Abstract
The mitochondrial transcription machinery synthesizes the RNA primers required for initiation of leading-strand DNA synthesis in mammalian mitochondria. RNA primers are also required for initiation of lagging-strand DNA synthesis, but the responsible enzyme has so far remained elusive. Here, we present a series of observations that suggests that mitochondrial RNA polymerase (POLRMT) can act as lagging-strand primase in mammalian cells. POLRMT is highly processive on double-stranded DNA, but synthesizes RNA primers with a length of 25 to 75 nt on a single-stranded template. The short RNA primers synthesized by POLRMT are used by the mitochondrial DNA polymerase γ to initiate DNA synthesis in vitro. Addition of mitochondrial single-stranded DNA binding protein (mtSSB) reduces overall levels of primer synthesis, but stimulates primer-dependent DNA synthesis. Furthermore, when combined, POLRMT, DNA polymerase γ, the DNA helicase TWINKLE, and mtSSB are capable of simultaneous leading- and lagging-strand DNA synthesis in vitro. Based on our observations, we suggest that POLRMT is the lagging-strand primase in mammalian mitochondria.
Keywords: DNA replication, mitochondrion, primase
Human mitochondria have a small double-stranded DNA (dsDNA) genome of 16.6 kb, which contains two origins of replication, OH and OL (1). According to the strand-displacement model for mitochondrial DNA (mtDNA) replication, DNA synthesis is continuous on both strands and takes place in a strand-asymmetric mode. DNA synthesis from OH is unidirectional and proceeds to displace the parental heavy strand (H-strand). After leading-strand synthesis has reached two-thirds of the genome, it activates OL and lagging-strand DNA synthesis then initiates in the opposite direction (2–4). Recently, analysis of mtDNA replication intermediates by atomic force microscopy revealed that the strand-displacement model is an oversimplification and that additional initiation sites for lagging-strand mtDNA synthesis exist on the light strand (L-strand) (5). However, individually, these multiple initiation sites may be less abundant, explaining why only OL was identified when 5′-end mapping was used to identify initiation sites for lagging-strand mtDNA synthesis (3).
The strand-asymmetric model for mtDNA replication has been challenged by two dimensional neutral/neutral agarose gel electrophoresis (2DNAGE), demonstrating the presence of conventional duplex mtDNA replication intermediates, indicative of coupled leading and lagging-strand DNA synthesis (6, 7). One specific class of replication intermediates identified by 2DNAGE incorporates RNA on the lagging strand across the entire genome (8). The idea has been put forward that the lagging strand is initially laid down as RNA and subsequently replaced by DNA in a maturation step involving the mitochondrial DNA polymerase (POLγ), but the exact mechanisms remain to be elucidated (8, 9).
The mitochondrial transcription machinery synthesizes the RNA primers required for initiation of leading-strand DNA synthesis at OH. Transcription from the mitochondrial light strand promoters (LSP) generates transcripts of almost genomic length, which are processed to yield the individual mRNA and tRNA molecules (10, 11). LSP-dependent transcription also generates the primers needed at OH (12), and RNA attached to the newly synthesized H strand has been detected in both mouse and human cells (13). Primer formation may include an RNase MRP-dependent cleavage of the transcribed L-strand RNA at the initiation sites of DNA replication (14). Alternatively, specific mtDNA sequences may direct premature transcription termination and primer formation near OH (15).
As described above, both the modified strand-asymmetric model and the strand-coupled model for mtDNA replication require initiation of lagging-strand DNA synthesis at multiple sites. However, the enzyme required for lagging-strand primer synthesis has not yet been identified. Bioinformatic and biochemical analysis of the mitochondrial DNA helicase, TWINKLE, suggest that this protein also harbors a primase activity in at least some nonmetazoan cells (16–18), but the primase activity has been lost in mammalian TWINKLE (17, 19). There have also been reports of an OL-specific primase activity in mitochondrial extracts, but the corresponding protein has not been identified (20).
In this report, we characterize mitochondrial RNA polymerase (POLRMT) and provide biochemical evidence suggesting that this polymerase is the lagging-strand primase in mammalian mitochondria. Our investigations were initially prompted by a previous report, which identified a primase activity in nuclear extracts of herpes simplex virus type 1-infected Vero cells (21). The observed primase synthesized short oligoribonucleotides, with the predominant products in the range of 30 to 60 nt. Based on the apparent molecular weight and the enzymatic characteristics of the observed activity, the authors concluded that the responsible enzyme most likely was POLRMT, but the enzyme was never directly identified. The biochemical analysis presented here supports this previous report and demonstrates that POLRMT has two distinct modes of action. The enzyme efficiently transcribes long regions of dsDNA, but becomes much less processive on single-stranded DNA (ssDNA), producing only short RNA molecules of 25–75 nt. The short RNA primers can be used by the mitochondrial POLγ to initiate DNA synthesis in vitro and this reaction is stimulated by mitochondrial ssDNA binding protein (mtSSB). We have demonstrated that the minimal mtDNA replisome (POLγ, mtSSB, and TWINKLE) can support leading-strand DNA synthesis on a double-stranded DNA template (22). We now find that addition of POLRMT to this system, leads to simultaneous leading- and lagging-strand DNA synthesis in vitro.
