Abstract
The ability to selectively induce a strong immune response against self-proteins, or increase the immunogenicity of specific epitopes in foreign antigens, would have a significant impact on the production of vaccines for cancer, protein-misfolding diseases, and infectious diseases. Here, we show that site-specific incorporation of an immunogenic unnatural amino acid into a protein of interest produces high-titer antibodies that cross-react with WT protein. Specifically, mutation of a single tyrosine residue (Tyr86) of murine tumor necrosis factor-α (mTNF-α) to p-nitrophenylalanine (pNO2Phe) induced a high-titer antibody response in mice, whereas no significant antibody response was observed for a Tyr86 → Phe mutant. The antibodies generated against the pNO2Phe are highly cross-reactive with native mTNF-α and protect mice against lipopolysaccharide (LPS)-induced death. This approach may provide a general method for inducing an antibody response to specific epitopes of self- and foreign antigens that lead to a neutralizing immune response.
Keywords: TNF-α, unnatural amino acids, vaccination, immunogenicity
A major challenge in modern vaccinology is the development of robust methods to selectively induce a strong immune response against self-proteins or to increase the immunogenicity of specific epitopes in foreign antigens that can elicit neutralizing antibodies but that are not immunodominant. A number of strategies are being pursued to address this challenge including the development of improved adjuvants, the introduction of foreign helper peptides into chimeric antigens, and the use of DNA vaccines (1–4). Interestingly, almost 50 years ago, Weigle (5) showed that rabbits immunized with a rabbit thyroglobulin that had been nonspecifically labeled with a diazonium derivative produced cross-reactive antibodies to native thyroglobulin. Although these early experiments produced a highly heterogeneous antigen, one interpretation is that chemical modification results in immunogenic epitopes that induce high-titer cross-reactive antibodies. Similarly, there is anecdotal evidence that T cell tolerance can be broken by autoreactive B cells, which are readily elicited by immunization with cross-reactive foreign antigens that differ from the self-antigen by one or a few amino acids (6).
In contrast to the relatively nonselective chemical methods for modifying proteins, it is now possible to make highly precise “chemical mutations” to protein structure by means of genetically encoded unnatural amino acids. More than 50 unnatural amino acids have been encoded in bacteria, yeast, or mammalian cells including metal-binding and posttranslationally modified amino acids, fluorescent and redox-active amino acids, and photo- and chemically reactive amino acids (7–9). More specifically, the phenylalanine derivative p-nitrophenylalanine (pNO2Phe, Fig. 1A) has been incorporated into proteins in bacteria in response to the amber nonsense codon with high fidelity and good efficiency for use as a spectroscopic distance probe (10). Nitroaryl groups have historically been used as highly immunogenic haptens (11), most likely because of the propensity of the electron-deficient pi system to interact with the Tyr and Trp side chains common to germ-line antibody combining sites. Because of their close structural similarity, we postulated that proteins containing either Phe → pNO2Phe or Tyr → pNO2Phe mutations might generate a robust immune response that would be cross-reactive with the native protein. Here, we show that immunization of mice with a Tyr86 → pNO2Phe mutant of murine tumor necrosis factor-α (mTNF-α) generates a high-titer antibody response to WT mTNF-α that efficiently protects mice against a lipopolysaccharide (LPS) challenge.
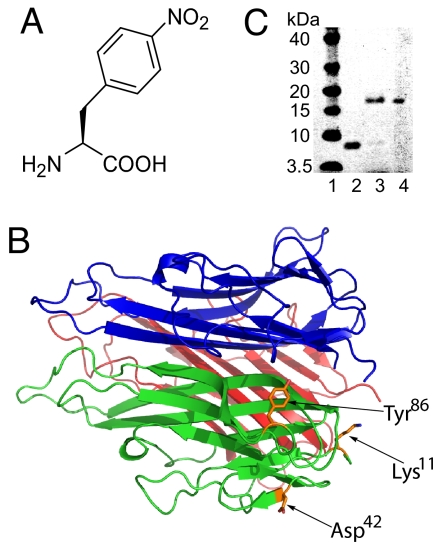
Fig. 1.
Incorporation of pNO2Phe into mTNF-α. (A) Structure of pNO2Phe. (B) X-ray crystal structure of mTNF-α trimer with Tyr-86, Asp-42, and Lys-11 indicated (PDB ID code 2TNF). (C) Expression of the Tyr86 amber mutant of mTNF-α in the absence (lane 2) and presence (lane 3) of 1 mM pNO2Phe with the pNO2Phe-specific mutRNACUA/aminoacyl-tRNA synthetase pair. Protein samples were purified by Ni-NTA affinity column under denaturing conditions and analyzed by SDS/PAGE with SimplyBlue staining. Lane 4 contains WT mTNF-α, and lane 1 is a molecular mass standard.
