Abstract
Parkinson's disease (PD) is a common neurodegenerative disorder thought to be associated with mitochondrial dysfunction. Loss of function mutations in the putative mitochondrial protein PINK1 (PTEN-induced kinase 1) have been linked to familial forms of PD, but the relation of PINK1 to mammalian mitochondrial function remains unclear. Here, we report that germline deletion of the PINK1 gene in mice significantly impairs mitochondrial functions. Quantitative electron microscopic studies of the striatum in PINK1−/− mice at 3–4 and 24 months revealed no gross changes in the ultrastructure or the total number of mitochondria, although the number of larger mitochondria is selectively increased. Functional assays showed impaired mitochondrial respiration in the striatum but not in the cerebral cortex at 3–4 months of age, suggesting specificity of this defect for dopaminergic circuitry. Aconitase activity associated with the Krebs cycle is also reduced in the striatum of PINK1−/− mice. Interestingly, mitochondrial respiration activities in the cerebral cortex are decreased in PINK1−/− mice at 2 years compared with control mice, indicating that aging can exacerbate mitochondrial dysfunction in these mice. Furthermore, mitochondrial respiration defects can be induced in the cerebral cortex of PINK1−/− mice by cellular stress, such as exposure to H2O2 or mild heat shock. Together, our findings demonstrate that mammalian PINK1 is important for mitochondrial function and provides critical protection against both intrinsic and environmental stress, suggesting a pathogenic mechanism by which loss of PINK1 may lead to nigrostriatal degeneration in PD.
Keywords: neurodegeneration, PARK6, genetic Parkinson's disease, knockout, parkinsonism
Parkinson's disease (PD) is the second most common neurodegenerative disorder affecting >1% of the population >65. The manifestations of the disease are caused by the degeneration of dopaminergic neurons in the substantia nigra pars compacta. The aetiology of the disease is still unknown but probably involves a combination of genetic and environmental factors. A growing number of clinical and experimental reports implicate mitochondrial dysfunctions and oxidative stress in the pathogenesis of PD (1, 2). Mitochondria isolated from PD patients display a reduced complex I activity and an increased capacity to produce reactive oxygen species (ROS) (3, 4). Accidental exposure to mitochondrial toxins leads to symptoms resembling those of PD via the selective death of dopaminergic neuron (5, 6).
The recent discovery of recessively inherited loss of function mutations in three distinct genes that are linked to early onset parkinsonism (7–9) has provided a unique opportunity to create in vivo models to identify early pathogenic changes, which may ultimately lead to neurodegeneration (10). Among these genes, PTEN-induced kinase 1 (PINK1) offers the most obvious link between mitochondrial dysfunctions and the disease. Bioinformatics analysis reveals that PINK1 is a putative serine-threonine kinase with a mitochondrial targeting motif at its N-terminal end (9). PINK1 has been localized in mitochondrial fractions, although it is not yet clear whether it is localized within the outer (11) or the inner membrane (12, 13). Given its probable subcellular localization, it is reasonable to think that PINK1 plays a role in the normal biology of mitochondria. In fact, two recent articles showed dramatic functional and structural impairments of mitochondria in Drosophila bearing null mutations for dPINK1 (14, 15). These impairments include fragmented cristae, loss of outer membrane, ATP depletion, and are accompanied with dopaminergic neuronal degeneration in one study (14). More recently, PINK1 has been shown to interact genetically with proteins involved in mitochondrial morphogenesis and specifically promotes mitochondrial fission (16, 17). However, questions remained whether the dramatic phenotypes observed in mutant flies would be conserved in a mammalian model carrying null mutations in the PINK1 gene.
Mutations in the PINK1 gene are recessively inherited and some of the missense mutations have been reported to be associated with decreased kinase activities, suggesting a loss-of-function pathogenic mechanism (18, 19). To study the function of PINK1 in mice, our group has generated PINK1 knockout mice, which exhibit deficits in evoked dopamine release and subsequent striatal synaptic plasticity impairment in the absence of dopaminergic neuronal degeneration (20). In the current study, we focus on the potential role of PINK1 in mitochondrial function and structural integrity. We find that loss of PINK1 function in mice does not cause major ultrastructural changes in their mitochondria, but instead leads to functional deficits in a dopaminergic circuit- and age- specific manner with increased sensitivity to oxidative stress.
Results
No Gross Structural Defects in Mitochondria of PINK1−/− Mice but Increased Numbers of Larger Mitochondria.
