Abstract
Histone deacetylase inhibitors (HDACi) and agents such as recombinant tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and agonistic anti-TRAIL receptor (TRAIL-R) antibodies are anticancer agents that have shown promise in preclinical settings and in early phase clinical trials as monotherapies. Although HDACi and activators of the TRAIL pathway have different molecular targets and mechanisms of action, they share the ability to induce tumor cell-selective apoptosis. The ability of HDACi to induce expression of TRAIL-R death receptors 4 and 5 (DR4/DR5), and induce tumor cell death via the intrinsic apoptotic pathway provides a molecular rationale to combine these agents with activators of the TRAIL pathway that activate the alternative (death receptor) apoptotic pathway. Herein, we demonstrate that the HDACi vorinostat synergizes with the mouse DR5-specific monoclonal antibody MD5-1 to induce rapid and robust tumor cell apoptosis in vitro and in vivo. Importantly, using a preclinical mouse breast cancer model, we show that the combination of vorinostat and MD5-1 is safe and induces regression of established tumors, whereas single agent treatment had little or no effect. Functional analyses revealed that rather than mediating enhanced tumor cell apoptosis via the simultaneous activation of the intrinsic and extrinsic apoptotic pathways, vorinostat augmented MD5-1-induced apoptosis concomitant with down-regulation of the intracellular apoptosis inhibitor cellular-FLIP (c-FLIP). These data demonstrate that combination therapies involving HDACi and activators of the TRAIL pathway can be efficacious for the treatment of cancer in experimental mouse models.
Histone deacetylase inhibitors (HDACi) are an exciting class of anticancer drugs currently in early phase clinical trials for the treatment of hematological malignancies and solid tumors (1, 2). These agents can elicit a range of biological responses that affect tumor growth and survival, including inhibition of tumor cell cycle progression, induction of tumor cell-selective apoptosis, suppression of angiogenesis, and modulation of immune responses. Although HDACi show promise as single agents, given their pleiotropic anticancer activities and the apparent lack of toxicity to normal cells, their use in combination with other agents may improve their breadth of application. Already, HDACi have been shown to function synergistically in vitro with a host of structurally and functionally diverse chemical compounds and biologically active polypeptides (1, 3). With a more complete understanding of the molecular mechanisms of action of HDACi, combination studies based on a strong mechanistic rationale are now possible.
Induction of apoptosis plays a key role in mediating the antitumor effects of HDACi in preclinical models (4–6), and the molecular events underpinning this process are now being elucidated. HDACi can induce tumor cell apoptosis through activation of either the extrinsic (death receptor) or intrinsic (mitochondrial) pathway depending on the cell type and/or the HDACi under investigation (1). Activation of the extrinsic pathway by HDACi occurs through transcriptional up-regulation of various TNF receptor super-family members and/or their cognate ligands. Indeed, studies by different groups using various genetic or biological means to inhibit death receptor signaling have demonstrated that death receptor signaling is required for HDACi-induced apoptosis (see ref. 1 and references therein). Conversely, we and others have demonstrated that whereas HDACi induce expression of death receptors, ligands, and down-regulate inhibitors of death-receptor signaling such as cellular c-FLIP (7) and XIAP (8), the intrinsic rather than the extrinsic pathway is necessary for HDACi-mediated apoptosis (1). We therefore propose that there is a mechanistic rationale for combining HDACi with death receptor stimuli–either the HDACi will augment death receptor-mediated apoptosis by hyperactivating the same pathway, or the simultaneous activation of the extrinsic and intrinsic apoptotic pathways will result in additive or synergistic killing.
Human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL, Apo-2L) interacts with two death-inducing receptors, DR4 and DR5, and with three “decoy receptors,” DcR1, DcR2, and the soluble receptor osteoprotegerin (9). Only one murine, death-inducing receptor has been identified (mouse DR5) that shares sequence homology with human DR4 and DR5 (10), and two murine decoy receptors have also been identified (11). In humans, TRAIL can induce tumor cell-selective killing by activating the death-receptor-mediated apoptotic pathway through binding to the TRAIL-R1/DR4 or TRAIL-R2/DR5 receptors, although apoptotic signaling may be regulated by expression of decoy receptors or activation of additional signaling pathways such as the NF-κB pathway (9). The therapeutic potential of TRAIL is based on its ability to induce apoptosis in a wide variety of human tumor cell lines in vitro and in vivo with seemingly little toxicity against normal cells (12). Moreover, recombinant soluble TRAIL can be safely introduced into nonhuman primates, and early phase clinical trials indicate that the agent is nontoxic to humans (13). Agonistic mAbs that functionally engage human and murine TRAIL receptors [HGS-ETR1/mapatumumab (anti-DR4 antibody) and HGS-ETR2/lexatumumab (anti-DR5 antibody) in humans, and MD5-1 (anti-DR5 antibody) in mice] have been generated and induce TRAIL receptor oligomerization and activate the extrinsic apoptotic cascade, culminating in target cell death (14–16). These agents have been tested in Phase I clinical trials and exhibit excellent safety profiles (17, 18). The use of mAb to target TRAIL receptors may have a therapeutic advantage over the use of recombinant TRAIL because they demonstrate a longer half-life in vivo, a higher affinity for the target receptor, no decoy receptor engagement, and they may provide a mechanism to induce long-term, tumor-specific T cell memory (through Fc receptor engagement) that prevents tumor recurrence (16).