Results
POLRMT Efficiently Synthesizes Short RNA Fragments on an ssDNA Template.
A previous report suggested that POLRMT possesses primase activity (21) and we decided to experimentally address this possibility. First, we analyzed the polymerase activity of recombinant POLRMT on ssDNA (Fig. 1A). We observed that POLRMT synthesizes relatively short RNA species on ssDNA (25–75 ribonucleotides), confirming previous results with partially purified native POLRMT (21). The RNA primers were strongly reduced (>95%) after RNase H treatment, indicating that the RNA primers were annealed to ssDNA (data not shown).
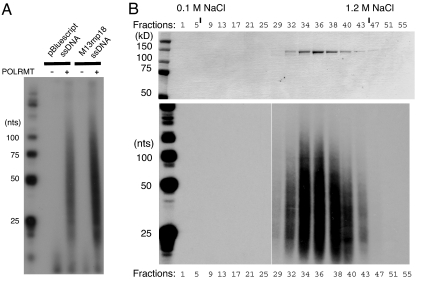
Fig. 1.
POLRMT synthesizes short RNA stretches on ssDNA templates. RNA synthesis by POLRMT was monitored as described in Material and Methods by labeling the RNA. (A) RNA synthesis by POLRMT (500 fmol) was carried out on pBluescript or M13mp18 ssDNA templates and the products were separated on a denaturing polyacrylamide gel (10%). (B) POLRMT peak fractions (10 μl) from purification over MonoS were separated by SDS/PAGE (10%) and revealed with Coomassie Brilliant Blue staining (Upper). The RNA synthesis of the protein fractions (5 μl) in the upper panel was analyzed in the presence of M13mp18 ssDNA (Lower). The beginning and the end of the linear salt gradient are indicated.
Even though we estimated the purity of our recombinant POLRMT preparation to be >95%, we decided to follow the activity during another step of purification to exclude the possibility that the short RNA primers were formed by a contaminant primase from the Sf9 insect cells. Examination of the purified protein showed that the DNA primase activity coincided perfectly with the peak of POLRMT protein during elution from a cation exchange column (Fig. 1B). These results support the conclusion that the observed oligoribonucleotides were produced by POLRMT.
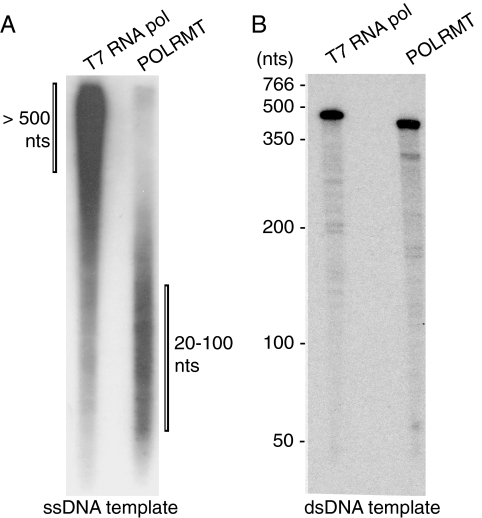
We demonstrated that POLRMT can efficiently synthesize long stretches of RNA on a dsDNA template (23) and we were therefore surprised to see preferential synthesis of short transcripts on an ssDNA template. To investigate whether this is a specific characteristic of POLRMT, we analyzed the T7 RNA polymerase in parallel. The T7 RNA polymerase was chosen because POLRMT displays significant sequence similarity and appears functionally related to this bacteriophage protein (24, 25). A direct comparison of POLRMT and T7 RNA polymerase revealed that the phage polymerase synthesizes much longer stretches of RNA on ssDNA (Fig. 2A). The majority of RNA species produced by the T7 RNA polymerase had a length of 500 nt or longer. On a linearized, dsDNA template, both POLRMT and T7 RNA polymerase synthesized full-length runoff transcripts of ≈400 nt (Fig. 2B). We conclude that the ability to preferentially synthesize oligoribonucleotides on ssDNA is specific to POLRMT.
Fig. 2.
RNA synthesis of POLRMT and T7 RNA polymerase on ssDNA and dsDNA templates. RNA synthesis by POLRMT (500 fmol) or by T7 RNA polymerase (0.8 units) were monitored as described in Material and Methods. (A) RNA products formed on the M13mp18 ssDNA template were separated on a denaturing polyacrylamide gel (10%). (B) RNA products formed by promoter-dependent transcription on a dsDNA template were separated on a denaturing polyacrylamide gel (10%). The positions of molecular size markers are indicated on the left. The length of the T7 RNA polymerase product is 458 nt and the POLRMT product is 396 nt.
POLγ Can Use POLRMT-Synthesized Primers to Initiate DNA Replication in Vitro.
