Abstract
Endothelial NOS (eNOS)-derived NO has long been considered a paracrine signaling molecule only capable of affecting nearby cells because of its short half-life in blood and relatively limited diffusion distance in tissues. To date, no studies have demonstrated that endogenously generated NO possesses a clearly defined endocrine function. Therefore, we evaluated whether enzymatic generation of NO in the heart is capable of modulating remote physiological actions and cell signaling. Mice with cardiac-specific overexpression of the human eNOS gene (CS-eNOS-Tg) were used to address this hypothesis. Cardiac-specific eNOS overexpression resulted in significant increases in nitrite, nitrate, and nitrosothiols in the heart, plasma, and liver. To examine whether the increase in hepatic NO metabolites could modulate cytoprotection, we subjected CS-eNOS-Tg mice to hepatic ischemia-reperfusion (I/R) injury. CS-eNOS-Tg mice displayed a significant reduction in hepatic I/R injury (4.2-fold reduction in the aminotransferase and a 3.5-fold reduction in aspartate aminotransferase) compared with WT littermates. These findings demonstrate that endogenously derived NO is transported in the blood, metabolized in remote organs, and mediates cytoprotection in the setting of I/R injury. This study presents clear evidence for an endocrine role of NO generated endogenously from eNOS and provides additional evidence for the profound cytoprotective actions of NO in the setting of I/R injury.
Keywords: eNOS, ischemia-reperfusion, nitrite, hepatic injury, nitroso
The discovery that a free-radical gas is endogenously generated and plays a profound role in cellular signaling and physiology (1, 2) has led to an intense effort toward elucidating the synthesis, storage, and transport of NO. The membrane permeable nature of NO allows it to freely diffuse to adjacent cells, and its high reactivity in the biologic milieu has helped define its role in cellular signaling. As such, understanding NO-mediated, cell-signaling mechanisms, both local to and distal to the site of production, is of vital importance given its diverse physiological actions and profound cytoprotective effects. The short (<2 ms) half-life of NO in blood, because of consumption by hemoglobin, limits the transport of NO to distant tissues (3). Additionally, the half-life of NO within normoxic tissues has been estimated at <0.1 s, with diffusion distance dependent on O2 concentration (4). These findings, coupled with the abundance of data (5, 6) that NOS activity is highly compartmentalized and intricately regulated, have suggested that the signaling actions of NO are restricted to the site of production, thereby classifying it as a paracrine or autocrine signaling molecule. However, recent evidence challenges this long-held notion with a variety of observations leading to the hypothesis that NO may serve as an endocrine-signaling molecule via its ability to be transported as nitrite (7) and/or S-nitrosothiol (RSNO) (8).
Although it was initially speculated that nitrite could be either a stored precursor of NO (9), the majority of research and general dogma in the field viewed nitrite as an inert oxidation product of NO metabolism. This hypothesis was widely supported until a recent paradigm shift was put in motion by observations of non-NOS derived NO production (10, 11). Although the mechanisms responsible for the consumption of NO and formation of nitrite are still poorly defined, nitrite and nitrate are now recognized to be a readily accessible storage form of NO that displays physiological activity (12). Likewise, the nitrosation of proteins may also serve as an important facilitator for the transport of bioactive NO (13). Evidence that nitrite/nitroso (RXNO)-species serve as NO transporters and facilitate remote physiological actions has been tested by using the administration of exogenous NO (14, 15). Additionally, our laboratory and others (16, 17) have reported that nitrite can be consumed under ischemic/hypoxic conditions to yield NO and thus impede cellular injury. The recent demonstration that modulation of dietary nitrite can have a profound effect on myocardial ischemia/reperfusion (I/R) injury is likewise supportive of an endocrine role for NO (18). However, to date, no reports have conclusively shown that endogenously generated NO (NOS-derived) is transported to remote tissues and exerts physiological activity. Therefore, we investigated whether NO, produced as a result of endothelial NOS (eNOS) overexpression in one organ (i.e., heart), could modulate I/R injury at a distant site (i.e., liver).
In the current study, we used a mouse with cardiac-restricted overexpression of eNOS to test this hypothesis. Overexpression of eNOS in the myocardium resulted in increased levels of NO metabolites in the circulation and subsequent transport and storage in distant organs. Importantly, the resulting NO biochemical changes in the liver significantly ameliorated hepatic I/R injury via formation of NO and related NO metabolites. These data provide evidence that eNOS-derived NO is transported in the vasculature, stored at sites distant from production, and modulates cellular injury during ischemia.
