Abstract
Viruses remodel the host cell to optimize their replication both by delivery of virion proteins into the cell and by de novo expression of viral proteins. The HSV particle contains several proteins that function to prepare the host cell for viral replication, including the VP16 transcriptional activator protein and virion host shutoff protein. HSV infection activates NF-κB pathways through Toll-like receptor (TLR) 2 and non-TLR pathways, and NF-κB activity is required for efficient viral replication. In a screen of the HSV proteome, we observed that the HSV UL37 tegument protein activates NF-κB signaling in a TLR2-independent manner. Expression of UL37 in transfected cells leads to IκB degradation and activation of both reporter genes and the endogenous IL-8 gene. This activation requires TNF receptor–associated factor 6 (TRAF6), and UL37 contains a TRAF6-binding domain that is required for interaction with TRAF6 and activation of NF-κB. A mutant virus encoding UL37 with an altered TRAF6-binding site shows reduced NF-κB activation in the early phase of infection. Therefore, the HSV UL37 virion structural protein can activate NF-κB through TRAF6. Activation of NF-κB by a virion tegument protein that is delivered into the host cell cytoplasm during viral entry represents a mechanism for activation of this pathway by a virus.
Keywords: host response, innate immunity, protein interactions
Innate immune responses have evolved to detect general classes of microbe-specific macromolecules and to mount a rapid signaling response that serves both to block microbial replication and to prime the more long-term and more specific adaptive response. Microbes in turn have evolved mechanisms for evading or blunting those innate responses so they can replicate to higher levels. In some cases microbes have evolved to use the host innate responses to their own advantages. For example, HIV replicates optimally in CD4+ T cells activated by cytokines (1, 2). Epstein-Barr virus, through its latency membrane protein 1, activates NF-κB signaling pathways to promote the growth of B-lymphoblastoid cells so that B cells can become its latent reservoir (3, 4). Similarly, human T-cell leukemia virus Tax protein causes growth and transformation of its host T cell through chronic activation of NF-κB by binding to IκB-γ kinase (IKKγ) and activating IKK activity (5, 6). Furthermore, HSV grows optimally in cells where NF-κB signaling is activated (7–9).
HSV undergoes a lytic replication cycle at mucosal surfaces and spreads into sensory neurons where it establishes a latent infection for the lifetime of the individual (10). The virus is cleared from the epithelium by the immune response, but the virus manages to evade and blunt the innate and adaptive immune responses to establish and maintain the latent infection. As part of the host innate response, HSV infection activates NF-κB signaling through Toll-like receptor (TLR) 2 (11, 12) and non-TLR2 pathways (7). In HSV-infected keratinocytes the induction of NF-κB is biphasic: The early wave occurs independently of viral replication, whereas the second wave requires gene expression (13). Soluble glycoprotein D (gD) can activate NF-κB activity (14), but it is not known if gD on the virion surface has the same activity. The NF-κB response induced by HSV is only partly antiviral in nature, because optimal viral replication requires NF-κB activity (7, 8). NF-κB activation is hypothesized to help activate transcription of at least some of the viral immediate-early genes (13). In this study we investigated one of the mechanisms of the early induction of NF-κB by HSV-1.
Results
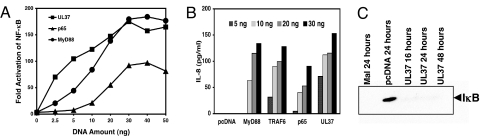
The HSV gene products that directly activate NF-κB through TLR2 or non-TLR2 pathways, other than potentially gD, have not been identified. To screen the HSV proteome for the capacity to activate NF-κB, we cloned the ORFs of HSV-1 strain KOS into a mammalian expression plasmid. Seventy HSV-1 ORFs were screened for their ability to activate an NF-κB reporter gene in human embryonic kidney (HEK) 293 cells expressing TLR2, and the viral UL37 gene product was one of the most active (results not shown). The UL37 protein is a component of the tegument layer of the HSV virion located between the envelope and the capsid (10) and is essential for viral replication (15). To our surprise, this signaling was independent of TLR2 because UL37 also activated NF-κB reporter gene expression in HEK293 cells that did not have the TLR2 gene transfected into them [supporting information (SI) Fig. S1]. This activity was dose dependent and was equivalent to that achieved by the transfection of equal amounts of plasmid DNAs expressing adaptor protein myeloid differentiation factor 88 (MyD88) or an NF-κB subunit, p65 (Fig. 1A). Consistent with its ability to activate the NF-κB reporter gene, UL37 activated the expression of the endogenous IL-8 gene in HEK cells, similar to overexpression of MyD88, TRAF6, or p65 (Fig. 1B). UL37 triggered the degradation of IκB in HEK cells, as observed with overexpression of the adaptor protein, MyD88 adapter-like protein (MAL) (Fig. 1C).