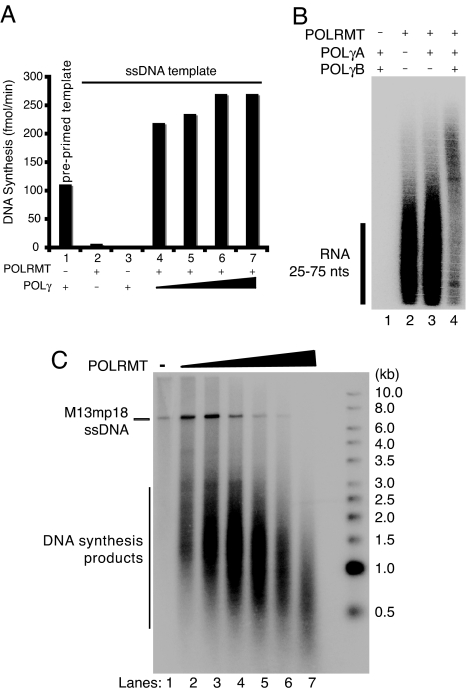
POLγ can initiate DNA synthesis from a preannealed primer on M13mp18 ssDNA (Fig. 3A, lane 1). In the presence of this template POLγ incorporates dNTPs into DNA at a rate of approximately 120 fmol/min. In the absence of a primer, POLγ is unable to initiate DNA synthesis on M13mp18 ssDNA (Fig. 3A, lane 3). However, in the presence of POLRMT, POLγ can efficiently incorporate dNTPs into DNA (Fig. 3A, lanes 4–7), suggesting that POLγ utilizes RNA primers synthesized by POLRMT to initiate DNA synthesis. POLRMT can synthesize multiple primers on each M13mp18 ssDNA molecule and higher levels of DNA synthesis are therefore observed in the presence of POLRMT (Fig. 3A, lanes 4–7), compared with the preannealed primer template (Fig. 3A, lane 1), which only contains one possible initiation site for DNA synthesis.
Fig. 3.
POLRMT primes DNA synthesis by POLγ on ssDNA templates. (A) RNA-primed DNA synthesis on an ssDNA template was monitored in the presence of [α-32P]dCTP for the labeling of leading-strand products. Reactions (20 μl) were incubated for 1 h at 37°C and aliquots were applied to DE81 filter papers for further analysis and scintillation counting as described in Material and Methods. Bar 1, Control experiment using preprimed M13mp18 ssDNA with POLγ (100 fmol); Bar 2, POLRMT (200 fmol); Bar 3, POLγ (100 fmol); Bar 4–7, POLRMT (200 fmol) and increasing amounts of POLγ (50, 100, 500, and 1000 fmol). (B) RNA-primed DNA synthesis on ssDNA was performed in the presence of [α-32P]UTP for labeling of RNA primers. Reactions (25 μl) contained M13mp18 ssDNA (35 fmol) and, when indicated, POLRMT (500 fmol), POLγA (100 fmol), or POLγB (300 fmol). After incubation for 1 h at 37°C, the products were separated on a denaturing polyacrylamide gel (10%) and detected by autoradiography. (C) The RNA-primed DNA synthesis assay was performed in the presence of [α-32P]dCTP. Reactions (20 μl) contained M13mp18 ssDNA (35 fmol), POLγ (100 fmol), and increasing amounts of POLRMT (0, 25, 50, 100, 250, 500, and 1000 fmol). After incubation for 1 h at 37°C the products were separated by electrophoresis on a denaturing agarose gel (0.8%) and detected by autoradiography.
To investigate the destiny of RNA primers during DNA synthesis, we used radiolabeled NTPs (Fig. 3B). When POLRMT was added in isolation we observed only RNA fragments of 25–75 nt (Fig. 3B, lane 2). Slightly longer products were detected after addition of the catalytic subunit of POLγ (POLγA), and the products were further extended upon addition of the processivity factor, POLγB. These longer products therefore represent a DNA molecule with a labeled RNA primer (Fig. 3B), demonstrating that POLγ can use POLRMT synthesized primers for initiation of DNA synthesis.
To further analyze whether the short RNA primers synthesized by POLRMT were used to initiate DNA replication, we next monitored the DNA products using radiolabeled dNTPs. We added increasing amounts of POLRMT to the reactions, keeping constant the concentrations of POLγ and ssDNA template. In the absence of POLRMT, no DNA synthesis was observed (Fig. 3C, lane 1). Addition of POLRMT resulted in increasing levels of DNA synthesis (Fig. 3C). Interestingly, the length of the DNA products became shorter with increasing amounts of POLRMT. This indicates that higher levels of primer synthesis lead to the synthesis of shorter DNA fragments, which would be expected, because DNA synthesis will only proceed until POLγ runs into the next RNA primer. In the presence of more primers, shorter stretches of ssDNA will be available for DNA synthesis.
mtSSB Stimulates Primer Utilization.