Results
Transgenic Overexpression of eNOS Restricted to Cardiomyocytes Increases Myocardial NO Metabolites (NOx).
Mice with cardiac-specific overexpression of human eNOS (CS-eNOS-Tg) have been characterized (19). These animals display robust eNOS immunoreactivity of myocardial tissue (Fig. 1A). Optical band density measurements of Western blots quantified a 65-fold increase in eNOS protein expression over nontransgenic, WT littermates (P < 0.001, Fig. 1A). In correlation with the observed high levels of eNOS protein, eNOS activity, as assessed by l-arginine to citrulline conversion, is significantly increased in CS-eNOS-Tg hearts (Fig. 1B). In addition, real-time PCR analysis of myocardial RNA isolated from CS-eNOS-Tg mice displayed no significant compensatory alterations in any of the murine NOS isoforms: eNOS, iNOS, or nNOS [supporting information (SI) Table S1].
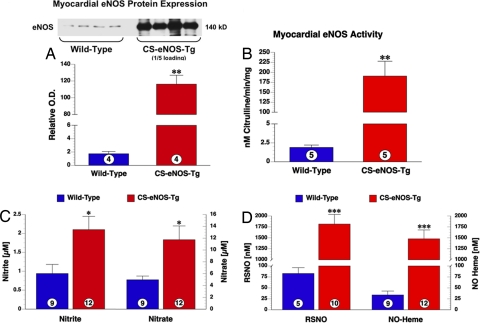
Fig. 1.
Cardiac eNOS protein and NO levels are elevated in CS-eNOS-Tg mice. (A) Myocardial tissue eNOS protein in WT and CS-eNOS-Tg mice. CS-eNOS-Tg samples were loaded at 1/5th the protein concentration (5 μg) of WT samples (25 μg). Optical density and volume analysis of the 140 kDa band revealed a 65-fold increase in CS-eNOS-Tg cardiac eNOS expression. (B) l-arginine to citruline conversion revealed a significant increase in NOS activity in CS-eNOS-Tg mice. (C) Nitrite (Left) and nitrate (Right) levels (μM) measured in hearts of WT and CS-eNOS-Tg mice. (D) RSNO and NO-heme levels (μM) in hearts of WT and CS-eNOS-Tg mice (*, P < 0.05; **, P < 0.01; ***, P < 0.001; numbers inside the circles denote number of animals per group).
Ion chromatographic analysis of nitrite revealed that CS-eNOS-Tg hearts have a significant 2.2-fold increase over WT mice (P < 0.05). Likewise, levels of nitrate were also increased 2.4-fold in the hearts of CS-eNOS-Tg mice (Fig. 1C). Gas-phase chemiluminescence revealed a substantial increase in RSNO in CS-eNOS-Tg hearts (P < 0.01, Fig. 1D). Additionally, overexpression of myocardial eNOS results in increased levels of NO-heme adducts (P < 0.001, Fig. 1D).
Myocardial eNOS-Derived NO Attenuates Myocardial I/R Injury.
It has been found that CS-eNOS-Tg mice exhibited a significant reduction in infarct size with improved left ventricular function after myocardial I/R injury (20). A subsequent analysis in the current study revealed similar results with CS-eNOS-Tg mice displaying a 50% reduction in infarct size per area-at-risk after 45 min of left coronary artery ischemia and 24 h reperfusion (P < 0.001, Table 1). The area-at-risk was similar between both groups.
Table 1.
Myocardial area-at-risk and infarct size in CS-eNOS-Tg
| Group | n | AAR/LV | INF/AAR | INF/LV |
|---|---|---|---|---|
| WT | 7 | 57.8 ± 2.8 | 52.1 ± 5.02 | 30.1 ± 3.3 |
| CS-eNOS-Tg | 7 | 54.4 ± 2.1 | 26.0 ± 1.9* | 14.3 ± 1.4* |
CS-eNOS-Tg mice and WT littermates were subjected to 45 min of left coronary artery ischemia and 24 h of reperfusion. No differences were observed in area-at-risk per left ventricle (AAR/LV) between groups. Analysis of myocardial infarct per area-at-risk (INF/AAR) and infarct per left ventricle (INF/LV) revealed a significant reduction in infarct size. *, P < 0.001 vs. WT.
eNOS Localized Within Cardiomyocytes Generates Increases in Plasma NOx.
