Abstract
NKT cell subsets can be divided based on CD4 and NK1.1 expression and tissue of origin, but the developmental and functional relationships between the different subsets still are poorly understood. A comprehensive study of 19 cytokines across different NKT cell subsets revealed that no two NKT subpopulations exhibited the same cytokine profile, and, remarkably, the amounts of each cytokine produced varied by up to 100-fold or more among subsets. This study also revealed the existence of a population of CD4−NK1.1− NKT cells that produce high levels of the proinflammatory cytokine IL-17 within 2–3 h of activation. On intrathymic transfer these cells develop into mature CD4−NK1.1+ but not into CD4+NK1.1+ NKT cells, indicating that CD4−NK1.1− NKT cells include an IL-17–producing subpopulation, and also mark the elusive branch point for CD4+ and CD4− NKT cell sublineages.
Keywords: cytokines, CD1d, thymus, T cell
NKT cells are CD1d-dependent T cells that mediate potent immunoregulatory functions in settings of autoimmunity, cancer, infection, and tolerance (1). Mouse NKT cells express the T cell antigen receptor (TCR) Vα14Jα18 chain coupled with Vβ8.2, Vβ7, or Vβ2, whereas human NKT cells have Vα24Jα18 coupled to Vβ11 (2). The biological function of NKT cells is paradoxical, because they rapidly produce large amounts of both T helper type 1 (Th1) and Th2 cytokines and promote cell-mediated immunity in some settings but suppress cell-mediated immunity in others (1).
One explanation for how NKT cells mediate these diverse functions is the existence of functionally distinct subsets. NKT cells can be divided broadly into CD4+ and CD4− subsets (2). In humans, this classification provides an important functional distinction, because CD4+ NKT cells make both Th1 and Th2 cytokines (such as IFN-γ, TNF, IL-4, IL-10, IL-13), whereas CD4− NKT cells primarily make Th1 cytokines (IFN-γ and TNF) (1, 3, 4). Mouse NKT cells also include CD4+ and CD4− subsets, but no clear distinction in cytokine production has been identified. This is surprising, because mouse CD4+ and CD4− NKT cells are distinct in their ability to regulate immune responses in vivo (5). However, careful subset analyses of NKT cell cytokine production in mice have been limited largely to IFN-γ and IL-4 production (5); these analyses are inadequate, because NKT cells are known to produce many other cytokines, including IL-5, GM-CSF, TNF, IL-10, IL-13, IL-21, and IL-17 (1, 6–8).
IL-17 production by NKT cells is particularly interesting. A relatively new lineage of differentiated effector CD4 T cells (Th17 cells) is defined by IL-17 production, and these cells play a critical role in the onset and progression of some forms of cell-mediated autoimmunity (9, 10). Differentiation of Th17 cells can occur in the presence of certain cytokine combinations, including TGF-β and IL-6 or TGF-β and IL-21 (9, 10). Th17 cells also up-regulate the IL-23 receptor, and IL-23 is important in Th17 expansion and maintenance (10). The master transcriptional regulator of Th17 development is RORγt; Th17 cells fail to develop in its absence, whereas its overexpression enhances Th17 development (11). It is unclear whether IL-17 production by NKT cells requires or is enhanced by the same differentiation signals, nor is it clear which subset of NKT cells produces this cytokine. Although Michel et al. (8) showed that NK1.1− NKT cells were the primary producers of IL-17, these cells were poor producers of IFN-γ and IL-4. This finding contrasts with our own data showing that NK1.1− NKT cells in the periphery are capable of potent IFN-γ and IL-4 production (12).
Herein, we investigate the cytokine-producing capability of NKT cell subsets. We demonstrate remarkable heterogeneity among NKT cell subsets and show that a previously unstudied subset of CD4−NK1.1− NKT cells is the main source of NKT-derived IL-17. Furthermore, transfer of thymic CD4−NK1.1− NKT cells to fetal thymus organ culture (FTOC) generates CD4− but not CD4+NK1.1+ NKT cells, demonstrating that these cells represent the elusive branch point of the CD4+ and CD4− NKT cell subsets.
Results
Extreme Diversity in Cytokine Production by Distinct NKT-Cell Populations.