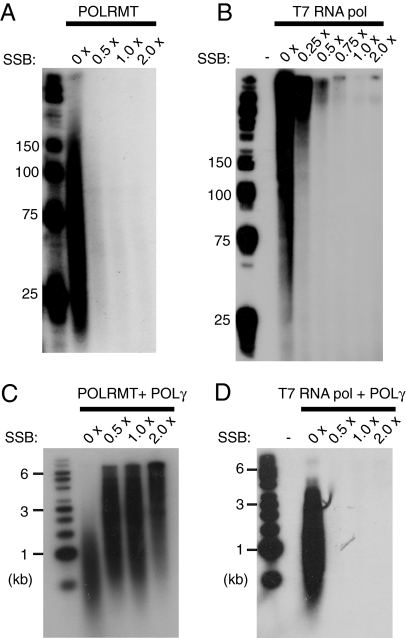
In vivo, the long-stretches of ssDNA formed during mtDNA replication are most likely coated by mtSSB. We therefore examined how mtSSB would influence POLRMT-dependent primer synthesis on ssDNA (Fig. 4A). Our experiments demonstrated that the primase activity was dramatically diminished when the ssDNA was fully coated with mtSSB. This inhibition was not specific to POLRMT, because a similar, negative effect was seen with T7 RNA polymerase (Fig. 4B). Interestingly, even if less RNA primers were produced when the ssDNA was fully coated with mtSSB, the primers produced could be efficiently used by POLγ to initiate DNA synthesis. In fact, addition of mtSSB stimulated the combined action of POLγ and POLRMT (Fig. 4C). DNA fragments of ≈500–1500 bp were produced in the absence of mtSSB (Fig. 4C, lane 2), whereas significantly longer products were produced in the presence of the mtSSB protein (Fig. 4C, lanes 3–5). In contrast, the heterologous T7 RNA polymerase failed to support DNA synthesis in the presence of mtSSB (Fig. 4D).
Fig. 4.
MtSSB influences POLRMT-dependent primer synthesis. (A) RNA synthesis on ssDNA was performed as described in Material and Methods. Reactions mixtures (25 μl) contained M13mp18 ssDNA (35 fmol), POLRMT (500 fmol), and increasing amounts of mtSSB (0 fmol, 8.4 pmol, 16.8 pmol, 33.6 pmol). Each mtSSB monomer covers approximately 60 nt (33, 34) and the saturation level for each protein concentration was calculated. The saturation level 1 × indicates that the ssDNA molecules should be completely covered with mtSSB. After the incubation for 1 h at 37°C, reaction products were separated by electrophoresis on a denaturing polyacrylamide gel (10%) and detected by autoradiography. (B) Reactions were performed as in (A), but in the presence of T7 RNA polymerase (1 unit). (C) RNA-primed DNA synthesis on ssDNA in the presence of [α-32P]dCTP for labeling of DNA. Reactions (25 μl) contained M13mp18 ssDNA (35 fmol), POLRMT (500 fmol), POLγA (100 fmol), POLγB (300 fmol), and increasing amounts of mtSSB [saturation levels are indicated in (A)]. The reactions were allowed to proceed for 1 h at 37°C and the reaction products were separated by electrophoresis on a denaturing agarose gel (1%) and detected by autoradiography. (D) Reactions were performed as in (C), but in the presence of T7 RNA polymerase (1 unit).
Synthesis of Leading and Lagging Strands in Vitro.
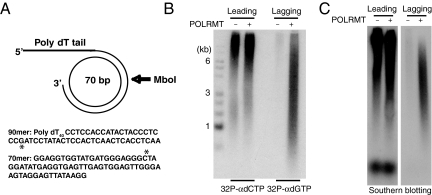
In earlier work, we have been able to reconstitute a minimal mammalian mtDNA replisome in vitro (22). In combination, POLγ and TWINKLE form a processive replication machinery, which is stimulated by mtSSB and can use dsDNA as a template to synthesize long stretches of single-stranded DNA. To investigate whether this machinery could support lagging-strand mtDNA synthesis, we used a synthetic minicircle template with a replication fork that has been described earlier (22). The template contains one C-rich and one G-rich strand, and offers several experimental advantages: the products of leading- and lagging-strand synthesis can be specifically radioactivity labeled; oligonucleotide probes can be designed that unambiguously detect leading and lagging strands; and restriction enzyme cleavage can be used to distinguish between single- and double-stranded DNA products (Fig. 5A).
Fig. 5.
Lagging-strand DNA synthesis in vitro. (A) Minicircle template for rolling-circle DNA replication. Radioactive dNTPs can be used to preferentially label the synthesis of leading ([α-32P]dCTP) or lagging ([α-32P]dGTP) strands. The single G and C in the templates for lagging and leading strands are indicated with an asterisk. (B and C) Products formed by rolling-circle DNA replication in vitro were analyzed by electrophoresis on an alkaline agarose gel as described in Materials and Methods. The reaction mixtures contained POLγ (200 fmol), TWINKLE (100 fmol), mtSSB (5 pmol), and POLRMT (200 fmol) as indicated. (B) POLRMT supports lagging-strand DNA synthesis. Lane 1, DNA size markers; lanes 2 and 3, leading-strand DNA synthesis labeled with [α-32P]dCTP; lanes 4 and 5, lagging-strand DNA synthesis labeled with [α-32P]dGTP. (C) Southern blot analyses of replication products. In lanes 1 and 2, the oligonucleotide probe is complementary to the leading strand; in lanes 3 and 4, the oligonucleotide probe is complementary to the lagging strand.