Combinations of HDACi and activators of the TRAIL and Fas pathways have been tested against human tumor cell lines in vitro resulting in additive or synergistic tumor cell death after combination treatment (see ref. 1 and references therein). The mechanistic basis for the synergistic effects remains unclear, and there have been no in vivo studies demonstrating therapeutic efficacy or associated toxicity of the combination in preclinical models. Herein, we demonstrate that the HDACi vorinostat (suberoylanilide hydroxamic acid, SAHA, Zolinza) and the anti-mouse DR5 mAb MD5-1 induce synergistic apoptosis of a variety of mouse tumor cell lines of different tissue origin in vitro. Using the 4T1.2 syngeneic mouse model of breast carcinoma, we clearly demonstrate that a combination therapy of vorinostat and MD51 displays synergistic antitumor activity over either agent alone and in the absence of any detectable toxicity. Finally, using 4T1.2 cells overexpressing either cytokine response modifier A (CrmA) or c-FLIP (to inhibit the extrinsic apoptotic pathway) or B cell/chronic lymphocytic leukemia lymphoma-2 (Bcl-2) (to inhibit the intrinsic pathway), we illustrate that the extrinsic apoptotic pathway is essential for the combination effect of vorinostat and MD5-1 in vitro and in vivo.
Results
MD5-1 and Vorinostat Function Synergistically to Induce Apoptosis In Vitro.
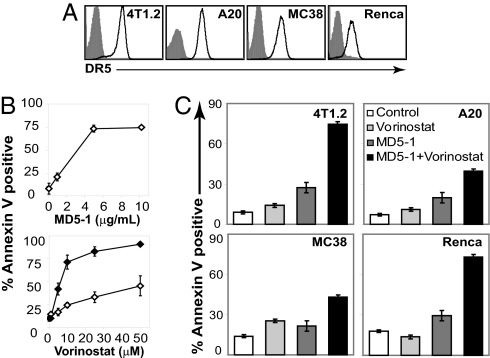
We surveyed mouse tumor lines for expression of DR5 and determined that the 4T1.2, Renca, A20, and MC38 cell lines each expressed the TRAIL receptor (Fig. 1A). Treatment of 4T1.2 (Fig. 1B), Renca, A20, and MC38 cells [supporting information (SI) Fig. S1A] with plate-bound MD5-1 anti-DR5 mAb for 24 h resulted in a dose-dependent induction of apoptosis. As reported (16), cross-linking of DR5 was essential for induction of apoptosis by MD5-1 (data not shown). In contrast to MD5-1, the HDACi vorinostat induced little or no apoptosis of 4T1.2 (Fig. 1B), Renca, and MC38 cells (Fig. S1B) in 24 h assays by using doses of drug up to 50 μM. Only A20 cells displayed substantial sensitivity to vorinostat after a 24 h incubation (Fig. S1B). Incubation of 4T1.2, Renca, and MC38 tumor lines for 48 h resulted in a dose-dependent increase in apoptosis reaching a maximum of ≈90% with 50 μM vorinostat (Figs. 1B and S1B).
Fig. 1.
In vitro sensitivity of tumor lines to Vorinostat and MD5-1. (A) DR5 expression on 4T1.2, A20, MC38, and Renca tumor lines was assessed by using flow cytometry (fluorescence intensity is represented on a logarithmic scale). Shaded histograms represent isotype-stained cells whereas open histograms represent staining with MD5-1 anti-DR5 mAb. (B) 4T1.2 mammary carcinomas were incubated with increasing concentrations of MD5-1 (Upper) and vorinostat (Lower) for 24 h (open diamonds) and 48 h (closed diamonds). (C) Tumor lines were incubated for 24 h with the following concentrations of vorinostat and MD5-1, respectively: 4T1.2 (5 μM, 1 μg/ml), A20 (1.5 μM, 0.5 μg/ml), MC38 (5 μM, 1 μg/ml), and Renca (10 μM, 0.5 μg/ml). A CI <0.5 was found for each tumor line, demonstrating a synergistic apoptotic relationship exists between vorinostat and MD5-1. Apoptotic cells were assessed by annexin V staining. Error bars indicate ± SEM of at least three independent experiments.
We next tested whether MD5-1 and vorinostat induced synergistic death of 4T1.2, Renca, MC38, and A20 cells. Using concentrations of each agent that alone induced minimal apoptosis in a 24 h assay, the two agents combined caused ≈75% of the 4T1.2 and Renca cells to undergo apoptosis (Fig. 1C). The degree of synergism for each of the tumor lines was assessed by calculation of the Combination Index (CI) (19). CI values <0.1 are considered very strong synergy; 0.1–0.3, strong synergy; and 0.3–0.7, synergy. The CI value for the 4T1.2 cell line was 0.105, and for Renca cell line, the value was 0.001. These data therefore demonstrate that triggering of the death-receptor pathway through the DR5 pathway, combined with inhibition of HDAC activity by vorinostat, results in a strong to very strong synergistic death of mammary and renal carcinoma cells, respectively. Enhanced induction of apoptosis was observed in the MC38 and A20 lines. The CI for A20 was 0.551, and for MC38 the CI was 0.283. We also assessed the kinetics of apoptosis induced by the combination of vorinostat and MD5-1. 4T1.2 cells that were treated with the combination demonstrated significant apoptosis as little as 8 h after combination treatment and steadily increased over the 24 h time course (Fig. S1C).