Results and Discussion
Murine TNF-α as a Model System.
mTNF-α was chosen as the target protein for this study because: (i) it is a well characterized cytokine involved in the regulation of infectious, inflammatory, and autoimmune phenomena (12); (ii) the biological properties of this protein have been extensively studied including its structure, function, and signaling mechanisms (12–16); and (iii) mTNF-α knockout mice are viable and show no apparent phenotypic abnormalities (15), suggesting that mice will survive a neutralizing immune response against TNF-α. In addition, anti-TNF-α antibodies (17, 18) and soluble chimeric TNF-α receptors (19, 20) are widely used in the treatment of autoimmune disease, and a number of approaches are being pursued to develop TNF-α-specific vaccines for clinical use. The latter include recombinant TNF-α molecules containing foreign immunodominant T-helper epitopes, TNF-α fusions to virus-like particles of the bacteriophage Qβ, and keyhole limpet hemocyanin-TNF-α heterocomplexes (4, 21, 22).
Based on the x-ray crystal structure of trimeric mTNF-α (13), a single Tyr86 → pNO2Phe mutant mTNF-α (pNO2Phe86mTNF-α) was selected as an immunogen for our initial studies (Fig. 1B). Tyr86 is highly conserved among different mammalian TNFs, and it has been determined that mutations at this site have no effect on protein folding and trimer formation but lead to a significant loss of cytotoxicity (23, 24) (which is advantageous for vaccination purposes).
Expression and Characterization of Mutant mTNF-α Proteins.
To express mTNF-α in Escherichia coli, plasmid pET26-mTNF was constructed that consists of an N-terminal His6 tag, a factor Xa cleavage site, and the mTNF-α gene downstream of the T7-lac promoter. The pNO2Phe86mTNF-α mutant was generated by substitution of the codon for Tyr86 with a TAG amber codon in plasmid pET26-mTNF. This mutated gene was then expressed in the presence of an orthogonal, amber suppressor tRNACUA/aminoacyl-tRNA synthetase pair derived from Methanococcus jannaschii that specifically inserts pNO2Phe into proteins in E. coli in response to the amber codon (10). The mutant protein (≈1 mg/liter in GMML minimum medium) was purified by Ni2+ affinity chromatography either under denaturing or native conditions, followed by cleavage of the His6 tag and size-exclusion chromatography. The composition and homogeneity of the mutant protein were subsequently analyzed by SDS/PAGE and mass spectrometry.
pNO2Phe86mTNF-α purified under denaturing conditions has a similar mobility on SDS/PAGE as WT mTNF-α (Fig. 1C); no full-length mTNF-α was observed when the mutant gene was expressed in the absence of pNO2Phe, indicating that there is no detectable incorporation of endogenous amino acids at position 86. The MS/MS analysis of an 8-mer tryptic fragment exactly matches the pattern for the incorporation of pNO2Phe at residue 86 [supporting information (SI) Fig. S1A and Table S1]. The MALDI-TOF spectrum (Table 1 and Fig. S2A) also shows a peak ([M-H]+:17287) that matches the expected molecular mass of pNO2Phe containing full-length mTNF-α ([M-H]+:17286). These results confirm the selective incorporation of pNO2Phe into the mutant mTNF-α.
Table 1.
MALDI-TOF mass spectrometry analysis of mTNF-α variants
| Protein | Species | Observed mass (calculated mass), Da |
|
|---|---|---|---|
| Full-length protein without His6 tag | Protein without Leu1Arg2 | ||
| pNO2Phe86mTNF-α | [M + H]+ | 17,287 (17,286) | 17,038 (17,017) |
| Phe86mTNF-α | [M + H]+ | 17,237 (17,241) | 16,972 (16,972) |
| WT mTNF-α | [M + H]+ | 17,255 (17,257) | 16,987 (16,988) |
To determine the effect of the pNO2Phe86 mutation on the quaternary structure of this protein, both WT mTNF-α and pNO2Phe86mTNF-α samples were analyzed by fast protein liquid chromatography (Fig. S1B). Both WT mTNF-α and pNO2Phe86mTNF-α show a similar retention time that corresponds to the molecular mass of the trimeric forms. Furthermore, both proteins were completely soluble at >10 mg/ml in PBS buffer (pH 7.5) at 25°C. As expected, WT mTNF-α activates NFκB signaling in a NFκB-luciferase reporter cell line. In contrast the pNO2Phe86 mutant (Fig. S3) has only 2% of the activity of WT mTNF-α in this assay, consistent with previous reports that Tyr86 is essential for receptor binding and that a variety of mutations at residue 86 lead to a significant decrease in activity (23, 24). One additional peak was also found in the MALDI-TOF spectrum that corresponds to the deletion of first 2 aa of pNO2Phe86mTNF-α (Table 1, Fig. S2A), presumably because of overdigestion during the factor Xa cleavage step. Because it is difficult to separate this truncated protein from full-length protein, and the deletion of the first two N-terminal amino acids only slightly affects TNF activity (24), the mixture was used directly to immunize mice both for the mutant mTNF-α and WT control. Additional experiments also indicated that the presence or absence of an N-terminal His6 tag had no influence on the immunization results.