NKT cell subpopulations defined by CD4 and NK1.1 expression were isolated from thymus, spleen, and liver and were tested for their ability to produce cytokines over a range of time points after stimulation with plate-bound anti-CD3/CD28 in vitro. These included IFN-γ, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-17, IL-21, GM-CSF and TNF (Fig. 1); macrophage inflammatory protein (MIP-1α), Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES) [supporting information (SI) Fig. S1]; IL-12, Monocyte Chemotactic Protein-1 (MCP-1), Monokine Induced by IFN-gamma (MIG), and Keratinocyte Chemokine (KC) were assayed also but were not detected (data not shown). Cytokine amounts are depicted on a logarithmic scale. Generally, thymus-derived NKT cells were by far the most potent cytokine producers (Fig. 1). In a notable exception, at 72 h the production of IFN-γ by liver-derived NKT cells was comparable to or higher than the production of IFN-γ by thymic NKT cells. This was confirmed by intracellular cytokine staining (ICS), which showed a higher percentage of IFN-γ+ cells from the liver fraction than from the thymus fraction at this time point (Fig. 2).
Fig. 1.
Distinct cytokine production by NKT cell subsets. NKT cells from spleens, livers, and thymuses of B6 mice were identified as α-GC/CD1d tetramer+ αβTCR+ cells and divided further on the basis of NK1.1 and CD4 expression as shown in the upper right dot plot (shown is a representative thymus sample). Purified NKT subsets were cultured in wells coated overnight with 10 μg/ml anti-CD3 and 10 μg/ml anti-CD28. An aliquot of supernatant was harvested at 24 and 72 h to assay for secreted cytokines. IL-17 and IL-21 were assayed by ELISA. All other cytokines were assayed by cytometric bead array. The absence of bars indicates undetectable amounts of cytokine, unless “not done” is stated. For thymus and liver NKT subsets, the results are derived from 5 or 6 separate cultures collected over three independent experiments. For spleen NKT cell subsets, results are derived from 3 or 4 separate cultures collected over two independent experiments. Bars depict mean ± standard error. All cytokine values are expressed in ng/ml. Total cells = absolute cell number.
Fig. 2.
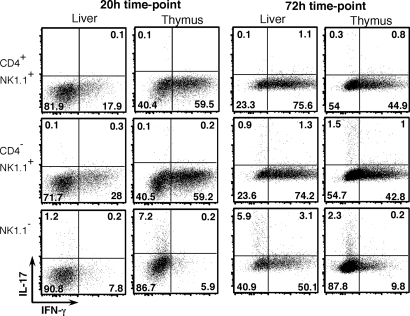
Costaining of NKT cell subsets for IFN-γ and IL-17. NKT cell subsets were isolated and stimulated on anti-CD3– and anti-CD28–coated plates as described in Fig. 1. GolgiStopTM was added to cultures 4 h before the indicated time point, and cells were stained for intracellular IFN-γ-APC and IL-17-PE. Numbers in each quadrant represent the percentage of cells in that quadrant, and results are representative of two independent experiments.
In separate experiments in which NKT cell subsets were stimulated by using α-GalCer–pulsed DC (Fig. S2), cytokine production by thymus NKT cell subsets was much lower, but the responses by peripheral NKT cells to the two types of stimulation were approximately comparable. A possible explanation is that a much higher proportion of thymic NKT cells express inhibitory receptors (13–15) that dampen NKT cell cytokine production when engaged by their ligands on the DC (16). Conversely, spleen CD4+NK1.1+ NKT cells showed higher cytokine production in response to α-GalCer–pulsed DC stimulation than to CD3/CD28 ligation, suggesting that spleen NKT cells have a greater requirement for DC-derived factors such as IL-12 and IL-18. Because of the differential regulatory mechanisms at play in these cultures, we favor data from CD3/CD28-stimulated NKT cells, because these cultures reveal the potential of each NKT subset to produce cytokines.
The most dramatic differences in anti-CD3/CD28 cultures were observed when comparing NK1.1− and NK1.1+ NKT cells (Fig. 1), particularly those from the thymus. In addition to higher IL-4 and lower IFN-γ levels that have been documented previously (17, 18), thymus NK1.1− NKT cells also produced substantially more IL-17, IL-10, and IL-21 but less IL-2, IL-3, IL-6, IL-9, GM-CSF, TNF, MIP-1α, and RANTES. Peripheral NK1.1− NKT cells generally were less active than their thymic counterparts, particularly at the 20-h time point, which is consistent with these cells being distinct populations of cells (12). By 72 h, however, cultures of peripheral NK1.1− NKT cells had accumulated levels of some cytokines (including IL-2, IL-4, IL-10, IL-13, IL-17, IL-21, IFN-γ, TNF, and MIP-1α) similar to or exceeding those of their thymic counterparts, suggesting slower induction and/or more sustained production. This was supported by ICS of NK1.1− NKT cells at the 72-h time point, showing that more liver NK1.1− NKT cells than thymic NK1.1− NKT cells were producing IFN-γ and IL-17 (Fig. 2). In contrast to human NKT cell subsets in which CD4+ NKT cells produce higher levels of Th2 cytokines (3, 4), we found that mouse CD4−NK1.1+ NKT cells and CD4+NK1.1+ NKT cells generally were comparable in their ability to produce IL-4 in response to anti-CD3/CD28; if anything, CD4−NK1.1+ NKT cells in thymus and liver were more potent producers of IL-10 and IL-13 (Fig. 1). The most striking difference between the CD4+ and CD4− subsets was IL-17 production, which was restricted almost entirely to the CD4− NKT cell fraction in each tissue tested.