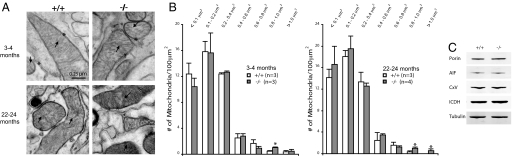
Striking morphological defects have been reported for mitochondria derived from indirect flight muscles in dPINK1−/− flies (14, 15). We therefore investigated whether such abnormalities would be conserved in a mammalian model. At 3–4 months of age, electron micrographs showed clearly defined, intact cristae and outer membranes in mitochondria derived from the striatum of both PINK1−/− and control mice (n = 3) [Fig. 1A and supporting information (SI) Fig. S1A]. Quantitative analysis revealed normal numbers of mitochondria in PINK1−/− striata (Fig. S1B). Citrate synthase activity, a common quantitative marker for intact mitochondria in tissue, confirmed unchanged levels of mitochondria in the PINK1−/− striatum (Fig. S1C). Although the average size of mitochondria also appears to be normal (data not shown), there is an increase in the number of larger mitochondria in the striatum of PINK1−/− mice (size bin of 0.8–1 μm2: +/+: 0.44 ± 0.11; −/−: 1.0 ± 0.10, expressed as the number of mitochondria per 100 μm2, n = 3, P < 0.05, Fig. 1B). We further looked for an effect of aging on the structure of mitochondria in 24-month-old PINK1−/− mice, and found that cristae and outer membrane structures appear normal in these aged PINK1−/− mice (Fig. 1A and Fig. S1D). Although the average size and the total number of mitochondria are unchanged (Fig. S1E), we found a similar increase in the number of larger mitochondria in two of the largest size bins in PINK1−/− mice (>1 μm2: +/+: 0.00; −/−: 0.57 ± 0.2, n = 4, P < 0.05; 0.8–1 μm2 +/+: 0.33 ± 0.15; −/−: 1.03 ± 0.17, P < 0.025, Fig. 1B).
Fig. 1.
Normal ultrastructure but increased numbers of larger mitochondria in the striatum of PINK1−/− mice. (A) Absence of gross ultrastructural defects in mitochondria of the PINK1−/− striatum at 3–4 (upper panels) and 24 (lower panels) months. Arrows indicate the presence of clearly defined and intact cristae. Asterisks show the presence of outer membranes. (B) Distribution of mitochondria in different size bins reveals increased numbers of larger mitochondria in the PINK1−/− striatum at 3–4 and 24 months (*, P < 0.05). (C) Western blot analysis of mitochondrial marker proteins shows normal levels of porin (for outer membrane), AIF (for intermembrane space), Complex V (CxV for inner membrane) and ICDH (for matrix) in the PINK1−/− striatum. Bands shown are representative of a total of 4 samples per genotype.
We also sought to use stable mitochondrial proteins as quantitative markers for mitochondrial integrity. In young PINK1−/− mice, western blot analysis showed normal levels of porin, apoptosis inducing factor (AIF), complex V (CxV), and isocitrate dehydrogenase (ICDH), which are markers for the outer membrane, intermembrane space, inner membrane and matrix, respectively (Fig. 1C). These results further confirmed the lack of gross mitochondrial defects in the striatum of PINK1−/− mice.
Impaired Mitochondrial Respiration in the Striatum of PINK1−/− Mice.
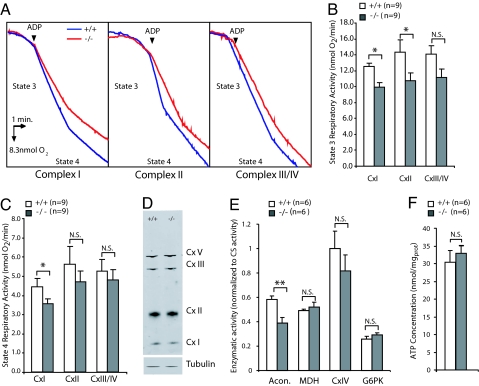
The absence of major structural or quantitative changes in PINK1−/− mice led us to explore functional aspects of mitochondrial biology. Respiration is the major function of mitochondria, providing energy to cells in the form of ATP. We measured the respiratory capacity of mitochondrial crude preparations using a polarographic method with substrates specific for each complex (glutamate/malate, succinate and TMPD/ascorbate for complex I, complex II and complex III/IV, respectively). We measured state 3, which represents the maximum respiration rate in the presence of ADP, and state 4, which represents O2 consumption by leakage of protons through the inner membrane after ADP exhaustion.
