Abstract
Apoptosis, a physiologically critical process, is characterized by a destruction of the cell after sequential degradation of key cellular components. Here, we set out to explore the fate of the physiologically indispensable nuclear envelope (NE) in this process. The NE mediates the critical nucleocytoplasmic transport through nuclear pore complexes (NPCs). In addition, the NE is involved in gene expression and contributes significantly to the overall structure and mechanical stability of the cell nucleus through the nuclear lamina, which underlies the entire nucleoplasmic face of the NE and thereby interconnects the NPCs, the NE, and the genomic material. Using the nano-imaging and mechanical probing approach atomic force microscopy (AFM) and biochemical methods, we unveiled the fate of the NE during apoptosis. The doomed NE sustains a degradation of both the mediators of the critical selective nucleocytoplasmic transport, namely NPC cytoplasmic filaments and basket, and the nuclear lamina. These observations are paralleled by marked softening and destabilization of the NE and the detection of vesicle-like nuclear fragments. We conclude that destruction of the cell nucleus during apoptosis proceeds in a strategic fashion. Degradation of NPC cytoplasmic filaments and basket shuts down the critical selective nucleocytoplasmic cross-talk. Degradation of the nuclear lamina disrupts the pivotal connection between the NE and the chromatin, breaks up the overall nuclear architecture, and softens the NE, thereby enabling the formation of nuclear fragments at later stages of apoptosis.
Keywords: atomic force microscopy, nuclear envelope, nuclear pores, nucleocytoplasmic transport
In contrast to the diversity of stimuli generating apoptosis, signaling and execution mechanisms are highly conserved (1). The execution of apoptosis is mainly driven by caspases, a family of cysteine proteases (2). Activation of caspases, in turn, occurs in many cases as a consequence of cytochrome c release from mitochondria (3). The apoptotic program can also be initiated artificially by delivering a load of exogenous cytochrome c into the cytosol (4, 5). Hallmarks of apoptosis are numerous. They comprise cell shrinkage; plasma membrane blebbing; detachment of chromatin from the NE; chromatin condensation; and partial degradation of key components of the NE, including a subset of NPC proteins (Nups) (6–9) and the nuclear lamina (10). The nuclear lamina is a fenestrated meshwork of lamin filaments (11) that, in higher cells, lines the nucleoplasmic face of the NE. It is closely connected with both the inner nuclear membrane and the underlying chromatin (12), and provides anchoring sites for NPCs (13). With respect to these connections, the nuclear lamina is believed to confer crucial overall structural and mechanical stability to the NE. Moreover, although the biochemical background of the cleavage of lamina proteins and Nups has been studied in considerable detail using biochemical methods, little is known about the resulting structural and mechanical changes of the NE during apoptosis. Investigations of structural and mechanical changes on biological samples such as the delicate NE can be carried out only with the help of atomic force microscopy (AFM). In contrast to electron microscopy, which is likewise a powerful tool for investigations at the nanoscale, AFM has the unique property of being capable of performing structural (14) and mechanical (15) investigations on biological samples under physiologically relevant conditions (16, 17). Here, we combine biochemical techniques and AFM to study changes in composition, structure, and mechanics of the NE during apoptosis, at the single molecule level and in physiologically relevant environments. For this purpose, Xenopus laevis oocytes were committed to apoptosis by microinjection of cytochrome c into the cytosol of oocytes, based on a previously published protocol (4).