Immunization Studies with WT and Mutant mTNF-α.
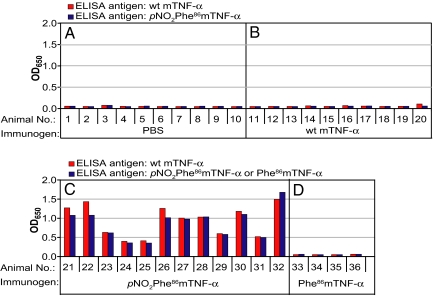
To determine the immunogenicity of the pNO2Phe86mTNF-α mutant, 32 C57BL/6 mice were randomized into three groups and injected with pNO2Phe86mTNF-α, WT mTNF-α, and PBS buffer by using the RIMMS (repetitive immunization at multiple sites) protocol (25). To avoid adverse effects due to cytotoxicity of mTNF-α, a dose of 5 μg of mTNF-α per injection was used throughout this study (26). Briefly, mice were injected eight times over 17 days. In each injection, 5 μg of protein in 200 μl of PBS was mixed 1:1 with complete Freund's adjuvant (CFA) for the first injection or with incomplete Freund's adjuvant (IFA) for the remaining injections at six specific sites proximal to peripheral lymph nodes. On day 21, antibody titers against pNO2Phe86mTNF-α and WT mTNF-α were determined by ELISA using a horseradish peroxidase conjugate of goat anti-mouse IgG secondary antibody. Mice immunized with either WT mTNF-α or PBS buffer alone had insignificant serum IgG titers against both pNO2Phe86mTNF-α and WT mTNF-α (Fig. 2). This is expected because WT mTNF-α is a self-protein and should be tolerated by the murine immune system. In contrast, mice immunized with pNO2Phe86mTNF-α were found to display markedly high serum IgG titers reacting against both pNO2Phe86mTNF-α (Fig. 2C, blue bars) and WT mTNF-α (Fig. 2C, red bars). Thus, a single pNO2Phe mutation (which alters the monomer molecular mass by 29 Da) induces a strong immunological response that results in antibodies that are highly cross-reactive with WT mTNF-α. Similar results were obtained with Bcl-2 transgenic mice (Fig. S4).
Fig. 2.
Serum titers for C57BL/6 mice immunized with PBS (A), WT mTNF-α (B), pNO2Phe86mTNF-α (C), or Phe86 mTNF-α (D). The protocol involved eight injections (5 μg of protein per injection) over a period of 17 days in the presence of complete Freund's adjuvant (CFA) for the initial injection and incomplete Freund's adjuvant (IFA) for the remainder. ELISAs were measured against WT mTNF-α (red bar) or pNO2Phe86mTNF-α (blue bar). For mice immunized with Phe86 mTNF-α, ELISAs were measured against WT mTNF-α (red bar) or Phe86 mTNF-α (blue bar). Before measurement, serum samples were diluted 1:1,000 with 1% BSA in PBS buffer.
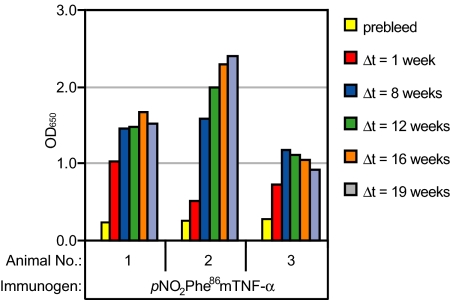
We also examined the immunogenicity in the absence of strong immunopotentiators and found that immunization with pNO2Phe86mTNF-α in the absence of any adjuvant also elicited significant anti-TNF-α titers (Fig. S5), suggesting that this approach may be applicable to therapeutic settings in which strong adjuvants are not desirable. Furthermore, the antibody response after immunizations with pNO2Phe86mTNF-α, remains quite robust after 19 weeks (Fig. 3). Such a long sustainability is highly desirable for clinical use, because current strategies often suffer from rapidly decreasing autoantibody titers when immunization ceases.