Combination Therapy Using MD5-1 and Vorinostat Causes Regression of Established Tumors.
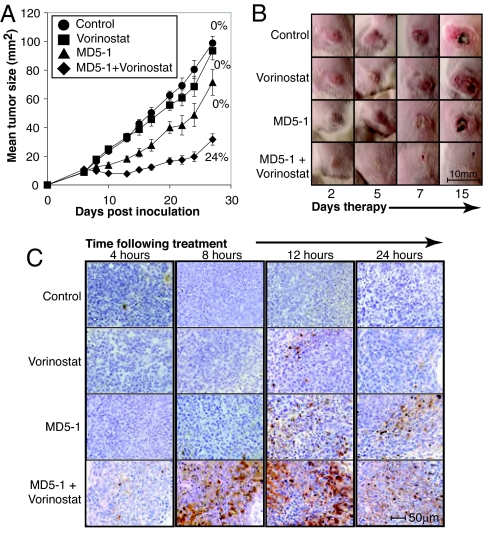
Our in vitro data confirm other reports demonstrating that HDACi can synergize with stimulators of death-receptor pathways to kill tumor cell lines (see ref. 1 and references therein). However, to our knowledge, there has not been any published data demonstrating combination effects between these agents in vivo. Moreover, it is unclear how toxic this form of combination therapy might be to normal tissues. The BALB/c-derived 4T1.2 tumor is an aggressive mammary carcinoma that spontaneously metastasizes to multiple organs within days of primary growth. BALB/c mice bearing established 4T1.2 s.c. tumors were treated with MD5-1 and vorinostat alone and in combination. Pharmacokinetic analysis demonstrated that vorinostat administered at 100 mg/kg i.p. into BALB/c mice achieved a Cmax of 2.0 μM and T1/2 of 3.7 h. Vorinostat alone had little to no effect on the growth of established 4T1.2 mammary carcinomas, and MD5-1 had minimal single-agent activity, mediating delayed tumor growth but no tumor regression (Fig. 2 A and B). In contrast, the combination of MD5-1 and vorinostat caused complete tumor regression of 6/25 4T1.2 tumors by day 14 of therapy and significantly suppressed the growth of the 19 other tumors (Fig. 2A). Of the six mice that were tumor free on day 14 of therapy, four mice remained tumor free (primary and metastases) for the remainder of the experiment (>100 d). To determine whether tumors that eventually grew out in mice treated with a combination of MD5-1 and vorinostat developed resistance to either agent alone, we resected the tumors and established tumor cell lines in culture. These lines showed no difference in sensitivity to MD5-1 or vorinostat when compared with parental 4T1.2 cells (Fig. S2A). These data indicated that the inability to achieve complete cures of all 4T1.2-bearing mice with the MD5-1/vorinostat combination therapy was not because of acquired resistance to either agent.
Fig. 2.
Vorinostat and MD5-1 therapy induces regression of established 4T1.2 mammary carcinomas. (A) BALB/c mice with established s.c. 4T1.2 tumors (>9 mm2, day 6) were treated with control antibody (n = 16), vorinostat (n = 16), MD5-1 (n = 16), or MD5-1 and vorinostat (n = 25). MAbs (50 μg) were given i.p. (4 doses in total, given every 4 d), and vorinostat was injected i.p. daily at 100 mg/kg. Combined mean tumor sizes from three independent experiments are shown. Error bars represent ± SEM. Complete tumor regressions were only observed in mice treated with the vorinostat/MD5-1 combination with 6 of 25 mice tumor-free at the completion of treatment. Percent complete response for each treatment is shown. (B) Images of representative s.c. 4T1.2 tumors during the course of therapy. (C) TUNEL staining of 4T1.2 tumor sections taken from mice after treatment with vehicle, vorinostat (100 mg/kg), MD5-1 (50 μg), or vorinostat and MD5-1 (100 mg/kg and 50 μg, respectively). Tumors were ≈9 mm2 when treatment commenced.
Our in vitro data (Fig. 1) demonstrated that MD5-1 and vorinostat induced synergistic apoptosis, and we hypothesized that the enhanced apoptosis mediated by the combination treatment would translate to additional therapeutic activity in vivo. As shown in Fig. 2C, established tumors from mice treated with vorinostat demonstrated little or no TUNEL staining over a 24 h time course, whereas MD5-1 alone induced some TUNEL staining with the largest number of TUNEL-positive tumor cells seen at the 12 h time point (Fig. 2C). Synergistic apoptosis mediated by MD5-1 and vorinostat in vivo was demonstrated by a substantial increase in the number of TUNEL-positive tumor cells observed at the 8 and 12 h time points (Fig. 2C).
Given the superior antitumor effects demonstrated by using a combination of MD5-1 and vorinostat compared with single agent treatment, we next assessed the effect of treatment with MD5-1, vorinostat, or a combination of both on the health of BALB/c mice by measuring the body weight of the mice during and after treatment with these agents. None of the treated groups lost >10% of their body weight through the course of the experiment, indicating a lack of any major systemic toxicity (Fig. S2B). Moreover, there was no detectable elevation in serum aspartate aminotransferase, alanine aminotransferase, or creatinine levels in any of the treatment groups (data not shown). Together, these data indicate that combination therapy by using MD5-1 and vorinostat is efficacious against breast carcinomas without overt toxicity to the host.