Fig. 1.
Activation of NF-κB activity by HSV UL37. (A) Stimulation of NF-κB reporter gene expression by HSV-1 UL37. Various amounts of UL37, p65, or MyD88 plasmid DNAs were cotransfected into HEK293 cells with 20 ng of NF-κB reporter plasmid and 5 ng of thymidine kinase control plasmid. At 24 h after transfection, the firefly and Renilla luciferase activities were measured using the Promega dual-glo luciferase assay system. For each experimental sample the ratio of firefly to Renilla luciferase activity was calculated (Relative Light Units) and normalized to the medium-alone value to give the NF-κB Fold Activation values shown. The data points shown are the average of triplicate culture wells. (B) Stimulation of IL-8 production by UL37. The indicated amounts of UL37, MyD88, p65, and TRAF6 plasmid DNAs were transfected into HEK293 cells. At 24 h after transfection, culture medium from 3 replicate wells per sample was collected and combined. IL-8 levels were measured with the BD OptEIATM human IL-8 ELISA KIT II (BD Biosciences). (C) Effect of UL37 on IκB levels. HEK293 cells in 15-cm plates were transfected with 2 μg of MAL plasmid, empty vector plasmid, or UL37 plasmid DNAs. Cells were collected at the times indicated, and the amount of IκB in whole-cell lysates was determined by Western blot analysis using anti-IκB antibody (Santa Cruz).
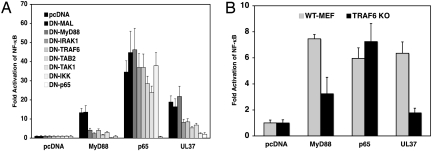
Activation of NF-κB through TLR2 involves the engagement of MyD88 and MAL, the recruitment of IL-associated kinase (IRAK) 4 and IRAK1, followed by the recruitment of TRAF6. TRAF6 then complexes with TAK1-binding protein 2 (TAB2), TGF-β activated kinase (TAK1), and TAB1, which activate IKK to phosphorylate IκB, causing its degradation and release of NF-κB for nuclear translocation (16). To determine if these signaling components were required for UL37 activation of NF-κB, a panel of dominant-negative (DN) mutant forms of signaling intermediates including MAL, MyD88, IRAK1, TRAF6, TAB2, TAK1, IKK, and p65 were cotransfected with UL37 plasmid to test their ability to inhibit the activity of UL37. We observed that overexpression of DN-p65 blocked the signaling induced by UL37, just as it blocked signaling by overexpression of MyD88 (Fig. 2A). Similarly, DN-IKK eliminated the NF-κB activation by UL37 but had no effect on p65 stimulation of NF-κB (Fig. 2A). Overexpression of DN-MAL or DN-MyD88 had no effect on UL37 signaling, but overexpression of DN-IRAK1, DN-TRAF6, DN-TAK1, or DN-TAB2 inhibited UL37 activation of NF-κB to the same extent that it inhibited MyD88 activation (Fig. 2). To confirm that TRAF6 was needed for the UL37 activation of NF-κB, we examined the effect of UL37 in TRAF6 knockout and WT mouse embryo fibroblasts (MEFs). UL37 activation of NF-κB was ≈5-fold lower in TRAF6 knockout MEFs than in WT MEFs (Fig. 2B). In total, these results indicate that UL37 activation of NF-κB uses a signaling pathway similar to that of the TLR2 pathway starting from TRAF6 and IRAK1.
Fig. 2.