We first used the minicircle template to investigate leading- and lagging-strand synthesis. Using only POLγ, TWINKLE, and mtSSB, we observed the production of long products (>10 kb), which were preferentially labeled with [α-32P]dCTP, supporting the interpretation that these were products of leading-stand DNA synthesis (Fig. 5B). When POLRMT was added to the reaction, we observed the long [α-32P]dCTP-labeled DNA fragments, but we also detected a second, shorter DNA population, which was preferentially labeled with [α-32P]dGTP, indicating that these were products of lagging-strand synthesis. To further validate that the short DNA fragments were lagging-strand products, we analyzed our reaction products by Southern blot analysis using strand-specific oligonucleotide probes. Our analysis showed that the shorter fragments were indeed produced by lagging-strand synthesis, whereas the longer products (>10 kb) were the products of leading-strand synthesis (Fig. 5C).
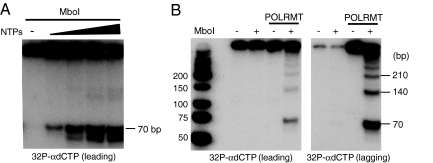
The 70-mer template used for rolling-circle DNA replication, contains one recognition site for the restriction enzyme MboI (Fig. 5A). We could therefore employ MboI digestion to distinguish between single- and double-stranded products from the rolling-circle DNA replication reactions. Restriction enzyme cleavage showed that nearly 100% of the products labeled with [α-32P]dGTP (lagging-strand), but somewhat <50% of the products labeled with [α-32P]dCTP (leading-strand) were double-stranded (Fig. 6A and data not shown). As would be expected for an RNA primed event, the synthesis of dsDNA was completely dependent on the presence of ribonucleoside triphosphates (Fig. 6A). To investigate whether the double-stranded DNA products were the products of rolling-circle DNA synthesis, we used subsaturating amounts of MboI for incomplete cleavage. This treatment gave rise to a ladder of fragments, with a unit length of 70 bp, corresponding to the length of the circular template. Double stranded fragments of 140 bp, 210 bp, and longer demonstrated that the labeled dsDNA products were produced during rolling-circle DNA replication (Fig. 6B).
Fig. 6.
Double-stranded DNA synthesis in vitro. (A) Production of dsDNA depends on NTPs. Rolling-circle DNA replication was performed as described in figure legend 5, but in the presence of varying amounts of NTPs (0 μM, 37.5 μM, 75 μM, 150 μM, and 300 μM). Products were labeled with [α-32P]dCTP, cleaved with MboI, separated by polyacrylamide gel electrophoresis, and detected by autoradiography. (B) Incomplete cleavage by MboI detects long double-stranded DNA products, indicative of rolling-circle DNA replication. The analysis was as in (A), but products labeled with either [α-32P]dGTP (lagging strand) or [α-32P]dCTP (leading strand) were cleaved with subsaturating amounts of MboI.
Discussion
That POLRMT is responsible for primer synthesis at OH was demonstrated >20 years ago (26). In contrast, the primase required for primer synthesis and initiation of mtDNA synthesis at OL and other initiation sites for lagging-strand synthesis has remained elusive. Here, we present a series of observations that demonstrates that POLRMT can function as a lagging-strand primase for mtDNA replication.
First, POLRMT is a processive polymerase on a dsDNA template, but is nonprocessive on an ssDNA template. On the ssDNA template, RNA primers with a length between 25 and 75 nt are synthesized. This is consistent with a previous report, which identified POLMRT in a search for a primase activity in Vero cells infected with herpes simplex virus (21). Second, POLRMT can efficiently support initiation of mtDNA synthesis on ssDNA coated with mtSSB. This activity seems to be specific to POLRMT, because the related T7 RNA polymerase is unable to support initiation of POLγ-dependent DNA synthesis in the presence of mtSSB. Third, addition of POLRMT allows the mtDNA replisome (POLγ, TWINKLE, mtSSB) to synthesize both leading- and lagging-strand DNA on a double-stranded template.
The findings presented here could easily be reconciled with the strand displacement model for mtDNA replication. During replication, this model foresees the generation of a long stretch of ssDNA between OH and OL. The exposed ssDNA is covered by mtSSB and is thus an excellent template for the POLRMT-dependent primase activity characterized here. Recently, analysis of mtDNA replication intermediates by atomic force microscopy revealed that lagging-strand mtDNA synthesis is initiated at several different sites throughout the L-strand (5). Even if DNA replication is initiated at multiple sites on the L-strand, the OL appears to be an especially strong initiation site. The presented results could also provide a molecular explanation for the conventional duplex mtDNA replication intermediates observed by (2DNAGE) analyses (27).