In addition to directly inducing tumor cell apoptosis, HDACi may indirectly cause tumor cell death by engaging the immune system (1). Moreover, it has been demonstrated that treatment with MD5-1 can stimulate antitumor immune responses (16). To determine whether the adaptive immune system played any role in mediating the antitumor activity of the vorinostat/MD5-1 combination, we compared and contrasted the effects of single agent and combination treatment on 4T1.2 tumors grown in BALB/c and SCID mice. 4T1.2 cells grew at equivalent rates in BALB/c and SCID mice, and the enhanced antitumor effects of the combination therapy compared with single-agent treatment demonstrated in BALB/c mice was also observed in SCID mice (Fig. S2C), indicating that the adaptive immune system plays little or no role in the primary therapeutic response elicited by vorinostat and MD5-1 used in combination.
To demonstrate that the combination of vorinostat and MD5-1 provided enhanced antitumor activities in vivo compared with vorinostat or MD5-1 alone, in tumors other than 4T1.2 mammary carcinomas, experiments were performed by using Renca tumors that, like 4T1.2, grew in BALB/c mice. As shown in Fig. S2D, the combination of vorinostat and MD5-1 again produced antitumor effects in vivo that were superior to that observed by using vorinostat or MD5-1 as monotherapies.
Mechanisms of Synergistic Antitumor Activity by Using MD5-1 and Vorinostat.
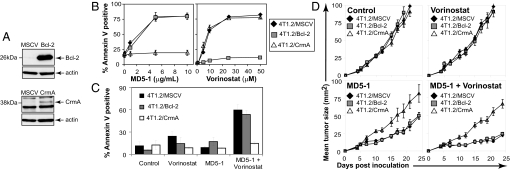
Our data indicate that vorinostat and MD5-1 cooperate to induce apoptosis of 4T1.2 cells, however, the molecular mechanisms of this synergistic cell death remained to be determined. A simple explanation would be that vorinostat merely increased expression of DR5 on 4T1.2 cells to enhance MD5-1-mediated apoptosis, as has been demonstrated (1). However, analysis of vorinostat-treated 4T1.2 cells demonstrated that the cell surface expression of TRAIL or DR5 was not altered (Fig. S2E). To identify the apoptotic pathways necessary for synergistic apoptosis by the MD5-1/vorinostat combination, we produced 4T1.2 cells overexpressing the viral serpin CrmA (to inhibit death receptor-mediated killing) or Bcl-2 (to inhibit apoptosis through the intrinsic pathway) by using murine stem cell virus (MSCV)-mediated gene transduction (Fig. 3A). Treatment of these cells with vorinostat resulted in dose-dependent apoptosis of all cell lines except the 4T1.2/Bcl-2 cells (Fig. 3B). In contrast, only 4T1.2/CrmA cells were resistant to MD5-1-mediated apoptosis (Fig. 3B). These data are consistent with findings demonstrating that vorinostat-mediated apoptosis is triggered by activation of the intrinsic apoptosis pathway and not the death-receptor pathway (see ref. 1 and references therein). Treatment of these cells in vitro with vorinostat and MD5-1 demonstrated a critical role for the extrinsic apoptotic pathway in apoptosis mediated by the combination treatment because 4T1.2 cells overexpressing CrmA were completely refractory to the synergistic induction of apoptosis (Fig. 3C). Conversely, similar levels of apoptosis were detected in 4T1.2/Bcl-2 and 4T1.2/MSCV cells treated with MD5-1 and vorinostat. Taken together, these data highlight a critical role for the extrinsic apoptotic pathway in mediating the observed synergy between MD5-1 and vorinostat. This suggests that vorinostat acts to modulate the downstream extrinsic apoptotic pathway effector molecules that enhance the sensitivity of the 4T1.2 mammary carcinomas to death receptor-mediated apoptosis.
Fig. 3.
Mechanisms of combination synergistic antitumor activity by using vorinostat and MD5-1. (A) 4T1.2 cells transduced with MSCV, MSCV-Bcl-2, or MSCV-CrmA were assessed by using Western blot analysis for expression of exogenous proteins. Overexpression of Bcl-2 and CrmA was confirmed by Western blot analysis by using anti-mBcl-2 and anti-CrmA antibodies. Equivalent protein loading was confirmed by reprobing blots with anti-actin mAb. (B) 4T1.2/MSCV, 4T1.2/Bcl-2, and 4T1.2/CrmA cells were assessed for sensitivity to MD5-1 (24 h, Left) and vorinostat (48 h, Right). (C) 4T1.2/MSCV, 4T1.2/Bcl-2, and 4T1.2/CrmA cells were cultured for 24 h with control antibody (1 μg/ml), vorinostat (5 μM), MD5-1 (1 μg/ml), or a combination of vorinostat and MD5-1 (5 μM and 1 μg/ml, respectively). Apoptotic cells were assessed via annexin V/PI staining and flow cytometry. The mean ± SEM of at least three independent experiments is shown. (D) SCID mice with established 4T1.2/MSCV, 4T1.2/Bcl-2, or 4T1.2/CrmA tumors (>9 mm2, day 6) were treated with control antibody (50 μg × 4; n = 6), vorinostat (100 mg/kg/d; n = 6), MD5-1 (50 μg × 4; n = 6), or MD5-1 and Vorinostat (50 μg × 4, 100 mg/kg/d, respectively; n = 8). Representative data from three independent experiments is shown.