NK1.1− NKT Cells Constitutively Express IL-23R and RORγt.
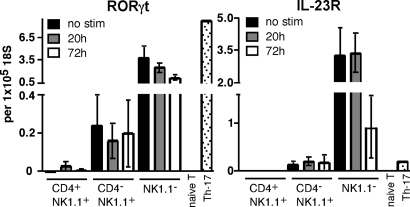
Given that Th17 development depends on expression of RORγt (11) and IL-23R (10), we investigated the expression of these factors by NKT cell subsets to determine whether they correlated with IL-17–producing capacity (Fig. 3). Quantitative RT-PCR analysis of thymic NKT cell subsets showed that both factors were constitutively expressed by NK1.1−NKT cells and, to a lesser extent, by CD4−NK1.1+ NKT cells, whereas CD4+NK1.1+ NKT cells and naïve conventional T cells showed little or no expression of these factors. The level of expression of these factors either did not change, or decreased, after activation in vitro.
Fig. 3.
Constitutive expression of RORγt and IL-23R by NK1.1− NKT cells. NKT cells were isolated from thymuses of B6 mice, separated into CD4+NK1.1+, CD4−NK1.1+, and NK1.1− fractions, and stimulated for 20 or 72 h on anti-CD3/CD28–coated plates as in Fig. 1. Naïve T cells (CD4+/CD62L+/CD44lo/CD25−) also were purified from spleen, and Th-17 cells were generated from naïve spleen after 3-d culture on anti-CD3–coated plates in the presence of TGF-β, IL-6, anti-IFN-γ, anti-IL-4, and 1 μg/ml soluble anti-CD28. RNA was isolated from all cell preparations, and expression of RORγt and IL-23R was determined relative to 18S ribosomal RNA. No stimulation and 20-h bars represent a total of 2 or 3 separate samples collected over two independent experiments, and 72-h bars represent 4–6 separate samples collected over three independent experiments. Bars depict mean ± standard error.
CD4−NK1.1− NKT Cells Are the Major Source of IL-17.
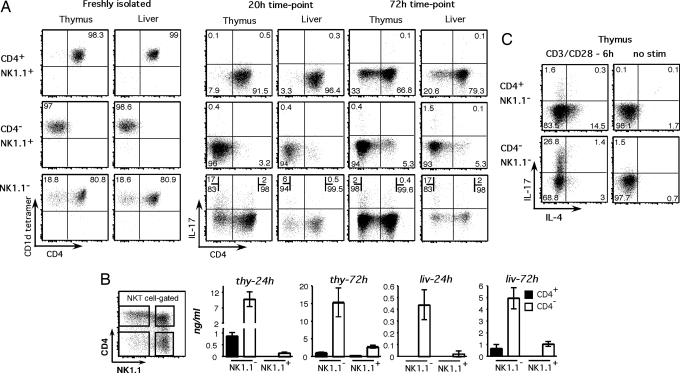
Most, but not all, NK1.1− NKT cells in the thymus and liver express CD4. Given that production of IL-17 in the NK1.1+ fraction of NKT cells was restricted largely to CD4− cells, we investigated whether CD4− cells were responsible for IL-17 production in the NK1.1− fraction. We first used ICS on sorted NKT cell subsets and confirmed our hypothesis that the CD4− fraction of NK1.1− NKT cells was much more capable of IL-17 production compared with the CD4+ fraction (Fig. 4A). We next sorted thymus- and liver-derived NK1.1− NKT cells into CD4+ and CD4− subsets to test them independently for IL-17 secretion (Fig. 4B). The CD4−NK1.1− NKT cells produced at least 10 times more IL-17 than the CD4+NK1.1− cells, and after 24 h thymus-derived cells were the most potent source of this cytokine. Similar results were observed in the liver: the CD4−NK1.1− NKT subset was the main source of IL-17, although optimal IL-17 production by liver-derived NKT cells seemed to be delayed compared with the thymus. The presence of IL-17 in the supernatants of the NK1.1−CD4− NKT cell cultures at 24 h suggested IL-17 was produced rapidly after stimulation. Indeed, ICS of NKT cells just 6 h after anti-CD3/28 stimulation showed very clear IL-17 production by 28% of CD4−NK1.1− NKT cells but by <2% of the CD4+NK1.1− fraction (Fig. 4C).