Fig. 3.
Determination of serum titer durability. Three Bcl-2 transgenic mice were immunized with pNO2Phe86mTNF-α. After a sequence of eight immunizations, bleeds were taken for ELISA analysis against pNO2Phe86mTNF-α at defined time points. Before each measurement, serum samples were diluted 1:100 with 1% BSA in PBS buffer. Δt corresponds to the time period between the last immunization and the bleed.
To verify that the immunological response is a result of the immunogenic nitroaryl group of the unnatural amino acid, a Tyr86 → Phe mutant mTNF-α (Phe86 mTNF-α) was generated. After confirmation of its trimeric quaternary structure by size-exclusion chromatography, mice were immunized with this mutant either in the presence or absence of CFA/IFA. In both cases no significant anti-TNF-α titers were generated, indicating that the pNO2 group is required to break immunological tolerance (Fig. 2D and Fig. S7). Furthermore, CD4+ T cells specific for pNO2Phe86 mTNF-α were elicited only when mice were immunized with this mutant protein and not when mice were immunized with WT mTNF-α or Phe86 mTNF-α (Fig. S6A). In contrast, no significant proliferation was observed when CD4+T cells from pNO2Phe86mTNF-α-immunized Bcl-2 mice were stimulated in vitro with WT mTNF-α (Fig. S6B). Preliminary epitope mapping experiments with mTNF-α mutants and peptide fragments of WT mTNF-α (data not shown) indicate that the polyclonal response to pNO2Phe86mTNF-α involves multiple protein epitopes. Together, these results suggest that insertion of pNO2Phe into the sequence of mTNF-α creates a T cell epitope, which enchances T cell help to trigger an effective immune response against this disease-associated self protein. It is possible that other immunization protocols (e.g., sequential immunization with the mutant and WT TNF-α) will also yield high-titer cross-reactive antibodies. These results are consistent with those of Dalum et al. (4), who incorporated immunodominant T-helper cell epitopes into mTNF-α to break immune tolerance. The current strategy, however, results in minor pertubations in a protein and should not disrupt its tertiary fold or dramatically affect expression, solubility or stability.
Extension to Mutations at Other Surface Sites.
The polypeptide sequence surrounding Tyr86 is not predicted to be a T cell epitope based on in silico sequence-based analysis of potential MHC class II DR epitopes in TNF-α (27). Nonetheless, to begin to explore the generality of this approach, we determined whether substitution of pNO2Phe at other sites might have a similar effect. The surface-exposed residue Asp42, which is not involved in trimerization or receptor binding, was therefore mutated to pNO2Phe. After confirming the mutation by SDS/PAGE and mass spectrometry, two groups of C57BL/6 mice were immunized with the pNO2Phe42mTNF-α and the Phe42 mTNF-α mutant (Fig. S8). Again, significant anti-TNF-α titers were elicited only by immunization with the pNO2Phe mutant protein. A similar result was obtained with mutation of another surface-exposed residue, Lys11, to pNO2Phe. These results suggest that pNO2Phe mutagenesis may be a fairly general approach to render specific self- or foreign antigens highly immunogenic and may not be limited to substitutions at surface-exposed Tyr or Phe residues. However, preliminary results indicate that incorporation of pNO2Phe is less effective at positions 104 and 19. Immunization of C57BL/6 mice with pNO2Phe104mTNF-α or pNO2Phe19mTNF-α resulted in the generation of antibodies that lacked significant cross-reactivity with native mTNF-α. Thus, context effects likely play a role in determining the nature of the immune response. Finally, it is likely that other immunogenic unnatural amino acids can also be used; alternatively for smaller antigens, immunogenic unnatural amino acids can be incorporated by semisynthesis or total peptide synthesis.
Vaccination Protects Mice in a LPS Challenge Mode.