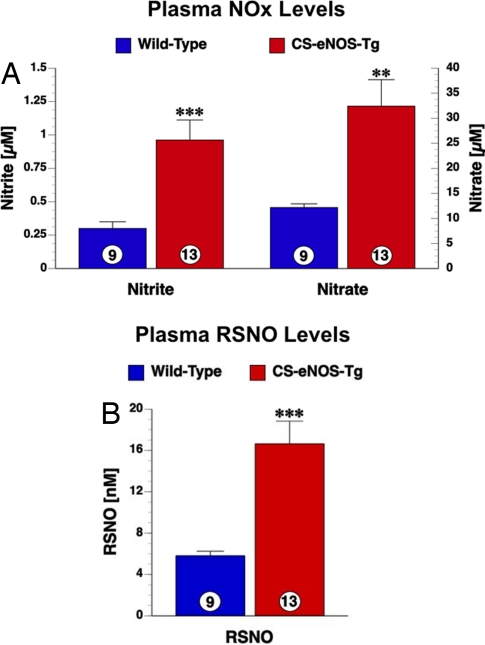
CS-eNOS-Tg mice display significant increases in all plasma NO metabolites. Plasma nitrite levels were increased 3.2-fold in CS-eNOS-Tg mice (P < 0.001, Fig. 2A). Analysis of plasma nitrate revealed a similar 2.7-fold increase in CS-eNOS-Tg mice compared with WT littermates. RSNO levels are also significantly increased by 2.9-fold in the plasma of CS-eNOS-Tg mice (P < 0.001, Fig. 2B).
Fig. 2.
Plasma NO metabolites are elevated in CS-eNOS-Tg mice. (A) Nitrite (Left) and nitrate (Right) levels (μM) measured in the plasma of WT and CS-eNOS-Tg mice. (B) RSNO levels (nM) in the plasma of WT and CS-eNOS-Tg mice (**, P < 0.01; ***, P < 0.001; numbers inside the circles denote number of animals per group).
Cardiac-Restricted Overexpression of eNOS Results in Increased Hepatic NOx Levels.
The observed elevations in plasma nitrite, nitrate, and nitrosothiols in CS-eNOS-Tg mice led us to analyze the liver as a distal representative organ for alterations in NO biochemistry. Steady-state hepatic nitrite was elevated 6.5-fold in CS-eNOS-Tg mice compared with WT littermates P < 0.05). Additionally, nitrate was increased 2.3-fold in CS-eNOS-Tg mice (Fig. 3A). RSNO proteins were also increased in CS-eNOS-Tg hepatic samples by 2-fold over WT mice (P < 0.01, Fig. 3B). Additionally, NO-heme adducts were increased by 3.3-fold (P < 0.01, Fig. 3B). Collectively, these biochemical data suggest a local change in NO related products.
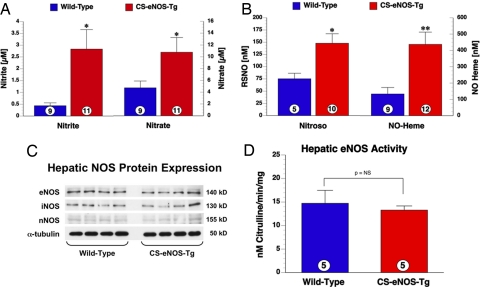
Fig. 3.
Hepatic NO metabolites are elevated in CS-eNOS-Tg mice with unaltered hepatic NOS expression. (A) Nitrite (Left) and nitrate (Right) levels (μM) measured in livers of WT and CS-eNOS-Tg mice. (B) RSNO and NO-heme levels (nM) in livers of WT and CS-eNOS-Tg mice. (C) Western blot analysis of all NOS isoforms (eNOS, iNOS, and nNOS) revealed no differences in immunoreactivity between WT and CS-eNOS-Tg hepatic samples. (D) There was no significant difference in eNOS activity in CS-eNOS-Tg liver samples (*, P < 0.05; **, P < 0.01; numbers inside the circles denote number of animals per group).
In an effort to determine the source of increased NOx and NO modified proteins, RNA expression was assessed by quantitative PCR for all murine NOS isoforms in hepatic tissue from WT and CS-eNOS-Tg mice. There was no significant difference in eNOS, inducible NOS (iNOS), or neuronal NOS (nNOS) message (GAPDH corrected) in CS-eNOS-Tg mice (Table S2). Western blot analysis of murine hepatic NOS protein expression also displayed no alteration in eNOS, iNOS, or nNOS (α-tubulin loading control, Fig. 3C). There was no significant difference between WT and CS-eNOS-Tg mice in terms of hepatic NOS activity (Fig. 3D) under basal conditions. Therefore, the observed changes in NO biochemistry that occur within the liver are a direct result of the increased synthesis of NO in the heart and transport in the plasma.