Identification of cellular signaling molecules needed for NF-κB activation by UL37. (A) Use of dominant-negative mutant genes. Plasmid DNAs (20 ng) encoding dominant-negative mutant forms of MAL, MyD88, IRAK1, TRAF6, TAK1, TAB2, IKK, or p65 were cotransfected with 20 ng of UL37, MyD88, or p65 plasmid DNAs, together with 20 ng of NF-κB reporter plasmid DNA and 5 ng of control plasmid DNA into HEK293 cells. At 24 h after transfection, the NF-κB fold activation was determined as in Fig. 1A. (B) Use of a TRAF6 knockout MEF cell line. WT MEF or TRAF6 knockout MEF cells were transfected with 100 ng of MyD88, p65, UL37, or empty vector plasmid, together with 100 ng of NF-κB reporter plasmid and 20 ng of control plasmid. At 24 h after transfection the NF-κB fold activation was determined as in Fig. 1A.
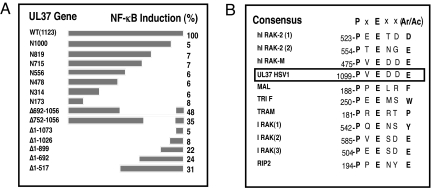
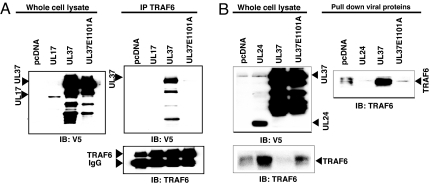
We constructed a series of mutant UL37 genes to map the essential portions of UL37 needed for NF-κB activation (Fig. 3A). We observed that the C-terminal 123-aa residues were essential for activation (Fig. 3). Analysis of the amino acid sequence within this region identified a potential TRAF6-binding motif of the general form PxExx(Ar/Ac) (17) within UL37: 1099PVEDDE1101A4 (Fig. 3B). The UL37 sequence is identical to the TRAF6-binding motif in IRAK-M (18), strengthening the possibility that this sequence is a TRAF6-binding domain. We therefore tested whether UL37 interacts with TRAF6 through immunoprecipitation and pulldown assays using extracts of cells transfected with a plasmid encoding a V5 epitope- and his-tagged UL37 protein. When we immunoprecipitated TRAF6 from the extracts, we observed coprecipitation of WT UL37 (Fig. 4A). However, the UL37 E1101A mutant protein, in which the essential glutamate residue of the putative TRAF6- binding site was replaced by alanine, coimmunoprecipitated with TRAF6 only at very low levels (Fig. 4A). A control HSV protein, UL17, did not coprecipitate with TRAF6 (Fig. 4A). In the reverse experiment, when tagged UL37 was recovered by affinity purification, TRAF6 was pulled down very efficiently (Fig. 4B). However, when the UL37 E1101A mutant protein was recovered by affinity purification, very little TRAF6 was pulled down (Fig. 4B). Similarly, very little TRAF6 was pulled down by a control HSV protein, UL24 (Fig. 4B). Therefore, UL37 interacted with TRAF6, and the putative TRAF6-binding motif was required for this interaction.
Fig. 3.
The UL37 C terminus is essential for NF-κB activation and contains a TRAF6-binding domain consensus sequence. (A) Mutant UL37 genes. A panel of C-terminal, N-terminal, and internal deletion mutations within the UL37 ORF was constructed as described in Materials and Methods. Plasmid DNAs encoding these mutant proteins were transfected into HEK293 cells along with the NF-κB reporter plasmid and the thymidine kinase control plasmid. NF-κB fold activation was determined as in Fig. 1A and expressed as the percentage of the WT NF-κB induction. (B) HSV-1 UL37 contains a potential TRAF6-binding site. The consensus TRAF6-binding site motif has been described previously (17). TRAF6-binding sites are compiled from Refs. 17 and 24.
Fig. 4.