Fig. 4.
IL-17 is produced primarily by a rare population of NK1.1−CD4− NKT cells. (A) Purified NKT cell populations from thymus and liver were stimulated as described in Fig. 1, and ICS was performed after 20 and 72 h. The first group of dot plots shows freshly isolated NKT cells for α-GC/CD1d tetramer and CD4 expression. The second and third groups represent cultured cells at 20 and 72 h, respectively, and depict CD4 versus IL-17. The numbers in each quadrant represent the percentage of total cells, except for stimulated NK1.1− NKT cells, for which the numbers represent the percentage of either CD4+ or CD4− NKT cells producing IL-17. Dot plots are representative of two experiments. (B) NKT cells from thymus and liver were purified into 4 subsets on the basis of NK1.1 and CD4 expression and cultured as described in Fig. 1. Supernatants were harvested at 24 and 72 h to assay for secreted IL-17 by ELISA. Thymus bars represent 8 separate cultures derived over four independent experiments, and liver bars represent 3–5 separate cultures derived over two independent experiments. The 24-h thymus graph contains a split y-axis. (C) NK1.1−CD4− and NK1.1−CD4+ thymus NKT cells were assayed for intracellular IL-17 expression after 6 h CD3/CD28 stimulation in 1 experiment. Shown is IL-4 versus IL-17 expression in stimulated and nonstimulated samples. Numbers represent the percentage of cells in that quadrant.
An interesting observation was that not all cells within the CD4/NK1.1-defined subsets were producing cytokines by ICS (Fig. 2 and Fig. 4). This may in part reflect the strength or timing of the in vitro stimulus so that not all cells were activated to the same extent. However, the exclusive production of IL-17 and IFN-γ by different cells also suggests that the subsets we have defined may be further divisible.
We also examined IL-17 production from lymph node, spleen, and lung lymphocytes after short-term (3 h) stimulation with Phorbol myristate acetate (PMA) and ionomycin, which supported our observations from the thymus and liver that IL-17–producing NKT cells were primarily NK1.1−CD4− (Fig. S3). Using an intrathymic FITC injection assay to track recent thymic emigrants, we found that recent thymic emigrant NKT cells were readily detectable in spleen, as published (18), but were almost undetectable in lymph nodes, where NKT cell production of IL-17 is most abundant (data not shown). This finding suggests that peripheral IL-17–producing NK1.1− NKT cells are part of the mature NKT cell pool rather than immature thymic emigrant cells. It is noteworthy that in these experiments NKT cells were not separated according to NK1.1 expression before stimulation, excluding the possibility that NK1.1 ligation somehow inhibits IL-17 production by the NK1.1+ fraction.
The finding that RORγt is constitutively expressed by some NKT cells suggests that these cells are primed to make IL-17, because transduction of RORγt into naïve CD4 T cells has been shown to elicit IL-17 production (11). Nonetheless, we tested whether NKT cells were susceptible to regulation via traditional Th17-inducing factors. Inhibition of IFN-γ and IL-4 augmented NKT cell expansion and IL-17 production within the NKT cell compartment (Fig. S4). Addition of TGF-β and IL-6 did increase the percentage of NKT cells producing IL-17, and CD4+ NKT cells made up a higher proportion of these cells. However, these factors impaired the recovery of NKT cells from these cultures, and hence their net effect on IL-17 production by NKT cells seemed to be negligible.
Given the differences in IL-17 production between CD4−NK1.1− and CD4+NK1.1− NKT cells, these subsets also were tested separately for production of other cytokines (Fig. S5). In general, CD4+NK1.1− and CD4−NK1.1− NKT subsets had similar cytokine profiles and resembled each other more closely than they resembled their NK1.1+ counterparts. Except for IL-17, which was produced at higher levels by CD4−NK1.1− NKT cells, the thymic CD4+NK1.1− fraction produced the same or higher levels of cytokines (IFN-γ, IL-4, IL-6, IL-10, IL-13) than the thymic CD4−NK1.1− NKT cells. Liver CD4−NK1.1− and CD4+NK1.1− NKT cells also produced similar levels of cytokines, except for IL-17.
CD4−NK1.1− NKT Cells Are Precursors to the CD4−NK1.1+ Lineage.