CS-eNOS-Tg Mice Are Protected Against Hepatic I/R Injury.
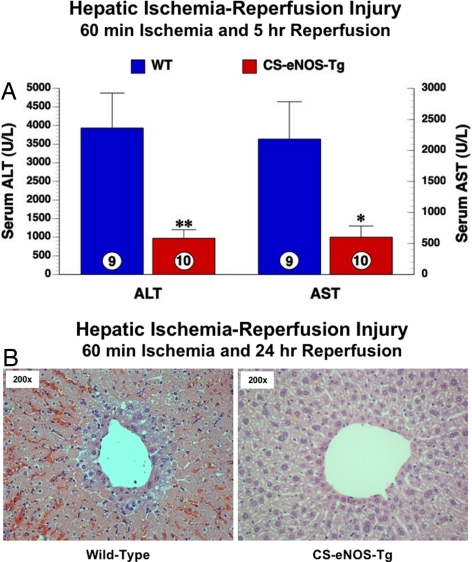
To demonstrate that the changes in hepatic NO biochemistry were able to impact cellular signaling by impeding a pathologic process, we subjected mice to hepatic I/R injury. After 60 min of ischemia and 5 h reperfusion, serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assessed as markers of hepatic injury. CS-eNOS-Tg mice display a 4.2-fold reduction in ALT (P < 0.01, Fig. 4A) after I/R injury. Serum AST is also reduced in CS-eNOS-Tg mice by 3.5-fold compared with WT littermates P < 0.05, Fig. 4A). In correlation, histological analysis (H&E staining 24 h after I/R) revealed a decreased level of pericentral necrosis and leukocyte infiltration in CS-eNOS-Tg mice compared with WT littermates.
Fig. 4.
CS-eNOS-Tg mice are protected against hepatic I/R injury. Mice were subjected to 60 min of hepatic artery and portal vein occlusion followed by 5 h reperfusion. (A) Serum ALT and AST levels (μM) were significantly lower in CS-eNOS-Tg mice (*, P < 0.05; **, P < 0.01; numbers inside the circles denote number of animals per group) (B) Hepatocellular injury was evaluated by H&E staining after 24 h of reperfusion. (Left) WT mice displayed robust cytological changes including extensive centrolobular injury as evidenced by a large degree of periportal hemorrhagic necrosis and leukocytic infiltrate. (Right) Pathology was attenuated in CS-eNOS-Tg mice (200× magnification).
Plasma Nitrite Levels Are Unaltered During Hepatic I/R Injury.
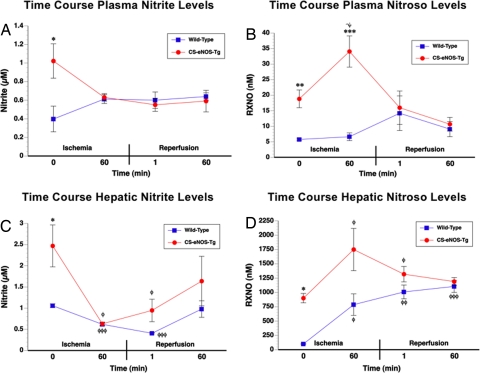
In an attempt to identify the protective endocrine NO metabolite(s), we conducted a series of experiments characterizing the underlying NO biochemistry during a time course of ischemia and reperfusion. The data reveal that despite the increased levels of basal nitrite in the CS-eNOS-Tg mice, there were no significant alterations in plasma nitrite levels over the entire time course of ischemia and reperfusion (Fig. 5A). The observed differences between WT and CS-eNOS-Tg mice at baseline (0 min) were further confirmed in these studies (P < 0.05).
Fig. 5.
Plasma and hepatic nitrite and RXNO level flux over the course of hepatic I/R injury. WT and CS-eNOS-Tg mice (n = 4–5 per group) were subjected to hepatic I/R and levels of plasma nitrite (A), RXNO species (B), hepatic nitrite (C), and hepatic RXNO species (D) were assessed at the following time points: 60 min ischemia, 60 min ischemia/1 min reperfusion, and 60 min ischemia/60 min reperfusion (*, P < 0.05 vs. WT; *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. baseline).
Plasma RXNO Levels Increase During Hepatic Ischemia in CS-eNOS-Tg Mice.