We first turned our attention to the striatum, because it contains abundant dopaminergic terminals with high concentrations of mitochondria. In addition, it is anatomically well defined and can be easily dissected to provide sufficient amounts of materials, whereas the substantia nigra pars compacta, in which dopaminergic neurons reside, is technically challenging to separate from other ventral midbrain nuclei and provides insufficient material for mitochondrial isolation. Interestingly, we found that state 3 activities for both complex I (+/+: 12.5 ± 0.4; −/−: 9.9 ± 0.6, n = 6, P < 0.01) and complex II (+/+: 14.4 ± 1.4; −/−: 10.78 ± 1.0, n = 9, P < 0.05) are significantly decreased in PINK1−/− mice, whereas complex III activity appear to be slightly lower as well (+/+: 14.1 ± 1.1; −/−: 11.2 ± 1.0, n = 9, P = 0.085, Fig. 2 A and B). The reductions in state 4 respiratory activities are less marked in PINK1−/− mice (Fig. 2C), although its activity for complex I is significantly reduced (+/+: 4.9 ± 0.4; −/−: 3.9 ± 0.2, n = 6, P < 0.05). Western blot analysis showed normal levels of complex I, II and III in the striatum of PINK1−/− mice (Fig. 2D). Thus, loss of PINK1 affects the functional capacity of these mitochondrial complexes but not their expression levels.
Fig. 2.
Impaired mitochondrial respiration in the PINK1−/− striatum. (A) Representative oxygraphs of striatal mitochondria preparations for complex I, complex II and complex III/IV at 3–4 months of age. After injection of limiting amounts of ADP (indicated by arrows) state 3 was measured. After exhaustion of ADP, O2 consumption slowed down representing state 4 of respiration. (B) State 3 respiratory activity was reduced for both complex I (n = 6) and complex II (n = 9) in PINK1−/− mice. (C) State 4 respiratory activity was reduced for complex I in PINK1−/− mice (n = 6) (*, P < 0.05). (D) Unchanged levels of complex I, complex II and complex III in the striatum at 3–4 months as measured by western blot. (E) Enzymatic activity of aconitase (acon.), malate dehydrogenase (MDH), cytochrome c oxidase (CxIV) and glucose-6-phosphate kinase (G6PK) measured from striatal lysates are shown as normalized to the citrate synthase activity. Aconitase activity was significantly lower in the PINK1−/− striatum (**, P < 0.005, n = 6), whereas other mitochondrial enzyme activities were unaffected. (F) Striatal ATP concentrations are similar in the striatum of PINK1−/− and wild-type mice.
Reduced Aconitase Activity in the Striatum of PINK1−/− Mice.
Aconitase, a key enzyme in the Krebs cycle, has recently been reported to be functionally impaired in DJ-1−/− mice (21). We explored the possibility that this defect might be shared with PINK1−/− mice. Using spectrophotometric methods, we found a significant 35% decrease of aconitase enzymatic activity in the striatum of PINK1−/− mice (+/+: 0.58 ± 0.03; −/−: 0.39 ± 0.04, normalized to the citrate synthase activity, P < 0.005, n = 6, Fig. 2E). Because it bears an iron sulfide cluster in its structure, aconitase, as well as complex I and complex II, are known to be sensitive to oxidative stress (22). In contrast, mitochondrial enzymatic activities known to be less sensitive to stresses, such as CS (Fig. S1C), cytochrome C oxidase (complex IV) and malate dehydrogenase (MDH) (Fig. 2E), are unaffected by the loss of PINK1 activity. Glucokinase, a nonmitochondrial protein known to be highly sensitive to oxidative stresses, is also unchanged in PINK1−/− mice (Fig. 2E), suggesting that the protection provided by PINK1 against oxidative stress might be limited to the mitochondrion.
To assess the impact of these functional deficits on the overall energy supply of the cell, we measured the level of ATP in the striatum by using the luciferine/luciferase assay. Absence of PINK1 does not lead to decreased ATP concentrations (+/+: 30.4 ± 3.2, −/−: 32.9 ± 3.2 nmol/mg, n = 6, P > 0.05, Fig. 2D). Therefore, the functional deficiencies caused by loss of PINK1 appear to be insufficient to create an overall energy crisis.
Age Dependant Impairment of Mitochondrial Respiration in the Cerebral Cortex of PINK1−/− Mice.