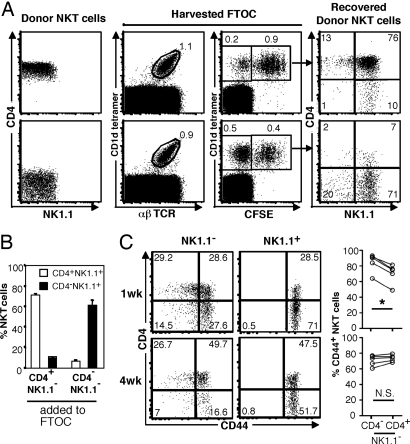
It is known that CD4+NK1.1− NKT cells in the thymus are immature precursors that can differentiate into NK1.1+ NKT cells when transferred into a thymus in vitro or in vivo (17–19), but it is not known whether this ability also extends to CD4−NK1.1− cells. Therefore, we added carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+ and CD4−NK1.1− NKT cells to separate NKT cell development cultures (12). Both subsets showed clear precursor potential in terms of their higher proliferation (CFSE dilution) and generated NK1.1+ NKT cells within a week of intrathymic culture. However, CD4−NK1.1− NKT cells generated CD4−NK1.1+ cells, whereas CD4+NK1.1− cells preferentially generated CD4+NK1.1+ cells (Fig. 5). When mature CD4+NK1.1+ NKT cells were added to this thymus differentiation system, they proliferated less and remained mostly (≈90%) CD4+NK1.1+ (data not shown), consistent with our earlier finding that these cells remain CD4+ after intrathymic transfer in vivo (19). Taken together, this indicates that the elusive branch point for CD4− NKT cells occurs before NK1.1 up-regulation.
Fig. 5.
CD4−NK1.1− NKT cells are precursors for CD4−NK1.1+ NKT cells. (A) E15 fetal thymus lobes were cultured for 1 week in standard FTOC conditions to allow development of CD4+CD8+ thymocytes. Lobes then were added to hanging drop cultures, combined with defined NKT subsets overnight, and returned to standard FTOC conditions for another 7 days before harvesting for analysis by FACS. CFSE labeling or a congenic marker together with α-GC/CD1d tetramer staining was used to identify donor NKT cells, and both doublet exclusion and unloaded tetramer exclusion were used to exclude false positives. Data are representative of 10 separate cultures for CD4+NK1.1− NKT cell repopulations and of 9 CD4−NK1.1− NKT cell repopulations derived over three independent experiments. (B) The percentage of mature NKT cell subsets from the given starting population that is present at the end of the culture period. Bars depict mean ± standard error. (C) NKT cells defined as α-GC/CD1d tetramer+ αβTCR+ cells were electronically gated, and NK1.1− and NK1.1+ subsets were examined separately for expression of CD44 versus CD4. Data shown are representative of 5 separate mice for each time point.
Immature NK1.1− NKT cells can be subdivided further into CD44low and CD44hi cells. When we analyzed these for CD4 expression, we found that in 1-week-old mice, the CD4−NK1.1− NKT cells were enriched for the more mature CD44hi cells (Fig. 5). Although this was less clear in 4-week-old mice, the intensity of CD44 expression still seemed to be higher in the CD4−NK1.1− subset than in the CD4+NK1.1− subset. These data suggested that CD4−NK1.1− NKT cells branch at a very early stage in NKT cell lineage development, just before or at the time CD44 up-regulation is starting to occur.
Discussion
Although it is well established that human NKT cell subsets, based on CD4 and CD8 expression, are functionally diverse in terms of cytokine production (3, 4), it is not clear whether similar diversity exists for mouse NKT cells. In this study, we have analyzed mouse NKT cell subsets based on the expression of CD4, NK1.1, and the tissue source. After examining different NKT cell subpopulations from thymus, spleen, and liver for production of 19 cytokines, our results show that mouse NKT cell populations exhibit remarkable diversity, with differences of 10- to 100-fold in the extent to which most cytokines were produced in response to anti-CD3/CD28 stimulation. Moreover, ICS suggested that further subpopulations exist, because IL-17 and IFN-γ production seemed to be mutually exclusive, even within the NK1.1− or CD4−NK1.1+ subsets. Although there were many interesting differences that should form the subject of future investigations into NKT cell diversity, the most intriguing finding was the potent production of IL-17 by CD4−NK1.1− NKT cells.