To determine whether the extrinsic apoptotic pathway played a critical role in mediating the therapeutic activities of the vorinostat/MD5-1 combination in vivo, the 4T1.2/MSCV, 4T1.2/Bcl-2, and 4T1.2/CrmA tumor lines were established in SCID mice. Mice bearing established 4T1.2/MSCV, 4T1.2/Bcl-2, and 4T1.2/CrmA tumors were treated with vehicle, vorinostat, MD5-1, or a combination of vorinostat and MD5-1 (Fig. 3D). 4T1.2/MSCV, 4T1.2/Bcl-2, and 4T1.2/CrmA grew s.c. at equivalent rates in vehicle-treated mice (Fig. 3D), whereas, consistent with data presented in Fig. 2A, vorinostat had little or no antitumor activity against any of the 4T1.2 tumor lines by using the dosing regimen of 100 mg/kg/d. Treatment with MD5-1 alone resulted in a slight decrease in the rate of growth of 4T1.2/MSCV and 4T1.2/Bcl-2 tumors, however no effect was observed against 4T1.2/CrmA tumors.
These data are consistent with the in vitro apoptosis results shown in Fig. 3B and the reported ability of c-FLIP, a negative regulator of caspase-8 activation, to protect experimental tumors from the therapeutic effect of MD5-1 alone (16). We produced 4T1.2/c-FLIP cells (Fig. S3A) and tested them for sensitivity to vorinostat, MD5-1, and the combination. These cells were sensitive to vorinostat but resistant to MD5-1(Fig. S3B) and the combination of vorinostat and MD5-1 in vitro (Fig. S3C). Moreover, we showed that MD5-1-mediated activation of caspase-8 and caspase-3 was enhanced in 4T1.2/Bcl-2 cells coincubated with vorinostat, and the activation of these caspases was completely inhibited in 4T1.2/c-FLIP cells (Fig. S3D). Importantly, the MD5-1/vorinostat combination was only effective against 4T1.2/MSCV and 4T1.2/Bcl-2 tumors in vivo (Figs. 3D and S3E). Taken together, these results indicated that vorinostat enhanced the ability of MD5-1 to kill 4T1.2 tumor cells via death-receptor pathway activation and demonstrated a clear and direct correlation between the induction of tumor cell apoptosis in vitro by the MD5-1/vorinostat combination and therapeutic efficacy in vivo.
Vorinostat-Mediated Down-Regulation of c-FLIP.
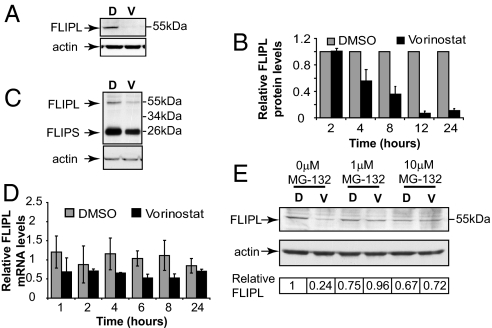
Our results indicated that enhanced expression of DR5 after vorinostat treatment was not the mechanism underpinning synergistic apoptosis of 4T1.2 tumor cells by the vorinostat/MD5-1 combination. Therefore, we assessed the expression of intracellular regulators of the TRAIL/DR5 death-receptor pathway after vorinostat treatment. As shown in Fig. 4A, treatment of 4T1.2 cells with vorinostat for 24 h resulted in a substantial decrease in expression of endogenous c-FLIP. The vorinostat-mediated decrease in c-FLIP levels occurred over time, with diminished levels of c-FLIP observed as little as 4 h after treatment with vorinostat (Fig. 4B). The kinetics of the observed decrease in expression of c-FLIP after vorinostat treatment was consistent with the sensitization of 4T1.2 cells to MD5-1-induced apoptosis shown in Fig. S1C. Consistent with the results observed in vitro, c-FLIP levels were also decreased in 4T1.2 tumors exposed to vorinostat in vivo (Fig. 4C). Vorinostat can both activate and repress gene expression (20), and HDACi have been shown to decrease the levels of c-FLIP mRNA (21). We therefore tested the levels of c-FLIP in 4T1.2 cells treated with vorinostat over a 24 h time course. As can be seen in Fig. 4D, there is a marginal decrease in c-FLIP mRNA over the time course, and the levels of c-FLIP do not decrease in a time-dependent manner. Inhibition of caspase activity by using zVAD-fmk had no effect on the vorinostat-mediated decrease in c-FLIP levels (data not shown). We therefore determined whether c-FLIP was targeted for proteasome-mediated degradation after treatment with vorinostat. Cotreatment of 4T1.2 cells with vorinostat and the proteasome inhibitor MG-132 blocked the decrease in c-FLIP levels seen after treatment of 4T1.2 cells with vorinostat alone (Fig. 4E). These results, coupled with our functional data demonstrating that 4T1.2/CrmA and 4T1.2/c-FLIP cells were refractory to the apoptotic and therapeutic effects of the MD5-1/vorinostat combination, indicate that vorinostat sensitized tumor cells to DR5-mediated apoptosis by decreasing the levels of c-FLIP and thereby relieving negative regulation of the death-receptor pathway.
