Rare, inherited mutations causing familial forms of Parkinson's disease (PD) have provided much insight into some of the molecular mechanisms that underlie both the genetic and sporadic forms of the disease. The role of mitochondria in sporadic PD has been debated for a little over 20 years, since the identification of complex I deficiency in the substantia nigra pars compacta (SNpc) (1). However, it was the identification of loss-of-function recessive mutations in the PINK1 gene, encoding a mitochondrial, putative protein kinase, that reignited interest in the pathophysiology of mitochondria and their potential role in parkinsonism (PARK6) (2). In Drosophila models of PINK1, it has been shown that PINK1 and parkin (the PARK2 locus) (3) act, at least in part, in a common pathway. These studies strongly suggested a role for PINK1 in normal mitochondrial function and implied that parkin was downstream of PINK1. Nevertheless, the role of PINK1 in mammalian mitochondrial function remained unclear. The role of DJ-1 a third recessive parkinsonism locus (PARK7) (4) is also likely to relate to mitochondrial function (5), although whether it too maps to the same pathway as PINK and parkin is not yet clear. In any event, these genetic data clearly show an important role for the mitochondrion in the recessive parkinsonisms, although the extent to which these findings will be relevant to sporadic PD remains unclear (1, 6).
The report by Gautier et al. (7) in this issue of PNAS focuses on mitochondrial functional defects in mice lacking expression of PINK1 (PINK1−/− mice). They show that mitochondrial respiration is impaired in the striatum of the PINK1−/− mice at 3–4 months. The next step will be to confirm these observations in the SNpc of the PINK1−/− mice. Furthermore, respiratory-chain dysfunction has been found in tissues outside of the brain of PD patients. In particular, data on skeletal muscles are still difficult to interpret (8). It will therefore be interesting to extend the present study to other PINK1−/− tissues.
A selective decrement in mitochondrial complex I in terms of the involvement of other components of the respiratory chain is generally agreed by the scientific community to be associated with sporadic PD. In the present study (7), defects in complex I and also in complexes II–IV were observed in the striatum of the PINK1−/− mice. These latter defects are relatively novel in a PD model. Most previous studies have demonstrated only a complex I deficiency, although some have also reported defects in complexes II–IV in PD patients (8). Future investigations will clarify whether the association of complex I with PD was effectively selective or whether other complex deficiencies could also play an important role in the pathogenesis of the disease. Even though the respiratory-chain dysfunction has been widely described in PD models, it will be important to elucidate the mechanism of impaired respiration in models without the use of complex I toxins. In addition, PINK1−/− mice (7) display reduced aconitase activity. It has been shown that aconitase activity was reduced in rat cerebellar granule neurons upon MPP+ treatment (9) and in the brain of DJ-1−/− mice (10). Therefore, it might be interesting to investigate whether mitochondrial aconitase may impart susceptibility to PD.
In contrast to mitochondria derived from indirect flight muscles in dPINK1−/− flies (11–13), no major structural defect was observed in mitochondria of PINK1−/− mice. Nevertheless, the number of larger mitochondria was increased in the striatum of the mice. This is an interesting observation because PINK1 has been suggested to promote mitochondrial fission and to regulate mitochondrial morphology in Drosophila and mammalian models (11–16). Mitochondrial fission in mammalian cells is mediated by DRP1, along with other proteins such as the mitochondrial protein FIS1. Notably, pharmacological inhibition of respiratory-chain complex I alters the organization of the mitochondrial network. It is widely accepted that an imbalance between fusion and fission activities is causative for many pathophysiological conditions. Furthermore, mutations in genes regulating mitochondrial morphology are causally linked to neurodegenerative diseases (17). PINK1's role in mitochondrial morphology needs to be further studied. Are DRP1 and FIS1 effectors of PINK1? Answering this question should help in the understanding of the dependence of neurons on a precise regulation of mitochondrial dynamics.
Although decreased in the striatum, mitochondrial respiration activities are normal in the cerebral cortex of PINK1−/− mice at 3–4 months. However, it is decreased in the cerebral cortex of animals at 2 years, suggesting that aging can exacerbate mitochondrial dysfunction in these mice. Mitochondria are a major source of reactive oxygen species (ROS). If mitochondrial ROS have important physiological roles (in signaling for example), they can cause oxidative stress in pathological conditions. Effectively, production of ROS is an important mechanism by which mitochondria contribute to the aging process. To prevent damage, mitochondria are equipped with an extensive array of antioxidative defenses to maintain a balance between ROS production and removal (18). It has been shown very recently that ROS production was increased in mitochondria of PINK1−/− neurons (19). It is therefore possible that PINK1, through the regulation of mitochondrial respiratory complexes and/or aconitase activity may play a role in the maintenance of the ROS balance.