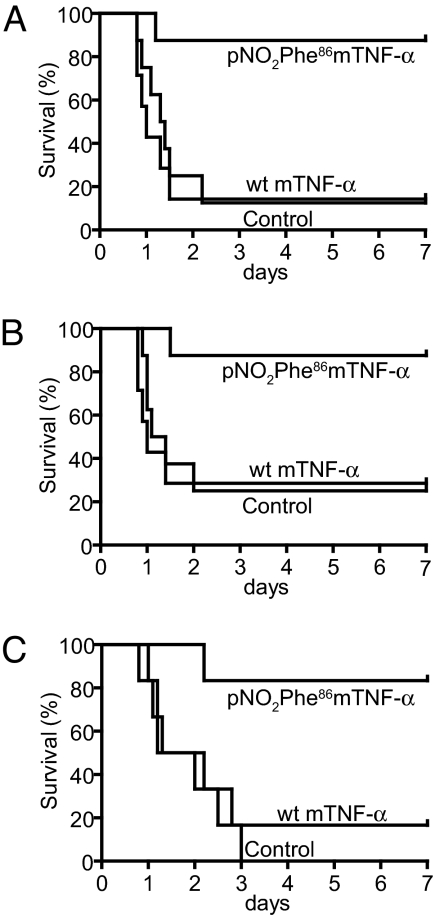
We next determined whether vaccination of mice with the pNO2Phe86mTNF-α would protect against a LPS challenge in a severe endotoxemia mouse model (28). Septic shock induced by LPS in this model is known to involve the production and release of TNF-α. C57BL/6 mice were immunized with PBS, WT mTNF-α, and pNO2Phe86mTNF-α. These mice were subsequently injected i.p. with LPS (8.5 mg/kg) 3 days after completion of the above immunization regime, and their survival rate was determined. As depicted in Fig. 4A, mice immunized with the pNO2Phe86mTNF-α mutant show a significantly greater survival advantage (87.5%) than those that received PBS or WT mTNF-α (12.5% survival rate) immunizations. Similarly, C57BL/6 mice receiving either pooled serum (100 μl) or purified IgG antibody (4 mg/kg) collected from Bcl-2 mice preimmunized with pNO2Phe86mTNF-α showed a significantly higher survival rate (83.3–87.5%) than those receiving pooled serum or IgG from Bcl-2 mice immunized with WT mTNF-α (16.7–25.0%) (Fig. 4 B and C). Hence, these results demonstrate that a single pNO2Phe group incorporated into a self-protein induces a robust cross-reactive antibody response against native protein that is protective in a disease model. We are currently extending these studies to other TNF-α dependent models including the collagen-induced arthritis (CIA) model and KRN transgenic mouse (K/BxN) model (29).
Fig. 4.
Immunization with pNO2Phe86mTNF-α improves survival of mice in a TNF-α-dependent severe endotoxemia model. Kaplan–Meier survival plots of mice receiving active or passive immunizations are shown. (A) Mice (eight per group) immunized with pNO2Phe86mTNF-α or WT mTNF-α are compared with seven mice receiving sham immunizations. Survival advantage of mice immunized with pNO2Phe86mTNF-α (P < 0.01) vs. WT is shown. (B) Mice (eight per group) injected with 100 μg of purified IgG from pNO2Phe86mTNF-α or WT immunized mice were compared with controls receiving saline injection. Survival advantage of mice immunized with pNO2Phe86mTNF-α (P < 0.01) vs. WT is shown. (C) Mice (six per group) received 100 μl of pooled serum from mice immunized with pNO2Phe86mTNF-α or WT mTNF-α. Survival advantage of mice immunized with pNO2Phe86mTNF-α (P < 0.01) vs. WT is shown.
Concluding Comments.
We have shown that it is possible to break immunological self-tolerance by the site-specific incorporation of pNO2Phe into a protein epitope. Although it has been known for some time that altered proteins can induce autologous antibodies, the ill-defined nature of the changes that render the proteins immunogenic complicate their production and therapeutic utility (30). For example, the chemically modified thyroglobulin preparations used in the studies of Weigle contained ≈50 azo linkages per molecule of thyroglobulin (31), resulting in a highly heterogeneous and possibly aggregated or partially unfolded antigen. Similarly, insertion of T cell epitopes at various positions in antigens can create proteins with altered tertiary structure, solubility, and stability compared with native protein. In contrast, the changes made here are chemically defined and confined to single residues. Moreover, these mutations do not appear to affect the overall quaternary structure of the protein nor its solubility. The resulting antibodies are therefore more likely to recognize the corresponding epitopes in the native protein. Finally, the pNO2Phe-containing mTNF-α mutants induced a protective cross-reactive immune response without the need for strong adjuvants and resulted in high titers for at least 4 months, attributes that may facilitate therapeutic applications of this methodology.