Results
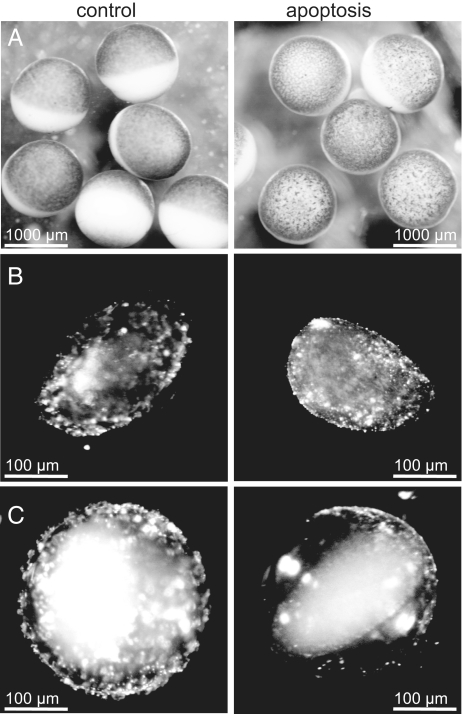
Previous studies have demonstrated that cytochrome c induces activation of caspases in Xenopus cell-free systems (18, 19) and in intact oocytes (4). In addition, oocytes were shown to reveal visible morphological cell-surface features, which mark cell death, on injection of cytochrome c into the cytosol (4). We initiated apoptosis in oocytes by direct microinjection of cytochrome c into the cytosol. Fig. 1 A shows the apoptosis-promoting effect of cytochrome c (≈2.5 h after microinjection), characterized by various features, with the growth of irregular blotchy patterns on the cell surface and the gradual loss of the sharp distinction between the animal (dark or pigmented) and the vegetal (white) poles counted among the most prominent ones. The observations described above clearly indicate that caspase activity has already begun, as further verified by DEVD proteolysis assays of cell-free extracts (see supporting information (SI) Fig. S1). Moreover, changes in the morphology of the cell surface were paralleled by marked changes of the surface and the interior of the cell nucleus, as can readily be seen under a conventional binocular microscope. NEs of nuclei, isolated manually 2.5 h after cytochrome c microinjection, seemingly flatten out (Fig. 1B Right), compared with control NEs (solvent-injected oocytes; Fig. 1B Left), which are fairly folded. To highlight this observation, we induced both control and apoptotic cell nuclei to swell by the removal of polyvinylpyrrolidone (PVP, Mr = 40 kD) from the nuclear isolation medium (NIM), where isolated nuclei were kept at all times. PVP compensates for the lack of macromolecules in NIM. It exerts a physiological oncotic pressure (as is the case in the cytosol of oocytes through the presence of yolk), which in turn prevents cell nuclei from swelling. As seen in Fig. 1C, whereas the NE of the swollen control nucleus (Left) retains its overall folded structure and its physical association with the chromatin, that of the swollen apoptotic nucleus (Right) almost perfectly flattens out and visibly loses its association with the chromatin. In addition, the ability of the nucleus to swell shows that the overall integrity of the NE remains intact. However, swollen apoptotic nuclei rendered very sensitive to mechanical damage soon burst open on their own (Fig. 2). Furthermore, vesicle-like nuclear fragments were observed to form spontaneously (Fig. 2). In a next step, NEs were structurally investigated with AFM at the nanoscale and in physiologically relevant environments (NIM). For mere structural investigations, NEs were chemically fixed and kept in NIM throughout. The purpose of fixation was to achieve a higher lateral resolution. For mechanical measurements, however, NEs were kept unfixed, as fixation was found to stiffen the NEs (data not shown). In the light of the observations made on both faces of the NE using AFM, it becomes obvious that the NE is disfigured during apoptosis. Below, we address changes in the nucleoplasmic face of the NE before moving on to describe changes occurring to the cytoplasmic face of the NE in parallel.
Fig. 1.
Macroscopic changes of Xenopus laevis oocytes and oocyte nuclei upon cytochrome c injection into the cytosol. (A) Control and apoptotic oocytes 2.5 h after injection of solvent or cytochrome c, respectively. (B) Nuclei prepared 2.5 h after injection of cytochrome c and kept in a nuclear isolation medium (NIM) containing 1.5% polyvinylpyrrolidone (PVP). PVP compensates for the lack of macromolecules in NIM and thereby exerts a physiological oncotic pressure, which in turn prevents cell nuclei from swelling. (C) Nuclei prepared similar to (B), but incubated in NIM free of PVP.
Fig. 2.
