Recycling Assays.
Reversible biotinylation assays were performed as described in Fournier et al. (25) with minor modifications (see SI Text).
NECD Binding Assay.
HeLa cells transiently transfected with a VSV-tagged ligand-expressing construct were incubated for 40 min at 37°C with a recombinant protein containing the first 12 EGF-like repeats of rat Notch1 fused to the Fc domain of human Ig (NECDFc; R&D Systems) preclustered with a FITC-conjugated anti-human IgG-Fc (Jackson ImmunoResearch). Cells were washed with PBS and analyzed by immunofluorescence as described previously (12). Quantitation and colocalization were performed by using AxioVision 4.6.3.
Notch1 Extracellular Domain Transendocytosis Assay.
HeLa cells were transfected with VSV-tagged ligand-expressing plasmids (VSV is localized in the extracellular domain of the ligands). Eight hours after transfection, MEFs stably expressing human Notch1 were seeded on HeLa cells (Notch1 is HA-tagged in its extracellular domain). After overnight coculture, cells were analyzed by immunofluorescence as described previously (12) using a Cy3-conjugated anti-VSV and an Alexa Fluor 488-conjugated anti-HA (Invitrogen).
Notch Activation Assay.
Bone marrow was isolated from 6-week-old C57BL/6 mice. After red cell depletion using NH4Cl, bone marrow-derived ScaI+ hematopoietic stem cells (HSC) were isolated by using ScaI-PE, an anti-PE microbead kit, and VarioMACS (Miltenyi Biotec) according to the manufacturer's protocol. T-lymphoid potential was tested by plating ScaI+ HSC on OP9 stromal cells that stably expressed Dll1, Dll1K17R, or Dll1–3. After 21 days of coculture, HSC were analyzed by flow cytometry with the following combination of antibodies: PE anti-CD8 and APC anti-CD4 (12). A 7AAD-GFP cell gate was set to exclude nonviable cells and Dll1-expressing 0P9 cells from the analysis. The data were analyzed by using FlowJo software.
Lipid Microdomain Isolation.
OP9 cells stably expressing Dll1 derivatives were lysed in buffer A (20 mM Tris, pH 7.5; 150 mM NaCl) containing 1% Brij 98 for 30 min on ice. Cell lysates were homogenized by passage through a 23-G needle 10 times, mixed with an equal volume of buffer B (85% sucrose/1% Brij 98 in buffer A), and transferred to centrifuge tubes. Successively, 2 ml of 30% sucrose in buffer A and 1 ml of 5% sucrose in buffer A were layered on the samples. After a centrifugation at 230,000 × g for 18 h, fractions were collected from the top to bottom and analyzed by immunoblotting with anti-Dll1CT, anti-Dll3ic, anti caveolin-1 (BD Transduction Laboratories), or anti β-tubulin (Sigma) antibody.
Supplementary Material
Acknowledgments.
We thank C. Brou for critically reading the manuscript, J. C. Aster (Harvard Medical School, Boston) for providing HA-tagged hNotch1 cDNA, M. Treier for 6× histidine-tagged ubiquitin constructs, A. Danckaert (Institut Pasteur, Paris) for her help with the quantification software. This work was supported in part by European Commission Framework Programme 6 Grant Rubicon. S.F.H. is supported by a grant from Ligue Nationale Contre le Cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.A.-T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800695105/DCSupplemental.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 3.Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- 4.Chitnis A. Why is delta endocytosis required for effective activation of notch? Dev Dyn. 2006;235:886–894. doi: 10.1002/dvdy.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Nichols JT, Miyamoto A, Weinmaster G. Notch signaling—constantly on the move. Traffic. 2007;8:959–969. doi: 10.1111/j.1600-0854.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 8.Katic I, Vallier LG, Greenwald I. New positive regulators of lin-12 activity in Caenorhabditis elegans include the BRE-5/Brainiac glycosphingolipid biosynthesis enzyme. Genetics. 2005;171:1605–1615. doi: 10.1534/genetics.105.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Six E, et al. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc Natl Acad Sci USA. 2003;100:7638–7643. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: A potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 11.Ladi E, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol. 2005;170:983–992. doi: 10.1083/jcb.200503113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Six EM, et al. The notch ligand Delta1 recruits Dlg1 at cell–cell contacts and regulates cell migration. J Biol Chem. 2004;279:55818–55826. doi: 10.1074/jbc.M408022200. [DOI] [PubMed] [Google Scholar]
- 13.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 14.Nichols JT, et al. DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol. 2007;176:445–458. doi: 10.1083/jcb.200609014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 16.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo BK, et al. Mind bomb-2 is an E3 ligase for Notch ligand. J Biol Chem. 2005;280:22335–22342. doi: 10.1074/jbc.M501631200. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- 19.Emery G, et al. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Jafar-Nejad H, et al. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 22.Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med. 2007;11:644–653. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geffers I, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol. 2007;178:465–476. doi: 10.1083/jcb.200702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Horst A, et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–1073. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 25.Fournier KM, Gonzalez MI, Robinson MB. Rapid trafficking of the neuronal glutamate transporter, EAAC1: Evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. J Biol Chem. 2004;279:34505–34513. doi: 10.1074/jbc.M404032200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.