This strategy should be applicable to other self-proteins, including those associated with protein-misfolding diseases or cancer. In addition, by introducing the pNO2Phe group at weakly immunogenic or otherwise silent epitopes, this approach may also enable the generation of a strong antibody response against regions of a pathogen that are predicted to result in neutralizing antibodies against viral, bacterial, or parasitic infections. Furthermore, the selective introduction of immunogenic amino acids into proteins may facilitate the generation of functional antibodies, e.g., agonists or antagonists of G protein-coupled receptors and other membrane-bound receptors for which it has historically been difficult to generate strong antibody responses. The structural basis for this phenomenon and exploration of its application to human disease remain to be elucidated.
Materials and Methods
Bacterial Strains and Plasmids.
E. coli XL1-Blue and BL21(DE3) were used as hosts for cloning and expression, respectively. pET26b was obtained from Novagen. E. coli strains were grown in minimal medium containing 1% glycerol and 0.3 mM leucine (GMML medium) or 2× YT medium. Restriction enzymes, T4 DNA ligase, dNTPs, and factor Xa protease were obtained from NEB. IPTG and 4–12% Bis-Tris Gels for SDS/PAGE were purchased from Invitrogen. pNO2Phe was purchased from Advanced ChemTech. Primers were purchased from Integrated DNA Technologies. DNA polymerase was obtained from Stratagene. The anti-TNF-α antibody was from R & D Systems, and recombinant mTNF-α was obtained from BioSource). Plasmid DNA was isolated by using Qiagen Plasmid Purification Kits and DNA purification after restriction digestion was performed by using QiaQuik PCR or gel purification kit.
Construction of mTNF-α Expression Vector, pET26-mTNF.
The murine tnf-α gene was amplified from plasmid pMuTNF (cat. no. 63169; American Type Culture Collection) by using PCR with the following primers: 5′-ATATACATATGCT CAGATCATCTTCTCAAAATTCG and 5′-AACAACCTCGAGTTATCACAGAGCA ATGACTCCAAAGTAGACC. The resulting PCR product was digested with NdeI and XhoI restriction enzymes and ligated into a pET26b vector (Novagen). The recombinant vector was then modified to append an N-terminal hexahistidine-tag (His6-tag), followed by a proteolysis site for factor Xa immediately before the first codon for mature WT mTNF-α. Site-specific incorporation of pNO2Phe into mTNF-α was carried out by mutating the codon for Tyr86, Lys11, or Asp42 to a TAG amber codon by using the Quick Change Mutagenesis Kit (Stratagene). The same kit was also used to prepare the mTNF-α mutants Phe86 mTNF-α and Phe42 mTNF-α. The sequences of all mTNF-α constructs were confirmed by DNA sequence analysis.
Expression of WT and Mutant mTNF-α.
To express the pNO2Phe86mTNF-α, pNO2Phe11mTNF-α, and pNO2Phe42mTNF-α mutants, E. coli BL21(DE3) cells were cotransformed with mutNO2PheRS, mutRNACUA and the respective mutant mTNF-α gene. The transformed cells were grown in the presence of 1 mM pNO2Phe in GMML medium at 37°C and induced with 1 mM IPTG when the OD600 reached 0.5. The cells were then continually shaken at 37°C for 12–16 h and then harvested. The cell pellet was stored at −80°C until use. WT mTNF-α, Phe86 mTNF-α, and Phe42 mTNF-α were expressed by essentially the same procedure. However, in contrast to the pNO2Phe mTNF-α mutants, these proteins were expressed in rich medium (2× YT medium) in the absence of pNO2Phe.
Purification of WT and Mutant mTNF-α Under Denaturing Conditions.