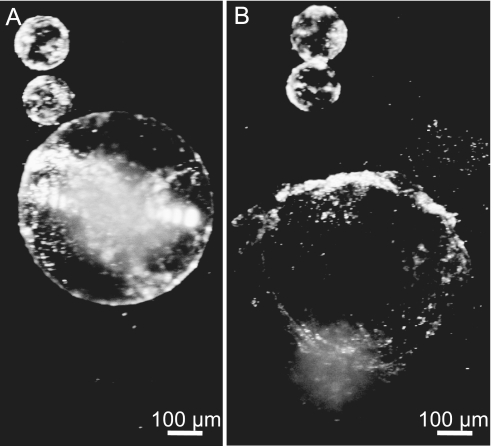
Mechanical destabilization of swollen apoptotic nuclei and spontaneous formation of vesicle-like nuclear fragments. (A) Swollen, but still intact, apoptotic nucleus and two vesicle-like nuclear fragments formed spontaneously from another, degraded nucleus. (B) A few minutes later, the apoptotic nucleus from A bursts open due to its mechanical instability.
Disfiguration and Softening of the Doomed NE upon Degradation of Its Prominent Structural and Functional Features, the Nuclear Basket and the Nuclear Lamina.
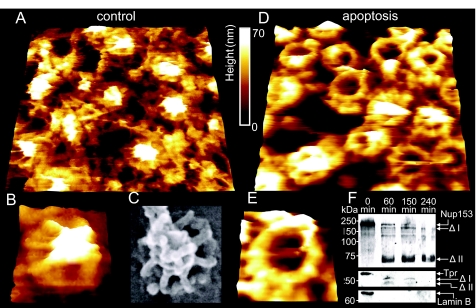
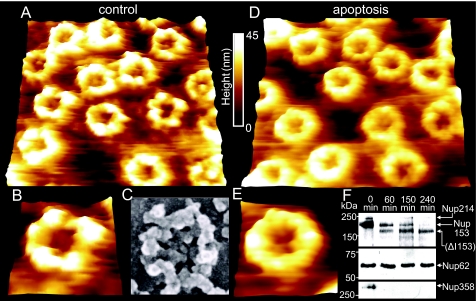
Fig. 3 shows fairly representative images of the nucleoplasmic faces of both control (Fig. 3 A and B) and apoptotic (Fig. 3 D and E) NEs. As can be seen in this figure, the control NE (Fig. 3A) is densely covered with a distinctive filamentous structure, the nuclear lamina, which is closely connected with NPCs. These in turn are decorated with a prominent intact nuclear basket, as shown in further detail in a magnified image (Fig. 3B). One of the most striking differences on comparison of the AFM images of control NEs with those of apoptotic NEs is that the latter feature NPCs that have either partially or completely lost the nuclear basket, as displayed in Fig. 3D and in a magnified image of a single NPC in Fig. 3E. The degradation of the nuclear basket implies cleavage of key basket proteins. In this respect, Nup153 is of particular interest due to its paramount importance for NPC structure and function and its proposed role in anchoring the NPC to the nuclear lamina (20). After induction of apoptosis in tissue culture cells, Nup153 was cleaved in a caspase-3-dependent manner and was absent from NPCs found in clusters (7, 21). Indeed, Western blot analysis using an antibody against the N-terminal domain of Nup153 (Fig. 3F) shows that the nuclear basket degradation observed with AFM is paralleled by cleavage of Nup153. Moreover, because the N terminus of Nup153 is known to reside on the nuclear ring moiety of the NPC (22, 23), it is likely that the anchoring structures visible in Fig. 3E consist of the N-terminal fragment visible in Fig. 3F (Nup153 Δ II). Another important structural component of the nuclear basket is Tpr. As proposed by Krull et al., Tpr is the major architectural component of the basket (23). Like Nup153, Tpr was found to be degraded upon induction of apoptosis in HeLa cells (7). Western blot analysis reveals an early cleavage of Tpr (Fig. 3F). Two fragments are visible, which, in contrast to the Nup153 fragments, seem to be further degraded and are no longer detectable after 240 min. These fragments may correspond to the filamentous basket structures which are still present at the NPCs after 150 min (Fig. 3 D and E).
Fig. 3.