Rapid IL-17 release by CD4−NK1.1− NKT cells may have important physiological consequences. Whereas Th17 cells generally are recognized as being the main source of IL-17 during immune responses (10), Th17 cells are differentiated effector CD4 T cells that develop from naïve CD4 T cells in the presence of cytokines such as TGF-β, IL-6, IL-21, and IL-23 over a matter of days. NKT cells, however, can produce IL-17 within 3 h and may therefore be a very important initial source of this proinflammatory cytokine. Consistent with this concept, Michel et al. demonstrated that lung NKT cells contributed to IL-17–dependent airway neutrophilia in response to LPS/α-GalCer instillation (8). However, in contrast to Michel et al., who showed that NK1.1− NKT cells produced IL-17 but not IFN-γ or IL-4, our results showed that the NK1.1− NKT cells do produce IFN-γ and IL-4. It is important to point out that our findings that NK1.1− NKT cells also produce IFN-γ and IL-4 are consistent with all other studies of this subset (e.g., refs. 12, 17, 18). These contrasting results may reflect different modes of stimulation, although in our experiments α-GalCer–pulsed DC, anti-CD3/CD28, and PMA/ionomycin were all capable of stimulating potent IL-17, IFN-γ, and IL-4 production by liver NK1.1− NKT cells. It also is conceivable that NKT cell exacerbation of some autoimmune diseases including collagen-induced arthritis (20, 21) and experimental autoimmune encephalomyelitis (22) are mediated via NKT cell production of IL-17, which is known to be a key factor in these diseases (10).
In line with the ability of NKT cells to produce IL-17 rapidly, RORγt and IL-23 receptor (IL-23R) were constitutively expressed by NK1.1− NKT cells. This finding is odd, considering that IL-23R generally is found on highly differentiated T cells including Th17 and memory T cells. Furthermore, a previous study using an EGFP reporter system suggested that RORγt is not expressed by NKT cells (23). It is not easy to reconcile this with our data, except that on close inspection it is apparent that a very small subset of NKT cells is positive for EGFP (23), which may correlate with CD4−NK1.1− NKT cells, which represent ≈5% of total NKT cells in the adult thymus.
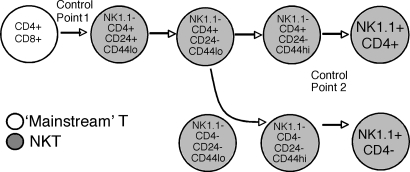
Collectively, thymic NK1.1− NKT cells have been considered to be immature precursors to their NK1.1+ counterparts, but the branching of CD4+ and CD4− NKT cells has proven difficult to define. Most NK1.1− NKT cells are CD4+, but the dramatic difference in IL-17 production by CD4−NK1.1− NKT cells and CD4+NK1.1− cells prompted us to assay their developmental potential separately. The results revealed that CD4−NK1.1− NKT cells are precursors to the CD4−NK1.1+ lineage, meaning that the branch point for these cells occurs before NK1.1 up-regulation (which we previously have referred to as “control point 2”) (24). The earliest NKT cells are defined as CD24+, and all these cells are NK1.1−CD4+ (25). As thymic NKT cells mature, they lose CD24 expression and up-regulate CD44 and NK1.1. Our data suggest that the CD4− branch point in NKT cells occurs before or during the transition from CD44lo to CD44hi within the NK1.1− stage (Fig. 6), because CD4−NK1.1− NKT cells vary in CD44 expression. However, given that, at least in 1-week-old mice, a larger proportion of CD4−NK1.1− NKT cells than CD4+NK1.1− NKT cells express CD44, these cells may be slightly more mature. This slight increase in maturity may explain their lower production of IL-4 and other cytokines that were expressed at high levels by the broader NK1.1− subset (17). Although the molecular basis for the divergence of CD4− NKT cells remains unclear, the marked difference in production of IL-17 by CD4− and CD4+ NKT cells, combined with other studies indicating their functional divergence (5), supports the concept that two separate sublineages of NKT cells diverge at the CD44loNK1.1− stage, during intrathymic NKT cell development.
Fig. 6.
Revised schematic of NKT cell development showing CD4− NKT cells branching at the NK1.1− stage.
In addition to subset-specific IL-17 production, we also demonstrated that NKT cells are remarkably heterogeneous for an array of other cytokines. Although the type of stimulation (anti-CD3/CD28 versus α-GalCer–pulsed DC) had some bearing on the cytokine profiles of some NKT cell subsets, the key point remains that different NKT cell subsets produce remarkably diverse cytokine profiles.