Although no alterations in RXNO levels were observed in WT mice, there was a significant increase in RXNO levels during ischemia in CS-eNOS-Tg mice (Fig. 5B, P < 0.05 vs. baseline, P < 0.001 vs. WT 60 min). The 1.8-fold increase in plasma RXNO levels after 60 min of ischemia completely returned to baseline levels after 1 min of reperfusion.
Hepatic Nitrite Is Consumed During Ischemia and Restored After Reperfusion.
Hepatic nitrite levels decreased 3.9-fold over the 60 min ischemia time in CS-eNOS-Tg mice (P < 0.5 vs. baseline, Fig. 5C). Likewise, nitrite levels also significantly decreased in WT mice during ischemia (P < 0.001 vs. baseline). Hepatic nitrite levels gradually returned to preischemic levels in WT and CS-eNOS-Tg mice over the course of reperfusion, and levels of nitrite were not significantly different from baseline at 60 min. There were no significant alterations in hepatic nitrate throughout the I/R time course study (data not shown).
Hepatic Nitrosothiol Formation Is Increased During Ischemia.
RXNO levels were increased in WT (7.8-fold) and CS-eNOS-Tg (1.9-fold) mice after 60 min of hepatic ischemia (P < 0.05 vs. baseline, Fig. 5D). These significant increases returned to preischemic levels in CS-eNOS-Tg animals after 60 min of reperfusion. However, WT nitrosothiol levels remained elevated throughout the course of reperfusion (P < 0.001, 60 min vs. baseline).
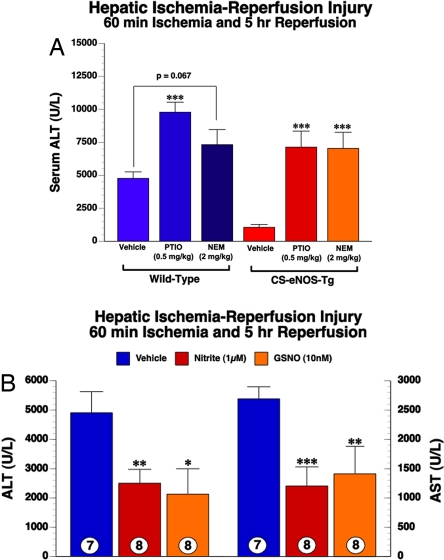
CS-eNOS-Tg Protection Against Hepatic I/R Is Dependent on Formation of NO and/or Nitrosothiols.
Reports have demonstrated that the cytoprotection elicited by nitrite during I/R is NO dependent (16, 17). Recent data demonstrates that RSNOs are cytoprotective in I/R, without the likely release of free NO (21), but through transnitrosation reactions. To address the suspected role of each NO species, we administered 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) before ischemia to scavenge any free NO produced from nitrite, and also administered N-ethylmaleimide (NEM) before ischemia to alkylate all free thiols. WT and CS-eNOS-Tg mice (n = 6 per group) were administered vehicle PTIO (0.5 mg/kg), NEM (2 mg/kg) 30 min before hepatic ischemia. Administration of either PTIO or NEM significantly exacerbated hepatic I/R injury in WT animals suggesting that inhibition of endogenous NO and nitrosothiol signaling exacerbates I/R injury, as suggested in ref. 25. As shown in Fig. 6A, both PTIO and NEM inhibited the protective effects in CS-eNOS-Tg mice, suggesting that both nitrite reduction to NO and thiols are sufficient for cytoprotection. Given the nonspecific actions of these less than ideal pharmacologic agents, we next administered both nitrite and S-nitrosoglutathione (GSNO) to WT control mice as a proof-of-principle experiment. Nitrite (1 μM plasma concentration) or GSNO (10 nM plasma concentration) were injected intravenously just before reperfusion. As shown in Fig. 6B, both nitrite and GSNO reduced hepatic injury ≈2-fold as depicted by decreased serum ALT and AST levels. These data are consistent with our NEM and PTIO experiments, revealing that both nitrite and RSNOs are sufficient for cytoprotection.
Fig. 6.