Fig. 4.
Vorinostat down-regulates c-FLIP expression. (A) 4T1.2 cells were treated with DMSO (D) or 5 μM vorinostat (V) for 24 h. Western blot analysis was performed on whole-cell lysates by using an anti-c-FLIP mAb. Equivalent protein loading was confirmed by reprobing the blot with anti-actin mAb. (B) 4T1.2 cells were treated with DMSO (D) or vorinostat (V) over a 24 h time course. Western blot analysis on whole-cell lysates was performed by using anti-c-FLIP and anti-actin mAbs. The expression of c-FLIP relative to the loading control (actin) was quantitated by using densitometry. Protein expression values were made relative to DMSO (normalized to one) at each of the tested time points. Data shown is the mean ± SEM of three independent biological replicates. (C) BALB/c mice bearing 4T1.2 tumors (≈9 mm2) were treated with vehicle (PEG:DMSO) or vorinostat (100 mg/kg) for 4 h. Tumors were harvested, and whole-cell lysates were produced for use in Western blot analysis as described in A. (D) 4T1.2 were treated with either DMSO or 5 μM vorinostat for the time points shown. Quantitative real time PCR was performed by using primers specific for c-FLIP. Fold change in c-FLIP mRNA levels normalized to the ribosomal protein L32 mRNA levels are shown. Data shown represents mean of three independent experiments ± SEM. (E) 4T1.2 cells were treated in vitro for 8 h with DMSO (D) or 5 μM vorinostat (V) and the proteosome inhibitor MG-132 (0.1 or 10 μM). Western blot analysis was performed on whole-cell lysates as described for A. A representative blot from three independent experiments is shown. Relative c-FLIP protein levels were determined as described in B and are shown.
Discussion
HDACi have demonstrated therapeutic activity against a variety of hematological malignancies with the most consistent responses observed in patients with cutaneous T cell lymphoma (2). Whereas few clinical trials using HDACi in patients with solid tumors have been performed to date, it is likely that HDACi might be best used for the treatment of these cancers in combination with other antitumor agents. HDACi can mediate a range of biological responses such as induction of tumor cell apoptosis, inhibition of cell cycle progression, enhancement of antitumor immunity, and suppression of angiogenesis (1), and it is becoming more clear which one or more of these activities is necessary to mediate the therapeutic activities of these agents. Using immunocompetent mouse models, we and others have recently demonstrated a direct correlation between the ability of HDACi to induce tumor cell apoptosis and their therapeutic efficacy (4, 5). Interestingly, our study demonstrated that activation of the intrinsic apoptotic pathway was necessary for HDACi-induced apoptosis and therapeutic activity (4), whereas Insinga and colleagues identified death-receptor signaling, and in particular, signaling mediated by the TRAIL pathway, as being the most important signaling event (5).
Herein, we have assessed the antitumor activities of vorinostat when used in combination with MD5-1, a mAb that binds to and activates mouse DR5 in vitro and in vivo. We demonstrate that vorinostat and MD5-1 induced synergistic apoptosis of mouse tumor lines of breast, renal, colon, and lymphoid origin. The enhanced tumor cell apoptosis mediated by the combination treatment was highlighted by a substantial decrease in the kinetics of tumor cell death compared with that observed with either agent alone. Importantly, we demonstrate that the combination of vorinostat and MD5-1 was therapeutically superior to single agent treatment, with tumor regression observed in ≈25% of the mice with established cancers treated with the MD5-1/vorinostat. The enhanced therapeutic activity observed by using the combination approach was concomitant with a demonstrable increase in in vivo apoptosis of the tumor mass as assessed by TUNEL staining.
The use of a syngeneic mouse model allowed us to assess potential toxicities associated with single-agent or combination treatment, which is something that has not been properly assessed in many studies of immunodeficient mice with human tumor xenografts receiving either recombinant human TRAIL or agonistic anti-human DR5 mAbs. Using the dosing regimen capable of mediating appreciable therapeutic responses, we observed no significant changes in the health of the treated mice. Importantly, liver function, which was highlighted as potentially being compromised by using agents that stimulate the TRAIL receptor pathway because of death of hepatocytes (22, 23), was unaffected by MD5-1 used alone and in combination with vorinostat. Our results, coupled with studies showing that TRAIL can be safely administered alone or in combination with other anticancer agents such as velcade (24), provide encouraging preclinical evidence that therapeutic strategies using TRAIL or agonistic mAbs targeting DR4 and/or DR5 may be safe in humans. Indeed, there have been recent promising safety and tolerability results from early phase clinical trials using antibodies specific for human DR4 (mapatumumab, HGS-ETR1) (17) or DR5 (lexatumumab, HGS-ETR2) (18). Whether a combination of HDACi and TRAIL signaling is well tolerated in humans requires additional testing.