Our data raise the question whether distinct cytokine profiles reflect different roles/functions for specific NKT cell subsets in immune responses. Few models have compared distinct NKT subsets directly, but we have shown that liver-derived NKT cells are superior to NKT cells from other organs in promoting tumor rejection and that CD4−NK1.1+ NKT cells from liver promote tumor rejection more effectively than CD4+NK1.1+ liver NKT cells (5). This is interesting in light of the current data that anti-CD3/28–stimulated liver NK1.1+ NKT cells produce high levels of IFN-γ but only low levels of Th2 cytokines such as IL-4, IL-10, and IL-13. Although thymus-derived NKT cells produce similar amounts of IFN-γ, the higher potential production of IL-4, IL-10, and IL-13 by these cells may explain why they could not promote tumor rejection. Consistent with this, inhibition of IL-4 and IL-10 improved the ability of NKT cells from thymus and liver to promote tumor rejection (5). Differential production of the proinflammatory cytokine IL-17 by CD4− and CD4+ liver NKT cells also may explain why the CD4− NKT cells are more potent in models of tumor rejection (5). Although studies that have explored the effect of IL-17 on tumor immunity have generated equivocal results (26), there is some evidence that this cytokine can enhance T cell–mediated tumor rejection (27, 28).
In summary, we have demonstrated that mouse NKT cell subpopulations exhibit remarkable diversity in their ability to produce cytokines. In particular, the production of the proinflammatory cytokine IL-17 by mouse CD4−NK1.1− cells, and to a lesser extent by CD4−NK1.1+ NKT cells, sharply distinguishes these cells from CD4+ NKT cells. The characterization of CD4−NK1.1− NKT cells also adds a new subpopulation to the NKT cell family and, at least for these cells in the thymus, provides a ‘missing link’ that represents the branch point for CD4− NKT lineage. Although this study represents a major step toward understanding NKT cell functional diversity and development, it raises important new questions about these cells: What is the developmental signal that results in branching of the CD4− lineage at the NK1.1− stage? Do the mutually exclusive IL-17+ and IFN-γ+ subsets of CD4−NK1.1− NKT cells represent distinct lineages, and do they both contain precursor potential? What is the relationship between similar (CD4/NK1.1-defined) subsets with distinct cytokine profiles in different tissues? What is the molecular basis for the extremely diverse cytokine profiles? Do the different subsets perform distinct functions associated with their unique cytokine profiles? The most important message from this study is that, as with conventional T cells and dendritic cells, studies into NKT cell biology and function must examine these cells as individual subpopulations, because they are too diverse to be examined as a homogeneous lineage.
Materials and Methods
Mice.
C57BL/6 mice were bred in house at the Department of Microbiology and Immunology Animal Facility, University of Melbourne, Australia. All mice used were between 5 and 7 weeks old unless otherwise stated, and all experiments were conducted in accord with the animal ethics guidelines of the University of Melbourne Animal Ethics Committee.
Lymphocyte Isolation.
Lymphocytes were isolated from the liver, thymus, and spleen as described in ref. 5. Thymic NKT cells were enriched from thymus by labeling thymocytes with anti-CD8 (clone 3.155) and anti-CD24 (J11D), and tagged cells were depleted by using rabbit complement (C-SIX Diagnostics) in the presence of DNase (Roche Diagnostics). Cells were spun over a histopaque gradient (1.083 g/ml; Sigma–Aldrich) at room temperature to collect viable cells. NKT cells were enriched from spleen by staining with phycoerythrin (PE)-conjugated CD1d tetramer and subsequent incubation with anti-PE microbeads (Miltenyi Biotech). Labeled cells then were passed through a magnetic column using the Miltenyi AUTOMACS system, and magnetized cells were collected and washed before being labeled for flow cytometric purification.
Antibodies and Flow Cytometry.
All antibodies used were from BD PharMingen. Antibodies included αβTCR-FITC (clone H57–597), NK1.1-PE/CY7 (PK-136), CD4-APC/CY7 (RM4–5), IL-17-PE (TC11–18H10), IL-4-APC (11B11), and CD44 FITC (IM7). Mouse CD1d tetramer loaded with α-GalCer (kindly provided by Kirin Pharma Company) was produced in house, using recombinant baculovirus encoding His-tagged mouse CD1d and mouse β2-microglobulin originally provided by M. Kronenberg (La Jolla Institute for Allergy and Immunology, San Diego, CA). For ICS, cells were cultured in GolgiStop (BD Biosciences) before being fixed and permeabilized by using the BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit. Flow cytometry was performed by using either an LSR-II or FACSCanto, and purification was performed on a FACSAria (BD Biosciences). Analysis was performed by using FlowJo software (Tree Star Inc.).
Cytokine Analysis from Cell Culture Supernatants.