Scavenging of NO or thiol blockade abolishes hepatic protection in CS-eNOS-Tg mice. (A) CS-eNOS-Tg mice (n = 6 per group) and WT littermates (n = 6 per group) were administered vehicle, the NO scavenger (PTIO, 0.5 mg/kg), or the thiol blocking agent (NEM, 2 mg/kg) 30 min before hepatic ischemia. The delivery of PTIO or NEM completely blocked the hepatic cytoprotection observed in CS-eNOS-Tg mice as evidenced by the increased serum ALT levels. (B) WT mice were injected intravenously (femoral vein) with saline vehicle (n = 7), nitrite (1 μM final plasma concentration; n = 8), or GSNO (10 nM final plasma concentration; n = 8) just before reperfusion. Both nitrite and GSNO significantly ameliorated hepatic I/R injury (*, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. vehicle).
Discussion
Although the cytoprotective effects of eNOS-generated NO have been well documented (19, 20, 22), these effects have, thus far, largely been attributed to local enzymatic production. Recent observations that the inhalation of NO gas at moderate to high levels (80 ppm) can result in systemic effects have begun to challenge the notion of NO being restricted to paracrine actions. NO gas has been shown to modulate a number of remote physiological and pathophysiological processes including systemic vascular resistance, intestinal blood flow, urinary flow, gastrointestinal I/R injury, and myocardial I/R injury (23–26). Additionally, it has been reported that modifications of dietary NOx (nitrate and/or nitrite) results in the alteration of systemic NO metabolite levels, and thus impacts physiological processes (11, 18, 27, 28). These observations that both administration (inhaled gas) and dietary intake are capable of modulating NO levels at sites distant from their application strongly support the notion that NO can be transported and exert distant physiological action (7). However, to date, no studies have clearly defined that NO derived from endogenous enzymatic sources (NO synthases) is transported to distant tissues resulting in biological effects far from the site of production. In the current study, we confirmed the hypothesis that eNOS-generated NO is transported in the vasculature, stored in distant organs, and modulates a pathophysiological process (I/R injury) thus enabling classification as an endocrine signaling molecule.
Using mice with robust overexpression of the human eNOS gene restricted to cardiomyocytes (CS-eNOS-Tg), we found that the levels of NO metabolite species are increased both in the plasma compartment and organs distant from production (i.e., liver). The observed increase in hepatic NO was not attributed to alterations in local NO production as assessed by both message and protein levels of all respective NOS isoforms (eNOS, nNOS, or iNOS). This was further confirmed by the observation of unaltered hepatic NOS activity. These data indicate that the observed increase in hepatic NO metabolites was solely because of increased eNOS activity and NO production within the heart of CS-eNOS-Tg mice. These results demonstrate that eNOS-derived NO is transported in the vasculature to distant organs. A number of reports have cited either nitrite (7) or RSNOs (8) as the predominant species responsible for intravascular transport of NO. Important to the current study, we have shown CS-eNOS-Tg mice to have no significant alteration in mean arterial blood pressure (20). If the transport species was authentic NO or RSNO, the normotensive phenotype of these mice would not be expected, circumstantially suggesting nitrite as the transport species. Findings supporting this ideology include: the respective concentration of blood and tissue nitrite (μM) in relation to other bioactive NO metabolites (nM), and the discovery of various nitrite reductases in many tissues, and the noted biological effects of nitrite administration (11). It is also important to note that nitrite levels in plasma also increase after nitrate ingestion or a nitrate load (29). Although much of the nitrate is eventually excreted in the urine, up to 25% is actively taken up by the salivary glands and is concentrated up to 20-fold in saliva (29). Therefore, the observed increase in steady-state nitrate in these mice could also contribute to the NOS-independent production of nitrite and ultimately NO.