Association of UL37 with TRAF6 is dependent on a motif similar to other TRAF6-binding proteins. HEK293 cells were transfected with 1 μg of indicated plasmid DNAs (encoding UL17, UL24, UL37, or UL37 E1101A proteins tagged with a V5 epitope and his6 tag) per well, and cell lysates were prepared for the following studies. Full-length UL37 is denoted by a filled triangle. Faster-moving species probably are UL37 breakdown products. (A) Immunoprecipitation with anti-TRAF6 antibody. Expression of the HSV proteins was confirmed by Western blot analysis of whole-cell lysates with anti-V5 antibody (Left). Immunoprecipitation of TRAF6 was performed with anti-TRAF6 antibody, which was confirmed by Western blot (Bottom Right). Although differing amounts of TRAF6 were observed in the different lysates in this experiment, this variation was not observed consistently. Coimmunoprecipitation of the HSV proteins was tested by Western blots of the immunoprecipitates with anti-V5 antibody (Top Right). (B) Pull-down of HSV proteins with metal affinity resin. (Top Left) Expression of HSV proteins was confirmed by Western blot analysis with anti-V5 antibody. (Bottom Left) Levels of TRAF6 in each of the whole-cell extracts was determined by Western blot analysis with anti-TRAF6 antibody. (Right) TYLON Metal Affinity Resin (Clontech) was used to recover HSV UL24, UL37, or UL37 E1101A proteins from whole-cell lysates. TRAF6 was detected in the pull-downs by Western blot analysis with anti-TRAF6 antibody. Percentage of TRAF6 pulled down: pcDNA, 1.5%; UL24, 0.12%; UL37, 74%; UL37E1101A, 1%.
UL37 associated with a complex that involved, in addition to TRAF6, TAB1, TAB2, and TAK1 because immunoprecipitation of these proteins also coprecipitated UL37 (Fig. S2). Small amounts of UL37 were coimmunoprecipitated with IRAK1, indicating that it may be a minor component of the complex, but UL37 was not associated with MyD88 or MAL. The interaction of IRAK1, TAB1, TAB2, and TAK1 with UL37 was dependent on the TRAF6-binding motif in UL37, arguing that the associations of the other proteins with UL37 were dependent on the binding of UL37 to TRAF6.
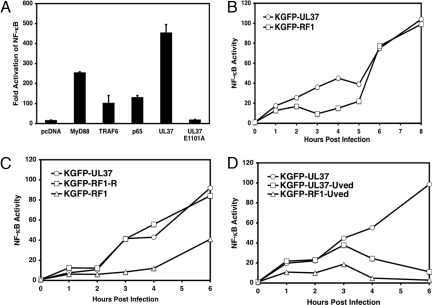
To test the role of the TRAF6-binding site in NF-κB activation, we transfected the WT or mutant UL37 plasmids into HEK cells with a reporter gene. WT UL37 activated the NF-κB reporter plasmid; the E1101A mutant protein did not (Fig. 5A). Therefore, UL37 must interact with TRAF6 to activate NF-κB signaling. To test the role of UL37 in activation of NF-κB in HSV-infected cells, we constructed a mutant HSV-1 strain that encodes the E1101A mutant form of UL37. Cells infected with a virus expressing WT UL37-GFP showed a biphasic induction of NF-κB DNA-binding activity (Fig. 5B), as observed previously (13). NF-κB activity was induced to moderate levels from 1 to 4 h and then was highly activated from 6 h onward (Fig. 5B). In contrast, the E1101A mutant virus (RF-1) showed a lower level of NF-κB activation from 1 to 5 h after induction but showed normal activation of NF-κB from 6 h onward (Fig. 5B). Despite the lack of NF-κB induction by the mutant virus, normal amounts of UL37 were incorporated into virus particles (X.L. and D.M.K., results not shown).
Fig. 5.
Mutational alteration of the UL37 TRAF6-binding motif abolishes NF-κB activation. (A) Transfection assay for NF-κB reporter gene activation. HEK293 cells were cotransfected with 20 ng of UL37, UL37 E1101A mutant, MyD88, or p65 plasmid DNAs with 20 ng of NF-κB reporter plasmid and 5 ng of control plasmid DNAs. NF-κB fold activation was measured as described in Fig. 1A. (B) Infection assay for NF-κB DNA-binding activity. HeLa cells were infected with KGFP-UL37 or KGFP-RF1 (UL37E1101A) mutant virus at multiplicity of infection (MOI) = 16, and the infected cells were collected at the indicated time after infection. A DNA-binding assay was used to measure NF-κB DNA-binding activity. Nuclear extracts were prepared and incubated with streptavidin-agarose beads coupled with IFNβ positive regulatory domain II (PRDII) oligonucleotides, and the bound p65 was collected and detected by Western blot using anti-NF-κB p65 antibody. The p65 bands were quantified by scanning as a measure of NF-κB DNA-binding activity. (C) Induction of NF-κB DNA-binding activity by a repaired virus. HeLa cells were infected with KGFP-UL37 virus, the mutant virus KGFP-RF1, or the repaired virus KGFP-RF1-R as described in B. At the times indicated, nuclear extracts were prepared, and DNA-binding activity was measured as described in B. (D) Induction of NF-κB DNA-binding activity by UV-inactivated viruses. HeLa cells were infected with KGFP-UL37, UV-inactivated KGFP-UL37, or UV-inactivated KGFP-RF1 virus, and NF-κB DNA-binding activity was measured at the times indicated.