Nanoscopic changes of the nucleoplasmic side of the NE 2.5 h after injection of cytochrome c or solvent, respectively, into the cytosol. (A) 730 × 730-nm AFM image of the nucleoplasmic side of the NE (control). (B) A magnification of (A), displaying a single NPC with intact nuclear basket. (C) SEM image of a single NPC (likewise from the nucleoplasmic side) for comparison (image taken from ref. 38, with permission of the Nature Publishing Group) (D) 730 × 730-nm AFM image of the nucleoplasmic side of an apoptotic NE. (E) A magnification of (D), displaying a single NPC bearing the remaining anchoring structures of the basket. (F) Western blots showing the degradation of Nup153 and Tpr, as well as a blot showing the degradation or removal of lamin B from the NE. Time points after cytochrome c injection are indicated, and putative degradation products are marked with a Δ.
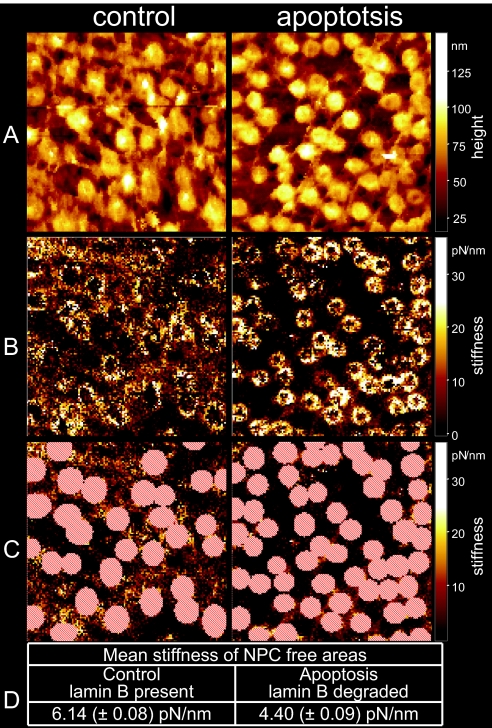
Up to this point, we have considered only the degradation of the nuclear basket, but this does not seem to be the only protein assembly that degrades during apoptosis, as implied in Figs. 3A and 3D. Another striking difference between Fig. 3A and Fig. 3D, apart from the presence or absence of the nuclear basket in control and apoptotic NEs, respectively, is that the nucleoplasmic face of the control NE is largely lined with the prominent nuclear lamina (filamentous network), whereas that of the apoptotic NE is largely deprived of any. This apparent difference is underlined by AFM-based surface roughness analysis, and direct evidence for the predicted degradation of the nuclear lamina in apoptotic NEs is provided by means of Western blot analysis. Surface roughness analysis is indeed performed on large NPC-free areas of the nucleoplasmic faces of both control and apoptotic NEs. NPC-free areas of the nucleoplasmic face of apoptotic NEs are observed to decrease their roughness by 44% (control: 10.1 ± 0.42 nm, apoptotic: 5.7 ± 0.31 nm) in comparison to NPC-free areas on the nucleoplasmic face of control NEs. This observation indicates a marked decrease in the content of the nuclear lamina of apoptotic compared to control NEs, which is indeed the case, as evident in Fig. 3F, which displays Western blot analysis and demonstrates an early disappearance of lamin B from apoptotic NEs. Because we don't see any degradation bands, we cannot rule out the possibility that lamin B was not cleaved but was just separated from the NE. In addition, we raised the question as to whether the degradation of the nuclear lamina results in impaired mechanics of the NE, as the nuclear lamina is largely assumed to confer not only structural but also mechanical stability to the NE. In an attempt to answer this question, we performed AFM-based nano-indentation measurements on the nucleoplasmic faces of both control and apoptotic NEs. For the nano-indentation measurements the so-called force volume AFM operation mode was applied (described in Materials and Methods and in more detail in SI Materials and Methods). In brief, we analyzed force curves as a function of the lateral position of the scanning AFM tip on top of the NE surface. These force curves show the indentation of the nucleoplasmic face of both control and apoptotic NEs as the tip loads the sample. By analyzing these force curves, we were able to determine the stiffness of the nucleoplasmic face of control NEs and to compare it to that of apoptotic NEs, with a lateral resolution on the scale of a few nanometers. Thereby, >16,000 force curves were analyzed for each image, to create stiffness maps (Fig. 4B). These maps (stiffness is color-coded, bright being stiffer than dark) show that the NPC-free areas of the nucleoplasmic face of apoptotic NEs are significantly softer (stiffness values are shown in Fig. 4D) than those of the NPC-free areas of control NEs (Fig. 4 B and C). In other words, the decrease in the nuclear lamina content in apoptotic NEs results in softening of the NE. Yet, the nucleoplasmic face of control NPCs appears to be softer than that of apoptotic NPCs (Fig. 4B). In this respect, it is most likely that the stiffness measured on the nucleoplasmic face of control NPCs largely represents that of the delicate basket, whereas the stiffness measured on the nucleoplasmic face of apoptotic NPCs represents that of the compact NPC ring, which is rendered free on basket degradation. Fig. S2 shows exemplary force-indentation curves of different areas from apoptotic and control NEs. The average stiffness values of the NPC-free areas are 6.14 pN/nm in the control and 4.40 pN/nm in the apoptotic case (Fig. 4D). In other words, the NPC-free nucleoplasmic face of control NEs is 28% stiffer than that of apoptotic NEs.