All purification steps were performed at room temperature. After thawing the cell pellet for 15 min on ice, the cell paste was resuspended in lysis buffer [100 mM NaH2PO4 (pH 8.0), 10 mM Tris·HCl, 8 M urea] at 5 ml per gram of wet weight. The cell suspension was sonicated on ice for 3 min. After centrifugation at 10,000 × g for 25 min, 10 ml of Ni-NTA His-Bind Resin (Novagen) was added to the supernatant and mixed on a rotary shaker for 60 min. The lysate–resin mixture was loaded onto a 5-ml polypropylene column (Qiagen) and washed twice with 40 ml of wash buffer A [100 mM NaH2PO4 (pH 6.3), 10 mM Tris·HCl, 8 M urea]. After another two washing steps with 10 ml of wash buffer B [100 mM NaH2PO4 (pH 5.9), 10 mM Tris·HCl, 8 M urea], elution was carried out with 100 mM NaH2PO4 (pH 4.5), 10 mM Tris·HCl, 8 M urea. The protein was concentrated with a 10K molecular mass cut-off Amicon Ultra-15 centrifugal filter device (Millipore) and loaded onto a HiPrep 26/10 desalting column (GE Healthcare) preequilibrated with factor Xa cleavage buffer [20 mM Tris·HCl, 200 mM NaCl, 1 mM EDTA (pH 7.4)]. Turbid fractions containing inclusion bodies were concentrated by several rounds of diafiltration using a 10K molecular mass cut-off Amicon Ultra-15 centrifugal filter device before addition of factor Xa (5% WT/WT). Quantitative removal of the N-terminal His6-tag was achieved within ≈3 days at room temperature as verified by SDS/PAGE analysis. After protease digestion, soluble factor Xa protease and the His6-tag peptide were separated from the inclusion bodies by centrifugation. The protein was then dissolved in ≈1 ml of solubilization buffer [8 M urea, 50 mM Tris·HCl (pH 8.0), 10 mM DTT] and injected onto a Superdex 75 10/300 GL column (GE Healthcare) preequilibrated with solubilization buffer. Two rounds of size-exclusion chromatography were carried out on an ÁKTA purifier instrument (GE Healthcare) at a flow rate of 0.3 ml/min. For refolding, the protein sample was dialyzed against renaturation buffer [240 mM NaCl, 10 mM KCl, 0.5% Triton X-100, 50 mM Tris·HCl, 1 mM EDTA (pH 8.0)] using a 10K molecular mass cut-off Slide-A-Lyzer dialysis cassette (Pierce). After refolding, the renatured protein was dialyzed against PBS.
Passive Immunization Protocols.
Mice were passively immunized 24 h before the endotoxin challenge. In the first experiment, mice received an i.p. injection of 100 μl of pooled serum from mice immunized with either pNO2Phe86mTNF-α or WT mTNF-α. A second cohort received 4 mg/kg of IgG purified from serum of mice immunized with either pNO2Phe86mTNF-α or WT mTNF-α. Control mice were injected with equal volumes of physiological saline.
Mouse Model of Severe Endotoxemia.
All experiments were performed in accordance with the National Institutes of Health Animal Protection Guidelines and were approved by The Scripps Research Institute Animal Care and Use Committee. Lipopolysaccharide (LPS, E. coli O111:B4; Calbiochem/EMD Biosciences) was dissolved in 37°C normal saline (0.9% wt/vol NaCl) by vortexing for 30 s before and after 2 minutes of sonication. Male C57BL/6 mice from The Jackson Laboratories were injected i.p. under 2% isoflurane at the age of 9 weeks with 7.5 mg/kg LPS for the passive immunizations or 15 weeks with 8.5 mg/kg LPS for the active immunization. All experiments were carried out in a room with alternating 12-h light/dark cycles under stable conditions of temperature (20°C-22°C) and relative humidity (40–60%). Kaplan–Meier curves were plotted, and survival differences were analyzed by using a log rank test.
For additional materials and methods, see SI Text.
Supplementary Material
Acknowledgments.
We thank Jason Mah and Linlin Li for carrying out ELISA experiments, Robin Nevarez and Jennifer Royce for carrying out immunizations of C57BL/6 and Bcl-2 mice, and Patrick Corpuz and Grady S. Hunt for technical assistance, Monique Stinson for performing T cell proliferation assays, and Dr. Sunia A. Trauger and Jon V. Apon for performing MS/MS analyses. We also acknowledge Drs. Benjamin M. Hutchins, Shoutian Zhu, and Ann E. Herman for discussions. This work was supported by the National Institutes of Health Grant GM62159, the Skaggs Institute for Chemical Biology, and postdoctoral fellowships from the Alexander von Humboldt Foundation (to J.G.) and the Deutsche Forschungsgemeinschaft (to F.N.). This is manuscript #19217 of The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804157105/DCSupplemental.