Bioinformatic analyses of TWINKLE homologues in eukaryotes have revealed conserved primase motifs, including a zinc finger motif, in all TWINKLE sequences outside of Metazoa (17). However, these motifs were lost in metazoan cells and human TWINKLE is not an active primase in vitro (19). Our findings suggest that POLRMT has replaced the TWINKLE associated primase activity in higher cells. Although RNA primers for DNA synthesis are usually synthesized by specialized primases, some replication systems have evolved to use RNA polymerases for primer synthesis and initiation of DNA replication (28, 29). The host Escherichia coli RNA polymerase synthesizes a transcript that is used to prime replication of the single-stranded genome of bacteriophage M13 (30, 31). Interestingly, in its single-stranded conformation, this origin forms an imperfect hairpin DNA, somewhat related to the predicted structure of OL. In future studies, we will attempt to address the mechanisms of OL activation, to see whether this is a preferred initiation site for POLRMT-dependent primer synthesis.
Materials and Methods
Recombinant Proteins.
TWINKLE, mtSSB, POLγA, POLγB, and TFAM were expressed and purified as described (22, 32). To generate POLRMT we coinfected insect cells with viruses expressing both POLRMT and TFB2M and purified the heterodimeric complexes to near homogeneity over Ni2+-agarose, as described in ref. 23. The peak of eluted proteins was dialyzed against buffer B [20 mM Tris–HCl (pH 8.0), 0.5 mM EDTA (pH 8.0), 1% glycerol, and 1 mM DTT] containing 0.2 M NaCl. The proteins were further purified on a 1-ml HiTrap heparin column (GE Healthcare) equilibrated in buffer B (0.2 M NaCl). After washing the column with three column volumes of buffer B (0.2 M NaCl), we used a linear gradient (10 ml) of buffer B (0.2–1.2 M NaCl) to elute the column. POLRMT and TFB2M were eluted as two distinct peaks from the heparin and we estimated the purity of the POLRMT peak fraction to be at least 95% by SDS/PAGE with Coomassie Brilliant Blue staining. In the experiment reported in Fig. 1B, we purified POLRMT over a third column. For this purpose the peak fractions of POLRMT from the heparin column were dialyzed against buffer C [25 mM NaPhosphate (pH 7.0), 0.5 mM EDTA (pH 8.0), 10% glycerol and 1 mM DTT] containing 0.1 M NaCl and purified over a Mono-S column equilibrated in buffer C (0.1 M NaCl). A linear gradient (10 ml) of buffer C (0.1–1.2 M NaCl) was used to elute the protein. In our experiment we refer only to the activity of POLRMT because the amount of TFB2M activity in the purified fractions was negligible (data not shown).
In Vitro Transcription on ssDNA.
The reaction mixture (25 μl), contained 35 fmol of single-stranded M13mp18 DNA (New England Biolabs), 10 mM Tris·HCl (pH 8.0), 20 mM MgCl2, 1 mM DTT, 100 μg/ml BSA, 400 μM ATP, 150 μM CTP, and GTP, 10 μM UTP, 0.2 μM α-32P UTP (3,000 Ci/mmol), 4 units of RNase inhibitor (Amersham Biosciences), and the indicated concentrations of T7 RNA polymerase (Roche) or purified POLRMT protein. We stopped the reaction after 30 min at 32°C by adding 200 μl of stop buffer [10 mM Tris·HCl (pH 8.0), 0.2 mM NaCl, 1 mM EDTA, and 0.1 mg/ml glycogen]. Further, we treated the samples with 0.5% SDS and 100 μg/ml proteinase K for 45 min at 42°C, and precipitated them by adding 600 μl of ice-cold ethanol. The samples were dissolved in 10 μl of loading buffer [98% formamide, 10 mM EDTA (pH 8.0), 0.025% xylene cyanol FF, 0.025% bromophenol blue], denatured for 5 min at 95°C, and analyzed on a denaturing polyacrylamide gel (10%) in 1 × TBE buffer.
In Vitro Transcription on dsDNA.
We cloned DNA fragments corresponding to bp 1–477 (LSP) of human mtDNA into pUC18 and used overlap extension PCR to delete CSB II (bp 289–319) (15). To introduce a T7 promoter upstream of the mtDNA sequence, we subcloned an mtDNA fragment of bp 1–393 lacking CSBII (bp 289–319) into pBluescript SKII. The in vitro transcription reaction on dsDNA was performed as described in ref. 23.
RNA-Primed DNA Synthesis and Rolling-Circle mtDNA Replication.
To label DNA products, during RNA-primed DNA synthesis on ssDNA, we used the following protocol. The reaction mixtures (25 μl) contained 35 fmol of single-stranded M13mp18 DNA (or 35 fmol of M13mp18 ssDNA preprimed with a 20 bp oligonucleotide, when indicated), 25 mM Tris·HCl (pH 8.0), 10.5 mM MgCl2, 1 mM DTT, 100 μg/ml BSA, 400 μM ATP, 150 μM CTP, 150 μM GTP, 150 μM UTP, 100 μM dATP, 100 μM dGTP, 100 μM dTTP, 4 units of RNase inhibitor, 10 μM dCTP, and 2 μCi α-32P dCTP (3,000 Ci/mmol) (Amersham Biosciences). To label RNA products, during RNA-primed DNA synthesis, we used similar conditions, but with the following modifications. All dNTPs were added to a final concentration of 100 μM together with 10 μM UTP and 0.2 μM α-32P UTP (3,000 Ci/mmol).