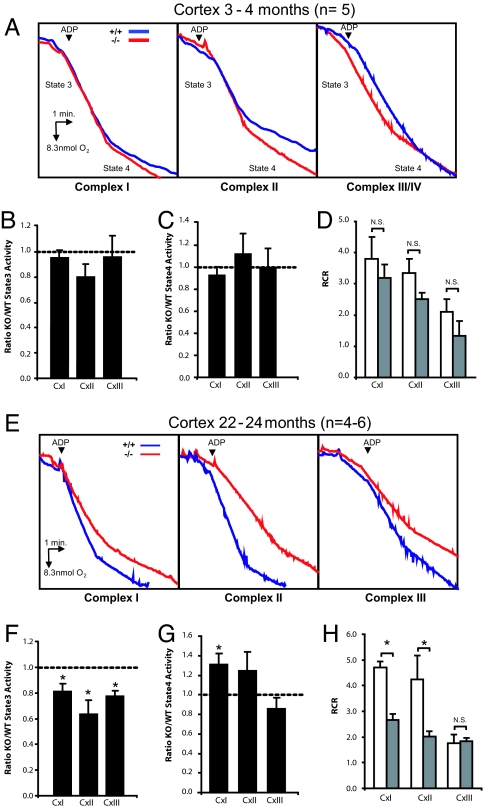
Although degeneration of dopaminergic neurons is a cardinal feature of PD, neurodegeneration is present in other brain subregions as well (23–25). We therefore examined whether loss of PINK1 function affects mitochondrial respiration in other brain regions, such as the cerebral cortex, which provides more abundant materials for isolating mitochondria than the striatum. To our surprise, at 3–4 months, state 3 activities for cortical mitochondria from PINK1−/− mice are not significantly altered for complex I (97.2 ± 6.4% of WT activity; n = 5, P > 0.05), complex II (81.5 ± 11.0 of WT activity, n = 5, P > 0.05) and complex III/IV (97.5 ± 17.5% of WT activity, n = 5, P > 0.05, Fig. 3 A and B), compared with wild-type (WT) mice. Similarly we were unable to observe any differences in state 4 activities for all 3 complexes (92.0 ± 7.8, 111.5 ± 17.8 and 99.0 ± 16.4% of WT activity for complex I, complex II and complex III/IV, respectively, n = 5, P > 0.05, Fig. 3C). These results indicate that mitochondrial respiration in the striatum is more sensitive to the loss of PINK1 function than in the cerebral cortex, possibly due to the abundance of dopaminergic terminals in the striatum.
Fig. 3.
Age-dependent impairment of mitochondrial respiration in the PINK1−/− cortex. (A) Representative oxygraphs of cortical mitochondria preparations for complex I, complex II and complex III/IV at 3–4 months of age. (B–D) State 3 (B) and state 4 (C) respiratory activities as well as respiratory control ratio (RCR) (D) were normal in the PINK1−/− cortex for all complexes. (E) Representative oxygraphs of cortical mitochondria preparation for complex I, complex II and complex III/IV at 22–24 months of age. (F) State 3 respiratory activity was reduced for all three complexes in the PINK1−/− cortex (*, P < 0.05, n = 4–6). (G) Contrary to state 3, state 4 respiratory activity was increased for complex I in the PINK1−/− cortex. (H) RCR is reduced for complex I and complex II in the PINK1−/− cortex.
Aging represents by far the greatest risk factor for idiopathic PD. To determine whether aging contributes to mitochondrial dysfunctions in PINK1−/− mice, we assayed the metabolic functions of cortical mitochondrial preparations at 22–24 months. We found that state 3 activities are impaired for complex I (81.3 ± 6.7% of WT activity, n = 4–6, P < 0.05), complex II (66.6 ± 10.2%, n = 4–6, P < 0.05) and complex III/IV (77.1 ± 4.4%, n = 4–6, P < 0.01, Fig. 3 E and F) in PINK1−/− mice. Unexpectedly, these differences are not reflected by decreases in state 4 activities but, on the contrary, by increased activities for complex I (131.0 ± 11.4 of WT activity, n = 4–6, P < 0.05, Fig. 3G). One possible explanation is that, at older ages, the integrity of the mitochondrial inner membrane is compromised, allowing more protons to reenter the matrix in the absence of ADP. This explanation is consistent with our observation of a significantly reduced respiratory control ratio in PINK1−/− mice (+/+: 4.61 ± 0.23; −/−: 2.67 ± 0.22, n = 4–6, P < 0.001, Fig. 3H).
Unchanged Levels of Oxidative Stress Markers in PINK1−/− Mice.
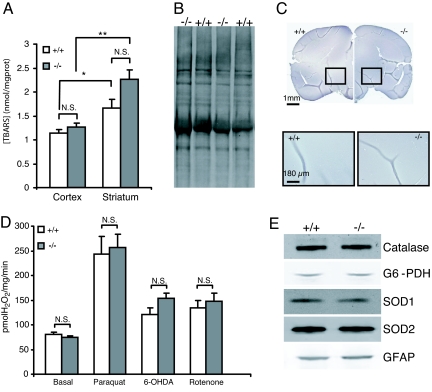
Complexes I and II as well as aconitase, which displayed reduced activities in PINK1−/− mice, are known to be sensitive to oxidative stress. To determine whether these defects are associated with increased oxidative stress, we analyzed both genotypic groups for accumulation of oxidative stress markers. We first assessed levels of thiobarbituric acid reactive species (TBARS), a marker of lipid peroxidation, in cortical and striatal mitochondrial preparations at 3–4 months. In both preparations we were unable to detect any differences between the genotypes (Fig. 4A). However, independent of the genotype, there is a significant increase in the striatum (1.95 ± 0.17 nmol/mg, n = 8, P < 10−3) relative to the cortex (1.21 ± 0.05 nmol/mg, n = 16), consistent with the presence of higher oxidative stress in the striatum due to dopamine metabolism.