The correlation between induction of apoptosis by vorinostat/MD5-1 and therapeutic efficacy was further strengthened by our functional studies designed to identify the apoptotic pathways required for the combination response. Consistent with our studies that used primary mouse lymphomas (4), vorinostat-induced apoptosis of mouse mammary carcinoma cells was blocked by overexpression of Bcl-2, whereas inhibition of the death-receptor pathway by overexpression of CrmA or c-FLIP had no effect. In contrast, MD5-1-induced apoptosis was almost completely inhibited in 4T1.2/CrmA and 4T1.2/c-FLIP cells but unaffected by overexpression of Bcl-2. However, of major interest was our finding that apoptosis and therapeutic activity, mediated by the combination of MD5-1 and vorinostat, was also significantly suppressed by CrmA and c-FLIP, whereas overexpression of Bcl-2 had little or no effect. These data clearly demonstrated that vorinostat enhanced the apoptotic activity of MD5-1 in the extrinsic pathway and is consistent with other studies that make similar conclusions (25, 26). However, there are other reports indicating that synergistic tumor cell apoptosis, mediated by the combination of HDACi and TRAIL activating agents, requires both the extrinsic and intrinsic pathways (27–29). These different results may merely reflect cell type-specific responses to HDACi and/or TRAIL, but the discrepancies underscore the need to use robust, tractable experimental systems to define the mechanisms of action of combination therapies using HDACi and agents that activate the TRAIL pathway.
We demonstrate enhanced therapeutic activity of a combination involving an HDACi and activation of the TRAIL pathway, and although the phenomena of enhanced in vitro apoptosis after cotreatment with HDACi and activators of the TRAIL pathway has been known for some time, there appears to be little consensus on molecular events that underpin additive or synergistic apoptosis after the combination treatment (see ref. 1 and references therein). Several studies have demonstrated that HDACi enhance expression of cell surface DR4 and/or DR5, giving rise to the hypothesis that this is a key event in enhanced TRAIL-mediated apoptosis after the addition of HDACi (25, 28, 30–32). However, a recent study provides compelling evidence that although HDACi can indeed induce expression of TRAIL and/or its cognate receptors, this event is not necessary to mediate synergistic apoptosis of target cells by using a combination of HDACi and recombinant TRAIL or anti-DR4/DR5 mAbs (33). Other studies have correlated synergistic tumor cell apoptosis by HDACi and activators of the TRAIL pathway with decreased expression of c-FLIP (8, 25), decreased expression of apoptosis inhibitors and Bcl-2 (25), inhibition of Cdc2 protein and subsequent down-regulation of survivin and XIAP (26), and modulation of NF-κB activity (28, 34, 35). It is not yet clear whether a common molecular event will be identified as being requisite to mediate synergistic apoptosis by HDACi and TRAIL activators, or whether the responses will depend on the cell type and/or HDACi under investigation.
Using a mouse mammary tumor model that spontaneously metastasizes and mimics human breast cancer, we have shown that combining the HDACi vorinostat with an agonistic mAb that specifically targets the DR5 death receptor results in synergistic apoptosis in vitro and in vivo and concomitant tumor regression. Moreover, such a combination therapy regimen was well tolerated. We demonstrated that activation of the death-receptor pathway, but not the intrinsic apoptotic pathway, was necessary for the apoptotic and therapeutic effects of the vorinostat/MD5-1 combination, and that the vorinostat-mediated down-regulation of c-FLIP observed in vitro and in vivo likely plays an important role in mediating the synergy. Our data imply that such combination therapies using HDACi and TRAIL or anti-DR4/DR5 mAbs are likely to be efficacious and may be delivered safely to patients. Overexpression of prosurvival Bcl-2 family proteins can inhibit the apoptotic activities of many chemotherapeutic agents (36), and our data demonstrating that a combination of vorinostat and MD5-1 can overcome such an effect raises the possibility that such combination treatments may be efficacious against tumors resistant to conventional chemotherapy regimens.
Materials and Methods
Apoptosis Assays.
Cells (2.5 × 104) were cultured in the presence of either Vorinostat (48-well plate, Greiner), plate-bound MD5-1, or in combination (96-well Protein A coated plates, Pierce). Cells were washed twice in PBS, labeled with allophycocyanin-conjugated annexin V (BD PharMingen) in the presence of propidium iodide (PI), and immediately analyzed by flow cytometry.
Therapy of Transplanted Tumors.
4T1.2 (7 × 104) or Renca (2 × 105) cells were injected s.c. into the hind flank of BALB/c or SCID mice. 4T1.2/MSCV, 4T1.2/Bcl-2, 4T1.2/CrmA, or 4T1.2/c-FLIP cells (7 × 104) were injected s.c. into the hind flank of SCID mice. Treatment commenced when tumors developed to a size of 9 mm2 (≈5–6 d after tumor inoculation). Mice were treated with Vorinostat (100 mg/kg/day i.p.) and/or with 50 μg MD5-1 or control antibody 4 times every 4 d. Tumor size was measured every 2–3 d, and data are represented as the mean ± SEM of at least six mice in each group.
Assessment of In Situ Apoptosis.
Established s.c. 4T1.2 tumors (≈9 mm2) were treated with vehicle, vorinostat (100 mg/kg), MD5-1 (50 μg), or a combination of both. Tumors were harvested 4, 8, 12, and 24 h after therapy, fixed in 10% neutral buffered formalin, and paraffin embedded. TUNEL positivity was assessed using the ApopTag Peroxidase in situ Apoptosis Detection Kit (Chemicon, #S7100).
For additional materials and methods, see SI Text
Supplementary Material
Acknowledgments.