To analyze leading-strand DNA synthesis during rolling-circle replication, we used a minicircle template (22), but otherwise followed the protocol used for labeling DNA products, during RNA-primed DNA synthesis on ssDNA. To monitor lagging-strand DNA synthesis on the minicircle template, we followed a similar protocol, but with the following modifications. The radioactive nucleotide [α-32P]dCTP was replaced with 2 μCi [α-32P]dGTP, and 10 μM dGTP and 100 μM dCTP were used. Replication factors were added as indicated in the figure legends and the reaction was incubated at 37°C for 90 min and terminated, as described. The pellets were dissolved in 20 μl of water for analysis by agarose gel electrophoresis or in 10 μl of loading buffer for analysis by denaturing polyacrylamide gel electrophoresis.
In Fig. 3A, incorporation of α-32P dCTP was measured by spotting 5 μl aliquots of the reaction mixture (after 90 min at 37°C) on Whatman DE-81 paper disks. The filters were washed (3 × with 2 × SSC and 1 × with 95% EtOH) and the remaining activity was quantified by scintillation counting.
Samples to be analyzed by restriction enzyme cleavage were treated with 100 μg/ml proteinase K and 0.5% SDS for 20 min at 37°C. The samples were diluted with H20 to 175 μl and extracted first with phenol/chloroform and then with chloroform. The aqueous phase (150 μl) was precipitated by addition of 2.5 M ammonium acetate and 2.5 volumes of ethanol. The pellet was dissolved in 20 μl of a buffer containing 100 mM NaCl, 50 mM Tris·HCl (pH 7.9), 10 mM MgCl2, and 1 mM DTT. MboI (1 or 5 units) was added to each reaction. The samples were then incubated for 4–8 h at 37°C and analyzed on a 10% polyacrylamide gel in 1× TBE buffer.
Southern Blot Analysis.
Rolling-circle DNA synthesis was carried out as described above but without the addition of radioactive nucleotides and in the presence of 100 μM each of dATP, dGTP, dCTP, and dTTP. The sample was divided in two parts and was loaded onto a 0.8% alkaline agarose gel. After electrophoresis, the DNA was transferred to a nylonfilter (N-Hybond+, Amersham Pharmacia). The membrane was cut in two pieces and hybridized with either the 50-mer 5′-TTGAGGTGAGTTGAGTGGAGTATAGGATCGGGAGGGTAGTATGGTGGAGG-3′ to detect leading-strand products or the 50-mer 5′-CCTCCACCATACTACCCTCCCGATCCTATACTCCACTCAACTCACCTCAA-3′ to detect lagging-strand products.
Acknowledgments.
This work was supported by the Swedish Research Council, The Swedish Cancer Society, European Commission (fp6 EUMITOCOMBAT), the Swedish Strategic Foundation, the Göran Gustafsson Foundation, and the Knut and Alice Wallenberg Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Falkenberg M, Larsson NG, Gustafsson CM. DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem. 2007;76:679–699. doi: 10.1146/annurev.biochem.76.060305.152028. [DOI] [PubMed] [Google Scholar]
- 2.Robberson DL, Kasamatsu H, Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci USA. 1972;69:737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapper DP, Clayton DA. Mechanism of replication of human mitochondrial DNA. Localization of the 5′ ends of nascent daughter strands. J Biol Chem. 1981;256:5109–5115. [PubMed] [Google Scholar]
- 4.Kang D, Miyako K, Kai Y, Irie T, Takeshige K. In vivo determination of replication origins of human mitochondrial DNA by ligation-mediated polymerase chain reaction. J Biol Chem. 1997;272:15275–15279. doi: 10.1074/jbc.272.24.15275. [DOI] [PubMed] [Google Scholar]
- 5.Brown TA, Cecconi C, Tkachuk AN, Bustamante C, Clayton DA. Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism. Genes Dev. 2005;19:2466–2476. doi: 10.1101/gad.1352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang MY, et al. Biased incorporation of ribonucleotides on the mitochondrial L-strand accounts for apparent strand-asymmetric DNA replication. Cell. 2002;111:495–505. doi: 10.1016/s0092-8674(02)01075-9. [DOI] [PubMed] [Google Scholar]
- 7.Holt IJ, Jacobs HT. Response: The mitochondrial DNA replication bubble has not burst. Trends Biochem Sci. 2003;28:355–356. doi: 10.1016/S0968-0004(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 8.Yasukawa T, et al. Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. EMBO J. 2006;25:5358–5371. doi: 10.1038/sj.emboj.7601392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanrooij S, Goffart S, Pohjoismaki JL, Yasukawa T, Spelbrink JN. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 2007;35:3238–3251. doi: 10.1093/nar/gkm215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 11.Clayton DA. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- 12.Xu B, Clayton DA. RNA-DNA hybrid formation at the human mitochondrial heavy-strand origin ceases at replication start sites: An implication for RNA-DNA hybrids serving as primers. EMBO J. 1996;15:3135–3143. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DY, Clayton DA. Properties of a primer RNA-DNA hybrid at the mouse mitochondrial DNA leading-strand origin of replication. J Biol Chem. 1996;271:24262–24269. doi: 10.1074/jbc.271.39.24262. [DOI] [PubMed] [Google Scholar]