Fig. 4.
Unchanged levels of oxidative stress markers in PINK1−/− mice. (A) Levels of lipid peroxidation in the mitochondrial fraction in the striatum and cortex measured by the TBARS assay were not significantly (N.S.) different between PINK1−/− and wild-type mice at 2–3 months (n = 6–10 for the cortex, P > 0.05; n = 4 for the striatum, P > 0.05; two-way ANOVA followed by Tukey's test). However, independently of genotype, there was significantly more lipid peroxidation in the striatum compared with the cortex (*, P < 0.025; **, P < 10−3). (B) Oxyblot analysis of striatal lysates at 22–24 months showed similar levels of protein carbonyls in both genotypes. Lanes shown are representative of a total of 6 samples per genotype. (C) 4HNE staining of the entire brain (Upper) and the substantia nigra (Lower) at 22–24 months appeared similar in both genotypes. (D) ROS production as measured by the Amplex red dye assay. Both genotypic groups showed similar ability to produce ROS either under basal conditions or in the presence of the ROS production enhancing toxins, paraquat, 6-OHDA and rotenone. (E) Western blot analysis of antioxidant proteins (Catalase, G6-PDH, SOD1 and 2) and GFAP in the striatum at 22–24 months. Representative results from six PINK1−/− and five wild-type mice are shown. Densitometric analysis shows no significant differences for all proteins tested.
Respiration deficiency in idiopathic PD brains has been associated with increased oxidation of complex I subunits (24). Therefore, we measured the level of protein carbonyls, a marker of protein oxidation. As measured by oxyblot, the total level of carbonyls in the striatum at 24 months was similar between PINK1−/− and control mice (n = 6, Fig. 4B). We then tested for the accumulation of another common marker of protein oxidation, 4-hydroxynonenal Michael adducts (4-HNE). Immunostaining of brain sections from PINK1−/− mice at 24 months failed to show any increases of protein oxidation in various brain subregions including the substantia nigra (Fig. 4C). Inflammation is a common feature of neurodegeneration, and is known to be present in PD patients and animal models (26). Using the presence of reactive astrocytes as a marker of inflammation, we compared brain sections of PINK1−/− and control mice at 22–24 months. No obvious difference in reactive astrocytes labeled by GFAP immunoreactivity was detected in the cortex and the SN between PINK1−/− and control mice (data not shown). This result was further confirmed quantitatively in the striatum at 24 months by western blot (Fig. 4E).
Mitochondria are the main site for production of reactive oxygen species (ROS) in the cell. It has been reported recently that the deletion of DJ-1 in mice enhances the capacity of mitochondria to produce ROS (21). Using the Amplex red dye fluorescence assay, we measured the capacity of isolated mitochondria to create H2O2. Although we were able to reproduce the result of increased ROS production in cortical mitochondria isolated from the cortex of DJ-1−/− mice (data not shown), we found that the loss of PINK1 does not lead to the same increased capacity in the striatum (Fig. 4D) or in the cortex (data not shown) under basal conditions or after administration of mitochondrial toxins, such as paraquat, 6-OH, dopamine and rotenone, which are known to enhance ROS production (Fig. 4D). The absence of increased oxidative markers could be due to compensatory mechanisms such as overexpression of antioxidant proteins. To test this hypothesis, we measured the levels of Mn-SOD, CuZn-SOD, Catalase and G6-PDH. No significant differences were detected in the striatum of PINK1−/− and control mice at 22–24 months (Fig. 4E).
Increased Sensitivity of Cortical Mitochondria to Exogenous Stressors in PINK1−/− Mice.
Given the absence of increases in oxidative stress markers in PINK1−/− mice, we tested whether in the absence of PINK1, mitochondrial functions are more sensitive to environmental stressors (27). We compared both genotypic groups for the effects of oxidative conditions on cortical mitochondrial respiration at 3–4 months. Under oxidative conditions (15-min preincubation of isolated mitochondria at 25°C with 100 μM H2O2), reduction of state 3 respiration activity was more pronounced in PINK1−/− mice than in their wild-type littermates for complex I (+/+: 80.4 ± 7.4; −/−: 57.6 ± 2.3, n = 6, P = 0.02, Fig. 5A). The same observation was made for state 3 respiratory activity of complex II (+/+: 65.7 ± 4.7; −/−: 49.8 ± 4.9, n = 9, P = 0.03, Fig. 5B).