We thank Dr. Victoria Richon (Merck) for help and advice on the project. R.W.J. is a Pfizer Australia Research Fellow and is supported by the National Health and Medical Research Council (NHMRC) Program Grant 251608, Cancer Council Victoria, Leukemia Foundation of Australia, and a research grant from Merck. A.J.F. is supported by The Cancer Research Institute Predoctoral Emphasis Pathway in Tumor Immunology. M.J.S. is a Senior Principal Research Fellow of the NHMRC and is supported by the Susan G. Komen Breast Cancer Foundation. K.T. and H.Y. are supported by the Ministry of Education, Science and Culture, Japan.
Footnotes
Conflict of interest statement: This work was supported in part by a collaborative research grant provided by Merck and Co. to the Johnstone laboratory and the Peter MacCallum Cancer Centre.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801868105/DCSupplemental.
References
- 1.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 2.Rasheed WK, Johnstone RW, Prince HM. Histone deacetylase inhibitors in cancer therapy. Expert Opin Investig Drugs. 2007;16:659–678. doi: 10.1517/13543784.16.5.659. [DOI] [PubMed] [Google Scholar]
- 3.Lindemann RK, Gabrielli B, Johnstone RW. Histone-deacetylase inhibitors for the treatment of cancer. Cell Cycle. 2003;3:779–788. [PubMed] [Google Scholar]
- 4.Lindemann RK, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci USA. 2007;104:8071–8076. doi: 10.1073/pnas.0702294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insinga A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 6.Nebbioso A, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Okamoto K, Yonehara S. Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through down-regulation of cellular FLIP. Cell Death Differ. 2005;12:10–18. doi: 10.1038/sj.cdd.4401507. [DOI] [PubMed] [Google Scholar]
- 8.El-Zawahry A, Lu P, White SJ, Voelkel-Johnson C. In vitro efficacy of AdTRAIL gene therapy of bladder cancer is enhanced by trichostatin A-mediated restoration of CAR expression and down-regulation of cFLIP and Bcl-XL. Cancer Gene Ther. 2006;13:281–289. doi: 10.1038/sj.cgt.7700905. [DOI] [PubMed] [Google Scholar]
- 9.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: Decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 11.Schneider P, et al. Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Biol Chem. 2003;278:5444–5454. doi: 10.1074/jbc.M210783200. [DOI] [PubMed] [Google Scholar]
- 12.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pukac L, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgakis GV, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: Induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130:501–510. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolcher AW, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 18.Plummer R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Peart MJ, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernhard D, et al. Inhibition of histone deacetylase activity enhances Fas receptor-mediated apoptosis in leukemic lymphoblasts. Cell Death Differ. 2001;8:1014–1021. doi: 10.1038/sj.cdd.4400914. [DOI] [PubMed] [Google Scholar]
- 22.Jo M, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 23.Corazza N, et al. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koschny R, et al. Bortezomib-mediated up-regulation of TRAIL-R1 and TRAIL-R2 is not necessary for but contributes to sensitization of primary human glioma cells to TRAIL. Clin Cancer Res. 2007;13:6541–6542. [Google Scholar]
- 25.Guo F, et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 2004;64:2580–2589. doi: 10.1158/0008-5472.can-03-2629. [DOI] [PubMed] [Google Scholar]
- 26.Kim EH, et al. Sodium butyrate sensitizes human glioma cells to TRAIL-mediated apoptosis through inhibition of Cdc2 and the subsequent down-regulation of survivin and XIAP. Oncogene. 2005;24:6877–6889. doi: 10.1038/sj.onc.1208851. [DOI] [PubMed] [Google Scholar]
- 27.Neuzil J, Swettenham E, Gellert N. Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: Role of Bcl-xL down-regulation. Biochem Biophys Res Commun. 2004;314:186–191. doi: 10.1016/j.bbrc.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 28.Singh TR, Shankar S, Srivastava RK. HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene. 2005;24:4609–4623. doi: 10.1038/sj.onc.1208585. [DOI] [PubMed] [Google Scholar]
- 29.Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003;2:1273–1284. [PubMed] [Google Scholar]
- 30.Nakata S, et al. Histone deacetylase inhibitors up-regulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23:6261–6271. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- 31.Earel JK, Jr, VanOosten RL, Griffith TS. Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res. 2006;66:499–507. doi: 10.1158/0008-5472.CAN-05-3017. [DOI] [PubMed] [Google Scholar]
- 32.Butler LM, et al. The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL. Int J Cancer. 2006;119:944–954. doi: 10.1002/ijc.21939. [DOI] [PubMed] [Google Scholar]
- 33.Inoue S, Twiddy D, Dyer MJ, Cohen GM. Up-regulation of TRAIL-R2 is not involved in HDACi mediated sensitization to TRAIL-induced apoptosis. Cell Death Differ. 2006;13:2160–2162. doi: 10.1038/sj.cdd.4401977. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmikanthan V, Kaddour-Djebbar I, Lewis RW, Kumar MV. SAHA-sensitized prostate cancer cells to TNFalpha-related apoptosis-inducing ligand (TRAIL): Mechanisms leading to synergistic apoptosis. Int J Cancer. 2006;119:221–228. doi: 10.1002/ijc.21824. [DOI] [PubMed] [Google Scholar]
- 35.Shetty S, et al. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol Cell Biol. 2005;25:5404–5416. doi: 10.1128/MCB.25.13.5404-5416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.