PINK1 can function through various mechanisms. In particular, chaperones may be key players in PD pathogenesis. PINK1 has been shown to interact with TRAP1, HSP90, and HtrA2 (18), all of which have a chaperone activity. It is therefore tempting to speculate that PINK1 might participate in the detoxification of proteins through its interactions with chaperone molecules.
PINK1 has been suggested to promote mitochondrial fission and to regulate mitochondrial morphology.
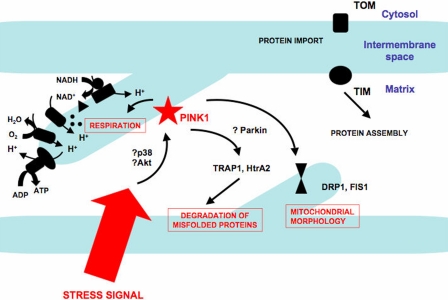
In the present study, Gautier et al. (7) show that cellular stress, such as H2O2 or mild heat shock could induce mitochondrial respiration defects in the cerebral cortex of the PINK1−/− mice. PINK1 has been shown to phosphorylate the mitochondrial chaperone TRAP1, protecting against oxidative stress-induced apoptosis (20) and to be important for the phosphorylation of HtrA2 upon activation of the p38 pathway, preventing mitochondrial stress (21). Assessing whether the p38 stress pathway can activate putative PINK1 function(s) in mitochondrial respiration and morphology will be a major step forward in our understanding of PD pathogenesis. In addition, the PI3K/Akt pathway has been shown to be implicated in the regulation of all three PINK1, Parkin, and HtrA2 proteins (22, 23). Lots of questions remain to be answered. Which signaling pathway(s) are responsible for PINK1 activation? Is parkin necessary for PINK1 to exert all its functions within the mitochondrion? Are there any PINK1 effectors other than HtrA2, TRAP1, and HSP90 (Fig. 1)? Elucidating these questions will provide further insight into the pathogenesis of at least the recessive forms of parkinsonism. The present report by Gautier et al. (7) is an important step along this path.
Fig. 1.
The PD-associated kinase PINK1 is important for mitochondrial function. In response to cellular stress, PINK1 might regulate mitochondrial respiration and morphology. Whether the protein Parkin, which has also been associated with PD and acts downstream of PINK1, is important in these functional mechanisms remains to be confirmed. Although it is not yet proven, PINK1 could also participate in the degradation of misfolded proteins within the mitochondria through its interactions with chaperone molecules such as HtrA2, TRAP1, and HSP90.
Footnotes
The authors declare no conflict of interest.
See companion article on page 11364.
References
- 1.Abou-Sleiman PM, et al. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 2.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1120–1122. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 3.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 4.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 5.Canet-Avilés RM, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy J, et al. Genetics of Parkinson's disease and parkinsonism. Ann Neurol. 2006;60:389–398. doi: 10.1002/ana.21022. [DOI] [PubMed] [Google Scholar]
- 7.Gautier CA, et al. Loss of PINK1 Causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schapira AH. Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim Biophys Acta. 1999;1410:159–170. doi: 10.1016/s0005-2728(98)00164-9. [DOI] [PubMed] [Google Scholar]
- 9.Shang T, et al. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J Biol Chem. 2004;279:19099–19112. doi: 10.1074/jbc.M400101200. [DOI] [PubMed] [Google Scholar]
- 10.Andres-Mateos E, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with Parkin. Nature. 2006;441:1162–1165. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 12.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by Parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exner N, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by Parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knott A, et al. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biskup S, Moore DJ. Detrimental deletions: mitochondria, aging and Parkinson's disease. BioEssays. 2006;28(10):963–967. doi: 10.1002/bies.20471. [DOI] [PubMed] [Google Scholar]
- 19.Wood-Kaczmar A, et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pridgeon JW, et al. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2008;5:1494–1503. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plun-Favreau H, et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- 22.Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, et al. Akt attenuation of the serine protease activity of HtrA2/Omi through phosphorylation of serine 212. J Biol Chem. 2007;282:10981–10987. doi: 10.1074/jbc.M700445200. [DOI] [PubMed] [Google Scholar]