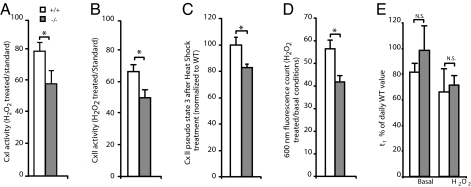
Fig. 5.
Increased sensitivity of cortical mitochondria to oxidative stress in PINK1−/− mice. (A) The reduction of state 3 respiration by complex I after 15 min of exposure to 100 μM H2O2 was more dramatic in mitochondria isolated from the PINK1−/− cortex at 3–4 months than from the control (*, P < 0.05, n = 5). Data are expressed as a percentage of the activity of the same sample measured previously in standard conditions. (B) Effect of 15 min of exposure to 500 μM H2O2 on complex II respiratory capacity of cortical mitochondrial preparation at 3–4 months. (*, P < 0.05, n = 9). Data are expressed as a percentage of the activity of the same sample measured previously in standard conditions. (C) Effect of exposure to a mild heat shock (10 min at 43°C before assay) on complex II respiratory capacity of cortical mitochondria preparation at 3–4 months. Data are normalized to WT activity under standard conditions (*, P < 0.05, n = 6). (D) Measure of the transmembrane potential of isolated mitochondria at 3–4 months after 15 min of exposure to 100 μM H2O2 by the JC-1 assay. (E) Mitochondria swelling assay. Data are fitted as the sum of exponentials. τ1 is given as the time constant of the first and main exponential. No difference between genotype was seen under basal conditions or after 15 min of exposure to 200 μM H2O2 (N.S.: not significant, n = 4).
We further examined whether a mild heat shock would influence metabolic activities. Mitochondria were incubated at 43°C before measuring respiration activities. The treatment probably affects the mitochondrial membrane integrity, thus we were not able to distinguish clearly between state 3 and state 4. We therefore measured the activity immediately after the addition of ADP and report it as a “pseudo state 3”. After treatment, succinate driven activity is significantly reduced in PINK1−/− mice (82.0 ± 3.0% of WT activity, n = 6, P < 0.05, Fig. 5C). To further confirm these results, we measured the effect of oxidative stress on mitochondrial transmembrane potential, which constitutes the driving force for proton to reenter the matrix. After 15 min of treatment with 100 μM H2O2, Δψ of PINK1−/− mitochondria energized with succinate are significantly reduced (+/+: 56.4 ± 3.9%; −/−: 41.7 ± 3.0 of Δψ under basal conditions, n = 4, P < 0.05, Fig. 5D).
It has been shown that under conditions of stress, cysteines of the mitochondrial permeability transition pore (MPT pore) undergo oxidation, thus increasing the probability for the pore to open and collapse the transmembrane potential (28). To test whether the observed Δψ collapse was due to an increased probability of the MPT pore opening, we performed the swelling assay. Mitochondrial preparations were provided with high concentrations of Ca2+, which leads to the opening of the MPT pore, accumulation of water inside the mitochondria and ultimately disruption of mitochondrial membranes. Under basal or stress (200 μM H2O2) conditions, we were unable to differentiate between genotypes for their swelling time constant (Fig. 5E). Together, our results show that independently of the MPT pore regulation, mitochondria lacking PINK1 are more sensitive to various stress conditions.
Discussion
In the present study, we report that loss of PINK1 results in mitochondrial dysfunctions in mice. In contrast to the severe structural defects found in the fly models, mitochondria in PINK1−/− mice appear to be structurally intact and preserved in total number, although there is a selective increase in larger mitochondria, which would be consistent with a role of PINK1 in the promotion of mitochondrial fission (Fig. 1, Fig. S1). Mitochondrial respiration, a key function of the organelle, is impaired in PINK1−/− mice. Interestingly, this functional deficit is present in a brain region-specific manner in young PINK1−/− mice with the impairment present in the striatum, which is rich in dopaminergic terminals, but absent in the cerebral cortex (Fig. 2), suggesting that loss of PINK1 and elevated oxidative stress associated with dopamine metabolism may serve as “two hits” for mitochondrial dysfunction observed in the striatum of PINK1−/− mice. Furthermore, at 2 years of age mitochondrial respiration in the cerebral cortex of PINK1−/− mice is impaired (Fig. 3), indicating that aging can also serve as the “second hit” to exacerbate mitochondrial dysfunction. Lastly, mitochondrial respiration defects can be induced in the cerebral cortex of young PINK1−/− mice by environmental cellular stress, such as exposure to H2O2 or mild heat shock (Fig. 5), again providing support for the “second hit” role of extrinsic oxidative stress and other cellular insults in causing mitochondrial dysfunction. Together, our findings demonstrate a key role of mammalian PINK1 in mitochondrial function. Specifically, PINK1 protects mitochondria from both intrinsic stress (e.g., dopamine metabolism and aging) and environmental insults (e.g., H2O2 and heat shock).