We performed two additional lines of experimentation to confirm that virion-associated UL37 can activate NF-κB in infected cells. First, to ensure that the phenotypic defect in the KGFP-RF1 mutant virus was caused by the UL37 gene mutation, we repaired the mutation by replacing the E1101A mutant gene with the WT UL37 gene. The rescued virus, KGFP-RF1-R, induced WT levels of NF-κB from 1 to 6 h after induction, confirming that UL37 was responsible for the phenotype. Second, to ensure that virion UL37 was responsible for the effect, we infected cells with UV-inactivated viruses because UV-inactivated HSV induces only early NF-κB activation (13). UV-inactivated KGFP-UL37 induced NF-κB DNA-binding activity at reduced levels from 1 to 3 h after induction only (Fig. 5D). UV-inactivated KGFP-RF1 mutant virus activated NF-κB DNA-binding activity at 1–6 h after induction to approximately one-half that of the WT virus (Fig. 5D). In total, at least one-half of the early virion-induced NF-κB DNA-binding activity was induced by UL37.
Discussion
HSV activation of NF-κB occurs in two waves, the first being independent of viral replication and the second requiring viral gene expression (13). We found that the UL37 protein located in the tegument layer between the virion lipid envelope and capsid protein shell can activate NF-κB. Virion envelope proteins can activate cytokine signaling by interaction with cell surface receptors (e.g., Ref. 14), but our observations provide evidence that an internal virion protein delivered into the cytoplasm can activate NF-κB. Influenza virus capsid and matrix proteins (19) and HCV core protein (20) can activate NF-κB when overexpressed, but there is no evidence that incoming virion proteins can activate signaling. Activation of NF-κB in HSV-infected cells also may be stimulated by the gD in the virion envelope interacting with the Herpesvirus entry mediator surface receptor (14), as observed in Fig. 5B from 1 to 2 h after induction. UL37 released into the cytoplasm then could induce the NF-κB activation that we observed from 2 to 5 h after induction (Fig. 5B). The combination of gD and UL37 could explain the early transient activation of NF-κB by UV-inactivated virions (13). The later activation that we observed after 5 h after induction could involve infected cell protein 27 or proteins whose expression requires infected cell protein 27 (21). The HSV UL37 protein has a virion structural and/or assembly role because it is essential for virion maturation (15), and its ability to induce NF-κB represents a possible second function.
The interaction of HSV with NF-κB is quite complex in that the virus and its gene products both initiate and inhibit NF-κB. It is likely that the early activation by virion proteins is necessary for full transcription of viral genes, whereas at later times viral gene products act to down-regulate host innate responses that would serve to reduce viral replication or induce adaptive immune responses. Conservation of the UL37 function in homologous genes encoded by other herpesviruses would support its importance in viral replication. Thus, it is noteworthy that the UL37 homologs encoded by several other herpesviruses also have putative TRAF6-binding domains. These homologs include HSV-2 UL37:1098PVEDDE1103, Cercopithecine herpesvirus 1 (monkey B virus) UL37: 1162PEEDDD1167, human cytomegalovirus UL47: 901PWESAP907, and human herpes virus 8 or Kaposi's sarcoma-associated herpes virus ORF63: 700PPEQPP705. Human cytomegalovirus is known to activate NF-κB very early in infection, probably because of effects of the virion particle (22). Thus, the UL47 protein may be in part responsible for this effect. The conservation of this potential binding site in several herpes viruses argues that this function is important in herpesviral replication or biology.