All cytokines were assayed by using BD Biosciences cytometric bead array flex set for mice (IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IFN-γ, TNF, MIP-1α, Monocyte Chemotactic Protein-1, Monokine Induced by IFN-gamma, Keratinocyte Chemokine, and RANTES) except for IL-17 and IL-21, which were detected by using ELISA from R&D Systems. Capture and detection antibodies in the IL-21 ELISA kit were used at 0.8 μg/ml (2 × recommended concentration), and IL-17 ELISA reagents were used as recommended.
Cell Culture.
For each experiment, tissues were pooled from 10–20 mice before NKT cells were purified and cultured separately. Cells were cultured in tissue culture media (as described in SI Methods) in a 96-well plate. For in vitro stimulation assays, no-azide low-endotoxin anti-CD3 (145–2C11; BD PharMingen) and anti-CD28 (37.51; BD PharMingen) were used at a concentration of 10 μg/ml to coat plates. For α-GalCer/DC and NKT cell cocultures, DCs were pulsed with 200-ng/ml α-GalCer for 3 h and washed twice before being mixed with NKT cells.
Fetal Thymus Organ Culture.
Thymic lobes were removed from embryos at day 15 of gestation and cultured for 7 days on the surface of filters (pore size, 0.45 μm) resting on Gelfoam sponges (Amersham Pharmacia) placed (and previously soaked) in 1 ml of FTOC medium (refer to SI Methods). After culture, the lobes were placed in Terasaki plates, 2 lobes per well, containing sorted and CFSE-labeled (as described in ref. 12) populations of NKT cells in 30 μl of RPMI-FTOC medium. The Terasaki plates were inverted gently, forming a hanging drop, and were incubated overnight. The lobes then were returned to standard FTOC conditions and cultured for 7 days in 1-ml cultures in RPMI-FTOC. Lobes were disrupted carefully using glass coverslips to release the cells for FACS analysis.
Supplementary Material
Acknowledgments.
We thank Alice Denton and Dr. Stephen Turner for help with quantitative RT-PCR, Ken Field for flow cytometric support, and David Taylor for animal husbandry. This research was funded by National Health and Medical Research Council (NHMRC) Program Grant 251608, renewed as 454569. J.M.C. and L.A.P. are supported by Cancer Research Institute postgraduate scholarships. S.C. and S.P.B. are supported by NHMRC Career Development Awards. F.M. is supported by an NHMRC Dora Lush Postgraduate Fellowship. D.I.G. and M.J.S. are supported by NHMRC Research Fellowships. We also thank the Picchi Brothers Foundation for generous contributions to the Flow Cytometry Facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801631105/DCSupplemental.
References
- 1.Godfrey DI, Kronenberg M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe NY, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coquet JM, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 7.Harada M, et al. IL-21-induced B epsilon cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med. 2006;203:2929–2937. doi: 10.1084/jem.20062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.McNab FW, et al. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J Immunol. 2007;179:6630–6637. doi: 10.4049/jimmunol.179.10.6630. [DOI] [PubMed] [Google Scholar]
- 13.Robson MacDonald H, Lees RK, Held W. Developmentally regulated extinction of Ly-49 receptor expression permits maturation and selection of NK1.1+ T cells. J Exp Med. 1998;187:2109–2114. doi: 10.1084/jem.187.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uldrich AP, et al. NKT cell stimulation with glycolipid antigen in vivo: Costimulation-dependent expansion, bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voyle RB, et al. Ligand-dependent inhibition of CD1d-restricted NKT cell development in mice transgenic for the activating receptor Ly49D. J Exp Med. 2003;197:919–925. doi: 10.1084/jem.20021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda M, Lohwasser S, Yamamura T, Takei F. Regulation of NKT cells by Ly49: Analysis of primary NKT cells and generation of NKT cell line. J Immunol. 2001;167:4180–4186. doi: 10.4049/jimmunol.167.8.4180. [DOI] [PubMed] [Google Scholar]
- 17.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 18.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(−) CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNab FW, et al. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 20.Chiba A, Kaieda S, Oki S, Yamamura T, Miyake S. The involvement of V(alpha)14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 2005;52:1941–1948. doi: 10.1002/art.21056. [DOI] [PubMed] [Google Scholar]
- 21.Ohnishi Y, et al. TCR Valpha14 natural killer T cells function as effector T cells in mice with collagen-induced arthritis. Clin Exp Immunol. 2005;141:47–53. doi: 10.1111/j.1365-2249.2005.02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahng AW, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa T, et al. Genetic evidence supporting selection of the valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 25.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28:207–212. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Benchetrit F, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 28.Hirahara N, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.