To further understand the flux of NO metabolites during I/R, plasma and hepatic samples were collected at various time points and assessed for nitrite, nitrate, and RXNO content. Consumption of hepatic nitrite was apparent in both WT and CS-eNOS-Tg mice with a maximal reduction after 60 min of ischemia in the Tg animals. Of interest is the fact that even though the CS-eNOS-Tg mice displayed significantly elevated levels of hepatic nitrite at baseline, after ischemia, these levels were reduced to the same extent as those measured in WT liver. This suggests that the period of ischemia was sufficient to maximally reduce hepatic tissue nitrite stores. Results suggest that as little as 3 min of total hypoxia is sufficient to largely consume tissue nitrite (30). After 60 min of reperfusion, nitrite levels were restored to near baseline levels. The temporal consumption of hepatic nitrite over the course of I/R was mirrored by large increases in hepatic RXNO levels during ischemia. These results point to tissue nitrosation as the predominant reaction, thus accounting for nitrite flux during ischemia. No significant change in plasma nitrite concentration was noted in either CS-eNOS-Tg or WT mice, further establishing the importance of nitrite stores within the tissue compartment. A modest increase in plasma RSNO was noted in CS-eNOS-Tg mice, which is likely explained as “spillover” from the tissue compartment given the high baseline levels of nitrite in the CS-eNOS-Tg mice and the observation that this did not occur in WT mice. The importance of tissue nitrite-mediated nitrosation during ischemia was made apparent by inhibiting RXNO species formation by irreversibly binding all thiols with the alkylating agent NEM before ischemia. The administration of NEM abolished all protective effects in the CS-eNOS-Tg mice, suggesting the modification of thiols as a central signaling event in nitrite-mediated cytoprotection. This concept, although controversial, has long been speculated, with initial findings by Ignarro and colleagues (30) that nitrite-mediated vasodilation required thiols, and further suggesting the possibility of a RSNO intermediate (31) in NO-mediated physiology. However, in our model, the scavenging of free NO with PTIO also blocked the hepatoprotection observed in CS-eNOS-Tg mice. This result suggests that free NO, resulting from nitrite reductase activity, may be responsible for mediating downstream nitrosation and eliciting cytoprotective signaling. The reduction of nitrite to NO has been cited to occur in a variety of biological tissues and has been prominently featured in protection against ischemic injury (32). Although this phenomenon was also observed in WT mice, the relative fold change in injury was still much greater in CS-eNOS-Tg mice. We also cannot discount the possibility of nonspecific effects of these agents independent of their intended usage in blocking NO flux. In a proof-of-principle experiment, we injected mice with either GSNO or nitrite to recapitulate the levels of RSNO and nitrite observed in the CS-eNOS-Tg mouse and found that both agents equally reduced I/R injury. Although these results did not allow further distinction as to the protective NO metabolite, they do corroborate our pharmacologic inhibition data. Although both agents were found to be hepatoprotective, our time course experiments reveal that nitrite is consumed to form nitrosothiols during ischemia. This data suggests that nitrite can be given to provide a source of NO and RSNO during ischemia, or low molecular weight RSNOs can be administered to transnitrosate protein thiols that may be involved in cytoprotective cell signaling. These are not mutually exclusive events but demonstrate the inherent biochemistry of nitrite and nitrosothiols and perhaps steady state equilibrium between the two molecules.
Recently, the nitrosation of many molecular targets has been shown to greatly impact a variety of cell-signaling pathways cited as critical to cytoprotection. S-nitrosylation has been shown to powerfully inhibit apoptosis (33), presumably by modification of cysteine residues within the catalytic domain of caspases. Additionally, the recent observation that S-nitrosylation can modulate G protein coupled receptor signaling represents another pathway by which RXNO formation may positively impact I/R injury (34). Yet another noted target for nitrosylation is PARP-1 (35) whereby cysteine modification inhibits transcriptional activity, a mechanism that has extensively been shown to positively impact I/R injury (36). A recent study by Gladwin and colleagues (37) proposed that nitrite's protective actions are mediated through inhibition of complex I of the mitochondrial electron transport chain, presumably via direct S-nitrosation. This mechanism could directly result in decreased reactive oxygen species levels associated with reperfusion injury, and inhibition of mitochondrial permeability transition, a primary event in necrotic cell death. Even though our data strongly suggests that nitrite-mediated nitrosation and reduction to NO are prominent protective signaling mechanisms at work, in our model, we do appreciate the other possible protective actions of free NO, such as altering redox status by scavenging superoxide (38).
In summary, we have shown that NO synthesized within a specified location (cardiomyocytes) is transported in the blood as a bioactive NO species, stored in distant tissues, and protects against ischemic injury in a NO and nitrosothiol-dependent fashion. This report provides direct evidence that endogenously generated NO is capable of exerting endocrine activity and therefore suggests the reassessment of the perception that NO is merely a paracrine signaling molecule.
Materials and Methods
All experimental procedures are described in SI Text.
Cardiac-Specific, eNOS-Transgenic Mice (CS-eNOS-Tg).
Mice were originally developed by Dr. Steffan Janssens (University of Leuven, Belgium), by using the human eNOS gene (39). The expression of this transgene is under the control of the α-myosin heavy-chain promoter, restricting expression to cardiac myocytes. This mouse was developed on a C57BL6/J background and has been well characterized (19, 20). Nontransgenic, WT littermates were used as controls in all experiments. Male mice were used at 8–12 weeks of age. All experimental procedures complied with the Guide for the Care and Use of Laboratory Animals (DHHS Publication No. 86–23, revised 1985, Animal Resources Program, DRR/National Institutes of Health, Bethesda). The Albert Einstein College of Medicine Animal Institute Care and Use Committee approved all experimental protocols.