Details of the mechanism by which UL37 activates NF-κB remain to be elucidated fully, but from our results we can propose the basic outline of this process. UL37 interacts with TRAF6, and this interaction is required for NF-κB activation. Furthermore, UL37-induced signaling requires TAK-1 and TAB-2, and UL37 is in a complex with TRAF6, TAB1, TAB2, and TAK1. This finding argues that UL37 binds to and activates TRAF6 signaling by promoting formation of the complex of proteins, leading to IKK activation, IκB degradation, and NF-κB activation. The role of this function in viral replication and latent infection also remains to be defined. The mutant virus defective for TRAF6 interaction shows an approximately 2-fold reduction in viral replication in Vero monkey cells (X.L. and D.M.K., unpublished results). Further studies of this mutant virus in cell culture and animal model systems are needed to determine the role of this UL37 function in viral infection.
Materials and Methods
Cell Culture and Reagents.
HEK293, WT MEF, and TRAF6 knockout MEF cells (provided by Tak Mak, University of Toronto) were grown in Eagle's DMEM containing 10% FBS (vol/vol) supplemented with 200 units/ml penicillin and 200 μg/ml streptomycin at 37°C in a humidified 5% CO2 incubator. The antibodies used were polyclonal rabbit and goat anti-human MyD88, MAL, IRAK1, TRAF6, TAB1, TAB2, TAK1 (Santa Cruz), and mouse monoclonal anti-V5 antibody (Invitrogen).
Plasmids and Viruses.
The HSV expression plasmids used in this report were constructed by PCR amplification of individual ORFs from HSV-1 strain KOS genomic DNA and insertion into the pcDNA3.1 (Invitrogen) vector. These constructs express the HSV proteins with V5 epitope and His6 tags. All plasmid inserts were sequenced at the Harvard-Dana-Farber Cancer Center sequencing facility, and HSV protein expression was verified by transfection and detection by Western blot analysis using anti-V5 antibody. The Stratagene QuikChange II Site-Directed Mutagenesis Kit (Stratagene) was used to construct the UL37 E1101A mutant gene. Expression vectors for DN-TAB (TAB2-C), DN-TBK1, DN-mTRAF6, and DN-IκB were provided by H. Sanjo at Osaka University, Japan, by Makoto Nakanishi at Nagoya University, Japan, by Zhijian Chen at the University of Texas Southwestern Medical Center (Dallas), and by Steven Bachenheimer at the University of North Carolina, Chapel Hill (8). The HSV-1 recombinant viruses KGFP-UL37 and KGFP-UL37E1101A were constructed by replacing the UL37 ORF with an ORF encoding a GFP-UL37 fusion protein or with a GFP-UL37 E1101A mutant fusion protein, respectively, by homologous recombination.
Reporter Assay.
HEK293 cells were seeded into 96-well plates at 5 × 104 cells the day before transfection. The cells were transfected with 20 ng of the NF-κB firefly luciferase reporter plasmid and 5 ng of thymidine kinase promoter–controlled Renilla luciferase reporter plasmid (Promega) together with the indicated amounts of expression plasmids using Genejuice (Novagen) transfection reagent. At 24 h after transfection, the firefly and Renilla luciferase activities were measured using the Promega dual-glo luciferase assay system. For each experimental sample the ratio of firefly to Renilla luciferase activity was calculated (in Relative Light Units) and normalized to the medium-alone value to give the NF-κB Fold Activation values shown.
IL-8 Secretion.
HEK293 cells were plated at 5 × 104 cells per well in 96-well plates on the day before transfection. The cells were transfected with the indicated amount of plasmid DNAs, and at 24 h after transfection cell cultural medium was collected and cleared of cell debris by centrifugation. Levels of IL-8 secreted from HEK293 cells were determined by an ELISA using an OptEIA human IL-8 ELISA set (BD Biosciences) according to the manufacturer's instructions.
Immunoprecipitation and Western Blot Analysis.
HEK293 cells in 6-well plates were transfected with 6 μg of the indicated plasmids using Lipofectamine 2000 (Invitrogen) in DMEM plus 1% FBS, according to the manufacturer's instructions. Cells were maintained in DMEM plus 1% FBS throughout the transfection. At 24 h after transfection, cells were collected and washed with cold PBS and subjected to immunoprecipitation using the indicated antibodies and an Immunoprecipitation Kit (Roche), or pull-down assays using TYLON Metal Affinity Resin (Clontech) per manufacturer's instruction. Proteins in the immune complexes were resolved by SDS/PAGE and analyzed by Western blotting.