Mitochondrial defects in PD patients were reported for the first time ≈20 years ago (3). Accumulating evidence suggest that mitochondrial defects might be causal to the pathogenesis of PD (2, 10). The presence of mitochondrial defects in mice carrying PINK1 germline deletions, which genetically recapitulate pathogenic mutations in PINK1-linked PD patients, provides further support for this notion. Actually, mitochondrial respiration defects similar to what we have found in PINK1−/− mice have been reported in other genetic mouse models of PD, such as parkin−/− mice (29, 30), and in fibroblasts derived from patients bearing PINK1 pathogenic mutations (31). However, the presence of complex II and complex III/IV defects are relatively novel in a PD mouse model, although they have been previously reported for mitochondria isolated from PD patients (23, 32, 33). It is also worth noting that these functional defects are relatively subtle and fail to create a major energy crisis as measured by ATP levels. Perhaps a more elaborate compensatory mechanism in mice provides a better protection for mitochondria from more severe damage due to loss of PINK1 or parkin.
Interestingly, our results showed brain subregion specificity and age dependence of mitochondrial defects in PINK1−/− mice. Region specificity for respiration defects has also been reported previously in parkin−/− mice (29). This cannot be explained by differential expression of PINK1, because it appears to be expressed quite uniformly across all brain subregions (34). Thus, the most likely explanation for the striatum specificity of the mitochondrial defects observed in PINK1−/− mice is that the striatum receives nigrostriatal dopaminergic projections and contains high concentrations of dopamine, which can degrade into cytotoxic oxyradicals and inhibit pyruvate/malate respiration (35). Indeed, we observed that mitochondria isolated from the striatum contain higher levels of lipid peroxidation markers compared with that of the cortex (Fig. 4A), providing further support for this interpretation. By two years of age, mitochondrial respiration is impaired in the cerebral cortex of PINK1−/− mice, perhaps due to the accumulation of oxidative insults accompanied with the aging process. Loss of PINK1 may undermine protective mechanisms against such intrinsic, physiological cellular stress.
In addition, PINK1−/− mice display reduced activities of complex I, complex II and aconitase. All three are present within the mitochondrion and share a high sensitivity to oxidative stress. This sensitivity is explained by the presence of Fe2S4 clusters in their three dimensional structures. These clusters are structurally and functionally essential to these protein complexes, and are extremely sensitive to oxidative stress. On the other hand, the more stable mitochondrial enzymatic activities, such as citrate synthase, Cytochrome C oxidase or malate dehydrogenase, are unaffected by PINK1 deletion. Hypersensitivity to oxidative stress is further supported by the observation that metabolic defects can be induced in mitochondria isolated from the cerebral cortex of young PINK1−/− mice by exposure to environmental agents for oxidative stress, such as hydrogen peroxide or a mild heat shock. These results are consistent with recent findings in HEK 293 cells (36).
Despite the mitochondrial functional defects and the increased sensitivity to oxidative stress, we did not detect an increase in markers of oxidative stress in PINK1−/− mice. This could be due to the higher sensitivity of the enzymatic activities measured by the functional assays, in contrast to the direct measurement of the accumulating levels of oxidized lipid or protein products. How can one explain such a protective role of PINK1 in mitochondria? One possibility, raised by studies in flies, is that PINK1 is involved in the regulation of mitochondrial fission and fusion (16, 17). Our finding that there is an increase in larger mitochondria in the striatum of PINK1−/− mice is consistent with a role of PINK1 in promoting mitochondrial fission. Although these mitochondria account for only a small percentage of total mitochondria (≈3%) in PINK1−/− mice, they represent a more significant percentage of total mitochondrial volume. It remains to be determined how promotion of mitochondrial fission by PINK1 would protect mitochondria from intrinsic and environmental insults and whether the mitochondrial defects underlie the impairment of dopamine release and striatal synaptic plasticity in PINK1−/− mice (20). In another scenario, PINK1 could protect mitochondria via the activation of mitochondrial chaperonin. TRAP1 and HtrA2, two mitochondrial proteins with chaperone functions, have been identified as putative downstream effectors for PINK1 (13, 36). Our respiration assay of isolated mitochondria in the presence of stressors supports a direct protective role of PINK1. Further investigation will be needed to elucidate the exact mechanism by which PINK1 protects mitochondrial function.