Supplementary Material
Acknowledgments.
The authors thank Mark R. Duranski for technical assistance. This work was supported by National Institutes of Health Grants 2RO1 HL-060849 (to D.J.L.) and F32DK077380 (to J.W.C.), American Diabetes Association Grant 7–04-RA-59 (to D.J.L.), and American Heart Association-National Grant 0735042N (to N.S.B.).
Footnotes
Conflict of interest statement: D.J.L. is a participant on a pending U.S. patent, filed on October 14, 2003 through the National Institutes of Health (patent no. 60/511, 244), regarding the use of sodium nitrite in cardiovascular disease. N.S.B. is on the scientific advisory board of TriVita, Inc., a nutritional company.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800700105/DCSupplemental.
References
- 1.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, et al. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 4.Kelm M, Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990;66:1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- 5.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 6.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 7.Gladwin MT, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 8.Stamler JS, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignarro LJ. Endothelium-derived nitric oxide: Actions and properties. FASEB J. 1989;3:31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- 10.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 11.Bryan NS, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 12.Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic Biol Med. 2006;41:691–701. doi: 10.1016/j.freeradbiomed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Stamler JS, et al. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon RO, III, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rassaf T, et al. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest. 2002;109:1241–1248. doi: 10.1172/JCI14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryan NS, et al. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens S, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94:1256–1262. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 20.Elrod JW, et al. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:1517–1523. doi: 10.1161/01.ATV.0000224324.52466.e6. [DOI] [PubMed] [Google Scholar]
- 21.Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E. Role of S-nitrosothiol transport in the cardioprotective effects of S-nitrosocysteine in rat hearts. Free Radic Biol Med. 2007;43:1086–1094. doi: 10.1016/j.freeradbiomed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Jones SP, et al. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci USA. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi Y, et al. Nitrosyl hemoglobin in blood of normoxic and hypoxic sheep during nitric oxide inhalation. Am J Physiol. 1998;274:H349–357. doi: 10.1152/ajpheart.1998.274.1.H349. [DOI] [PubMed] [Google Scholar]
- 24.Kubes P, Payne D, Grisham MB, Jourd-Heuil D, Fox-Robichaud A. Inhaled NO impacts vascular but not extravascular compartments in postischemic peripheral organs. Am J Physiol. 1999;277:H676–682. doi: 10.1152/ajpheart.1999.277.2.H676. [DOI] [PubMed] [Google Scholar]
- 25.Troncy E, et al. Extra-pulmonary effects of inhaled nitric oxide in swine with and without phenylephrine. Br J Anaesth. 1997;79:631–640. doi: 10.1093/bja/79.5.631. [DOI] [PubMed] [Google Scholar]
- 26.Hataishi R, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379–384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 27.Dejam A, Hunter CJ, Gladwin MT. Effects of dietary nitrate on blood pressure. N Engl J Med. 2007;356:1590. doi: 10.1056/NEJMc070163. [DOI] [PubMed] [Google Scholar]
- 28.Jansson EA, et al. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic Biol Med. 2007;42:510–518. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Ignarro LJ, Edwards JC, Gruetter DY, Barry BK, Gruetter CA. Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980;110:275–278. doi: 10.1016/0014-5793(80)80091-3. [DOI] [PubMed] [Google Scholar]
- 31.Ignarro LJ, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 32.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannick JB, Miao XQ, Stamler JS. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272:24125–24128. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- 34.Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Kuncewicz T, Dubinsky WP, Kone BC. Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J Biol Chem. 2006;281:9101–9109. doi: 10.1074/jbc.M511049200. [DOI] [PubMed] [Google Scholar]
- 36.Gero D, Szabo C. Role of the peroxynitrite-poly (ADP-ribose) polymerase pathway in the pathogenesis of liver injury. Curr Pharm Des. 2006;12:2903–2910. doi: 10.2174/138161206777947579. [DOI] [PubMed] [Google Scholar]
- 37.Shiva S, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aslan M, Freeman BA. Oxidases and oxygenases in regulation of vascular nitric oxide signaling and inflammatory responses. Immuol Res. 2002;26:107–118. doi: 10.1385/IR:26:1-3:107. [DOI] [PubMed] [Google Scholar]
- 39.Janssens SP, Shimouchi A, Quertermous T, Bloch DB, Bloch KD. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992;267:14519–14522. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.