NF-κB DNA-Binding Assay.
A modified version of oligonucleotide DNA precipitation (23) was used to measure NF-κB DNA-binding activity. HEK293 cells were transfected with plasmids or infected with virus as indicated, and at the times indicated cells were harvested and the cytoplasmic and nuclear extracts were prepared with NE-PER Nuclear and Cytoplasmic Extraction reagents (PIERCE Biotechnology) per the manufacturer's instructions. Cleared nuclear extracts (20 μl) were incubated with 25 μl of streptavidin-agarose beads (Invitrogen) coupled with 5′ 6-bp extended IFNβ PRDII oligonucleotides (5′-GGATCCGGAATTTCCCGGAATTTCCC-3′ and 5′-GGATCCGGGAAATTCCGGGAAATTCC-3′) (IDT) in 500 μl of binding buffer (23). The bound p65 was collected and detected by Western blot using anti-NF-κB p65 (C-20) antibody (Santa Cruz).
Supplementary Material
Acknowledgments.
We thank Elliott Kieff and members of our laboratories for helpful comments on the manuscript. This work was supported by National Institutes of Health Grant AI64349.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801617105/DCSupplemental.
References
- 1.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 2.Williams SA, Greene WC. Regulation of HIV-1 latency by T-cell activation. Cytokine. 2007;39:63–67. doi: 10.1016/j.cyto.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosialos G, et al. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 5.Chu ZL, DiDonato JA, Hawiger J, Ballard DW. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 6.Geleziunas R, et al. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, et al. Herpes simplex virus type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 8.Gregory D, Hargett D, Holmes D, Money E, Bachenheimer SL. Efficient replication by herpes simplex virus type 1 involves activation of the I{kappa}B kinase-I{kappa}B-p65 pathway. J Virol. 2004;78:13582–13590. doi: 10.1128/JVI.78.24.13582-13590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yedowitz JC, Blaho JA. Herpes simplex virus 2 modulates apoptosis and stimulates NF-kappaB nuclear translocation during infection in human epithelial HEp-2 cells. Virology. 2005;342:297–310. doi: 10.1016/j.virol.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Philadelphia: Lippincott, Williams and Wilkins; 2007. pp. 2501–2602. [Google Scholar]
- 11.Kurt-Jones EA, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurt-Jones EA, et al. The role of toll-like receptors in herpes simplex infection in neonates. J Infect Dis. 2005;191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- 13.Amici C, et al. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J Biol Chem. 2006;281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- 14.Medici MA, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: Role of nuclear factor kappaB. J Biol Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- 15.Desai P, Sexton GL, McCaffery JM, Person S. A null mutation in the gene encoding the herpes simplex virus type 1 UL37 polypeptide abrogates virus maturation. J Virol. 2001;75:10259–10271. doi: 10.1128/JVI.75.21.10259-10271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 17.Ye H, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;18:443–447. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 18.Wesche H, et al. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–19410. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 19.Flory E, et al. Influenza virus-induced NF-kappaB-dependent gene expression is mediated by overexpression of viral proteins and involves oxidative radicals and activation of IkappaB kinase. J Biol Chem. 2000;275:8307–8314. doi: 10.1074/jbc.275.12.8307. [DOI] [PubMed] [Google Scholar]
- 20.You LR, Chen CM, Lee YH. Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha. J Virol. 1999;73:1672–1681. doi: 10.1128/jvi.73.2.1672-1681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hargett D, Rice S, Bachenheimer SL. Herpes simplex virus type 1 ICP27-dependent activation of NF{kappa}B. J Virol. 2006;80:10565–10578. doi: 10.1128/JVI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalik TF, et al. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchjorsen J, et al. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 2005;79:12944–12951. doi: 10.1128/JVI.79.20.12944-12951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansell A, Brint E, Gould JA, O'Neill LA, Hertzog PJ. Mal interacts with tumor necrosis factor receptor-associated factor (TRAF)-6 to mediate NF-kappaB activation by toll-like receptor (TLR)-2 and TLR4. J Biol Chem. 2004;279:37227–37230. doi: 10.1074/jbc.C400289200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.