References
- 1.Makela PH, Kayhty H. Evolution of conjugate vaccines. Exp Rev Vaccines. 2002;1:399–410. doi: 10.1586/14760584.1.3.399. [DOI] [PubMed] [Google Scholar]
- 2.Restifo NP. The new vaccines: Building viruses that elicit antitumor immunity. Curr Opin Immunol. 1996;8:658–663. doi: 10.1016/s0952-7915(96)80082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldridge J, et al. Vaccine Adjuvants: Immunological and Clinical Principles. Totowa, NJ: Humana; 2006. [Google Scholar]
- 4.Dalum I, et al. Therapeutic antibodies elicited by immunization against TNF-alpha. Nat Biotechnol. 1999;17:666–669. doi: 10.1038/10878. [DOI] [PubMed] [Google Scholar]
- 5.Weigle WO. The induction of autoimmunity in rabbits following injection of heterologous or altered homologous thyroglobulin. J Exp Med. 1965;121:289–308. doi: 10.1084/jem.121.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamula MJ, Lin RH, Janeway CA, Jr, Hardin JA. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J Immunol. 1992;149:789–795. [PubMed] [Google Scholar]
- 7.Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science. 2001;292:498–500. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 8.Chin JW, et al. An expanded eukaryotic genetic code. Science. 2003;301:964–967. doi: 10.1126/science.1084772. [DOI] [PubMed] [Google Scholar]
- 9.Xie J, Schultz PG. A chemical toolkit for proteins—an expanded genetic code. Nat Rev Mol Cell Biol. 2006;7:775–782. doi: 10.1038/nrm2005. [DOI] [PubMed] [Google Scholar]
- 10.Tsao ML, Summerer D, Ryu Y, Schultz PG. The genetic incorporation of a distance probe into proteins in Escherichia coli. J Am Chem Soc. 2006;128:4572–4573. doi: 10.1021/ja058262u. [DOI] [PubMed] [Google Scholar]
- 11.Keinan E. Catalytic Antibodies. Weinheim, Germany: Wiley–VCH; 2005. [Google Scholar]
- 12.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 13.Baeyens KJ, De Bondt HL, Raeymaekers A, Fiers W, De Ranter CJ. The structure of mouse tumour-necrosis factor at 1.4 A resolution: Towards modulation of its selectivity and trimerization. Acta Crystallogr D. 1999;55:772–778. doi: 10.1107/s0907444998018435. [DOI] [PubMed] [Google Scholar]
- 14.Pennica D, Hayflick JS, Bringman TS, Palladino MA, Goeddel DV. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci USA. 1985;82:6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Vilcek J, editors. Tumor Necrosis Factors: Structure, Function, and Mechanism of Action. New York: Dekker; 1992. [PubMed] [Google Scholar]
- 17.Knight DM, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 18.Present DH, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 19.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams RO, Ghrayeb J, Feldmann M, Maini RN. Successful therapy of collagen-induced arthritis with TNF receptor-IgG fusion protein and combination with anti-CD4. Immunology. 1995;84:433–439. [PMC free article] [PubMed] [Google Scholar]
- 21.Spohn G, et al. A virus-like particle-based vaccine selectively targeting soluble TNF-alpha protects from arthritis without inducing reactivation of latent tuberculosis. J Immunol. 2007;178:7450–7457. doi: 10.4049/jimmunol.178.11.7450. [DOI] [PubMed] [Google Scholar]
- 22.Le Buanec H, et al. TNFalpha kinoid vaccination-induced neutralizing antibodies to TNFα protect mice from autologous TNFα-driven chronic and acute inflammation. Proc Natl Acad Sci USA. 2006;103:19442–19447. doi: 10.1073/pnas.0604827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XM, Weber I, Chen MJ. Site-directed mutational analysis of human tumor necrosis factor-alpha receptor binding site and structure-functional relationship. J Biol Chem. 1992;267:24069–24075. [PubMed] [Google Scholar]
- 24.Van Ostade X, Tavernier J, Fiers W. Structure–activity studies of human tumour necrosis factors. Protein Eng. 1994;7:5–22. doi: 10.1093/protein/7.1.5. [DOI] [PubMed] [Google Scholar]
- 25.Kilpatrick KE, et al. Rapid development of affinity matured monoclonal antibodies using RIMMS. Hybridoma. 1997;16:381–389. doi: 10.1089/hyb.1997.16.381. [DOI] [PubMed] [Google Scholar]
- 26.Libert C, et al. Identification of a locus on distal mouse chromosome 12 that controls resistance to tumor necrosis factor-induced lethal shock. Genomics. 1999;55:284–289. doi: 10.1006/geno.1998.5677. [DOI] [PubMed] [Google Scholar]
- 27.Steed PM, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- 28.Niessen F, et al. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 29.Ditzel HJ. The K/BxN mouse: A model of human inflammatory arthritis. Trends Mol Med. 2004;10:40–45. doi: 10.1016/j.molmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Lerner RA, Dixon FJ. The induction of acute glomerulonephritis in rabbits with soluble antigens isolated from normal homologous and autologous urine. J Immunol. 1968;100:1277–1287. [PubMed] [Google Scholar]
- 31.Weigle WO. The production of thyroiditis and antibody following injection of unaltered thyroglobulin without adjuvant into rabbits previously stimulated with altered thyroglobulin. J Exp Med. 1965;122:1049–1062. doi: 10.1084/jem.122.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.