- 14.Lee DY, Clayton DA. RNase mitochondrial RNA processing correctly cleaves a novel R loop at the mitochondrial DNA leading-strand origin of replication. Genes Dev. 1997;11:582–592. doi: 10.1101/gad.11.5.582. [DOI] [PubMed] [Google Scholar]
- 15.Pham XH, et al. Conserved sequence box II directs transcription termination and primer formation in mitochondria. J Biol Chem. 2006;281:24647–24652. doi: 10.1074/jbc.M602429200. [DOI] [PubMed] [Google Scholar]
- 16.Seow F, et al. The plastidic DNA replication enzyme complex of Plasmodium falciparum. Mol Biochem Parasitol. 2005;141:145–153. doi: 10.1016/j.molbiopara.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Shutt TE, Gray MW. Twinkle, the mitochondrial replicative DNA helicase, is widespread in the eukaryotic radiation and may also be the mitochondrial DNA primase in most eukaryotes. J Mol Evol. 2006;62:588–599. doi: 10.1007/s00239-005-0162-8. [DOI] [PubMed] [Google Scholar]
- 18.Shutt TE, Gray MW. Bacteriophage origins of mitochondrial replication and transcription proteins. Trends Genet. 2006;22:90–95. doi: 10.1016/j.tig.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Farge G, et al. The N-terminal domain of TWINKLE contributes to single-stranded DNA binding and DNA helicase activities. Nucleic Acids Res. 2008;36:393–403. doi: 10.1093/nar/gkm1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong TW, Clayton DA. Isolation and characterization of a DNA primase from human mitochondria. J Biol Chem. 1985;260:11530–11535. [PubMed] [Google Scholar]
- 21.Tsurumi T, Lehman IR. Release of RNA polymerase from vero cell mitochondria after herpes simplex virus type 1 infection. J Virol. 1990;64:450–452. doi: 10.1128/jvi.64.1.450-452.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23:2423–2429. doi: 10.1038/sj.emboj.7600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falkenberg M, et al. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- 24.Tiranti V, et al. Identification of the gene encoding the human mitochondrial RNA polymerase (h-mtRPOL) by cyberscreening of the Expressed Sequence Tags database. Hum Mol Genet. 1997;6:615–625. doi: 10.1093/hmg/6.4.615. [DOI] [PubMed] [Google Scholar]
- 25.Gaspari M, Falkenberg M, Larsson NG, Gustafsson CM. The mitochondrial RNA polymerase contributes critically to promoter specificity in mammalian cells. EMBO J. 2004;23:4606–4614. doi: 10.1038/sj.emboj.7600465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang DD, Hauswirth WW, Clayton DA. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985;4:1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt IJ, Lorimer HE, Jacobs HT. Coupled leading- and lagging-strand synthesis of mammalian mitochondrial DNA. Cell. 2000;100:515–524. doi: 10.1016/s0092-8674(00)80688-1. [DOI] [PubMed] [Google Scholar]
- 28.Kramer MG, Khan SA, Espinosa M. Plasmid rolling circle replication: Identification of the RNA polymerase-directed primer RNA and requirement for DNA polymerase I for lagging strand synthesis. EMBO J. 1997;16:5784–5795. doi: 10.1093/emboj/16.18.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zenkin N, Naryshkina T, Kuznedelov K, Severinov K. The mechanism of DNA replication primer synthesis by RNA polymerase. Nature. 2006;439:617–620. doi: 10.1038/nature04337. [DOI] [PubMed] [Google Scholar]
- 30.Geider K, Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974;249:3999–4005. [PubMed] [Google Scholar]
- 31.Zenkin N, Severinov K. The role of RNA polymerase sigma subunit in promoter-independent initiation of transcription. Proc Natl Acad Sci USA. 2004;101:4396–4400. doi: 10.1073/pnas.0400886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korhonen JA, Gaspari M, Falkenberg M. TWINKLE Has 5′ -> 3′ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J Biol Chem. 2003;278:48627–48632. doi: 10.1074/jbc.M306981200. [DOI] [PubMed] [Google Scholar]
- 33.Thommes P, Farr CL, Marton RF, Kaguni LS, Cotterill S. Mitochondrial single-stranded DNA-binding protein from Drosophila embryos. Physical and biochemical characterization. J Biol Chem. 1995;270:21137–21143. doi: 10.1074/jbc.270.36.21137. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Curth U, Urbanke C, Kang C. Crystal structure of human mitochondrial single-stranded DNA binding protein at 2.4 A resolution. Nat Struct Biol. 1997;4:153–157. doi: 10.1038/nsb0297-153. [DOI] [PubMed] [Google Scholar]