In summary, our study shows in a mammalian model that the putative mitochondrial protein PINK1 indeed plays an important role in the maintenance of basic functions of mitochondria. The absence of dopaminergic neurodegeneration in PINK1−/− mice further supports the notion that mitochondrial defects may represent a causal and early pathogenic event of PD. Understanding how they lead to greater cellular damages and ultimately neurodegeneration will provide tremendous insight into the pathogenesis of PD.
Materials and Methods
Mitochondrial Respiration Assay.
Mitochondrial respiration assay was performed as previously described (30).
Mitochondrial ROS Production Assay.
ROS production was measured as the increase in fluorescence of Amplex Red dye (Invitrogen). Cortical and striatal mitochondria preparations were made as previously described adjusted to 1 mg/ml in OEB buffer (0.25M sucrose, 5 mM Mops pH 7.4, 5 mM KH2PO4, 5 mM MgCl2). 15 μl of this preparation was placed in a well and the reaction was started by the addition of 100 μl of assay buffer (10 μM Amplex Red, 10 mM succinate, 0.2 units/ml HRP in OEB) and followed over time on a fluorescence plate reader.
Electron Microscopy, Enzymatic Assays, and Western Analysis.
Statistical Analysis.
Pooled results were expressed as means ± SEM. Unless otherwise specified, significance was determined by the non paired Student t test. Significance was set at P ≤ 0.05.
Supplementary Material
Acknowledgments.
We thank Dr. Chris Carr (Massachusetts Institute of Technology) for helping with the respiration data analysis. This work was supported by National Institute of Neurological Disorders and Stroke Grant R01NS41779 (to J.S.), an American Parkinson's Disease Association fellowship (to C.A.G.), and an Ecole Polytechnique (France) fellowship (to C.A.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11041.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802076105/DCSupplemental.
References
- 1.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. and discussion (2003) S36–S38. [DOI] [PubMed] [Google Scholar]
- 2.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann NY Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 3.Schapira AH, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow RH, et al. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 5.Hertzman C, et al. Parkinson's disease: A case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- 6.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 7.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 8.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 9.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 10.Shen J, Cookson MR. Mitochondria and dopamine: New insights into recessive parkinsonism. Neuron. 2004;43:301–304. doi: 10.1016/j.neuron.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi S, et al. PINK1 protein in normal human brain and Parkinson's disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- 12.Silvestri L, et al. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 13.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS Biol. 2007;5:e17. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 15.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 16.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sim CH, et al. C-terminal truncation and Parkinson's disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet. 2006;15:3251–3262. doi: 10.1093/hmg/ddl398. [DOI] [PubMed] [Google Scholar]
- 19.Beilina A, et al. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci USA. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andres-Mateos E, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 23.Bindoff LA, et al. Mitochondrial function in Parkinson's disease. Lancet. 1989;2:49. doi: 10.1016/s0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- 24.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benecke R, Strumper P, Weiss H. Electron transfer complexes I and IV of platelets are abnormal in Parkinson's disease but normal in Parkinson-plus syndromes. Brain. 1993;116(Pt 6):1451–1463. doi: 10.1093/brain/116.6.1451. [DOI] [PubMed] [Google Scholar]
- 26.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz A, Caro P, Gomez J, Barja G. Testing the vicious cycle theory of mitochondrial ROS production: Effects of H2O2 and cumene hydroperoxide treatment on heart mitochondria. J Bionenerg Biomembr. 2006;38:121–127. doi: 10.1007/s10863-006-9011-8. [DOI] [PubMed] [Google Scholar]
- 28.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 29.Stichel CC, et al. Mono- and double-mutant mouse models of Parkinson's disease display severe mitochondrial damage. Hum Mol Genet. 2007;16:3377–3393. doi: 10.1093/hmg/ddm083. [DOI] [PubMed] [Google Scholar]
- 30.Palacino JJ, et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 31.Hoepken HH, et al. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25:401–411. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Haas RH, et al. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino H, Nakagawa-Hattori Y, Kondo T, Mizuno Y. Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson's disease. J Neural Transm Park Dis Dement Sect. 1992;4:27–34. doi: 10.1007/BF02257619. [DOI] [PubMed] [Google Scholar]
- 34.Taymans JM, Van den Haute C, Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–961. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 35.Gluck MR, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: Implications for Parkinson's disease and catecholamine-associated diseases. J Neurochem. 2004;91:788–795. doi: 10.1111/j.1471-4159.2004.02747.x. [DOI] [PubMed] [Google Scholar]
- 36.Plun-Favreau H, et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.