Fig. 4.
Force volume and stiffness analysis of control and apoptotic NEs (nucleoplasmic face, 1.5 × 1.5 μm). (A) Height image of the force volume measurement, in which height points represent the return points after a load of 310 pN. (B) Stiffness analysis of the force volume measurements, showing a stiffening of the NPC and a softening of the NPC-free areas. (C) For the determination of the stiffness of NPC-free areas, NPC-containing areas (marked red) were omitted from the analysis. (D) Comparison of mean stiffness of NPC-free areas in control and apoptotic NEs.
The Cytoplasmic Face of the Doomed NE Is Deprived of the NPC Filaments.
We also investigated structural changes on the cytoplasmic face of the NE during apoptosis. Fig. 5A shows the cytoplasmic face of a control NE, and Fig. 5B is a magnification revealing an individual NPC. The NPC rim is observed decorated by distinctive elevated structures, which most likely represent the cytoplasmic filaments, seen in further detail in the magnified image. At this point, we would like to stress that AFM fails to image such filaments in an elevated position because they are far too soft to resist the loading force (even reduced to a minimum). The filaments end up naturally collapsing around the NPC rim and are probably detectable only as elevated structures. Fig. 5D shows the cytoplasmic face of an apoptotic NE, and Fig. 5E is a magnification revealing an individual NPC. Closer inspection of both figures indicates that the cytoplasmic rim of apoptotic NPCs is smoother than that of control NPCs, probably as a consequence of degradation of the cytoplasmic filaments. Indeed, surface roughness measurements of the NPC rim show a decrease in roughness of 33% (control: 5.8 ± 0.22 nm, apoptotic: 3.9 ± 0.27 nm). Again, these observations correspond with the Western blots in Fig. 5F, which show a degradation of Nup358 and Nup214, two major constituents of the cytoplasmic filaments (24). Alternatively, because we don't observe any degradation bands in the Western blots, removal of both Nups from the NE is conceivable. As Nup214 is required for the attachment of Nup358 to the NPC (25), its elimination from the NE may affect the presence of Nup358 at the NPC. Thus, the observed loss of Nup358 might partially be explained by the elemination of Nup214 from the NE.
Fig. 5.
Nanoscopic changes of the cytoplasmic face of the NE 2.5 h after injection of cytochrome c or solvent into the cytosol. (A) 730 × 730-nm AFM image of the cytoplasmic side of the NE (control). (B) A magnification of (A), displaying a single NPC featuring the cytoplasmic filaments. (C) SEM image of a single NPC for comparison (image taken from ref. 38, with permission) (D) 730 × 730-nm AFM image of the cytoplasmic side of an apoptotic NE. (E) A magnification of (D), displaying a single NPC bearing remainders of cytoplasmic NPC filaments. (F) Western blots showing the degradation or removal of Nup214 (using the polyspecific mab414) and Nup358 from the NE. Time points after cytochrome c injection are indicated. Mab414 also labels Nup153 and Nup62, which is not cleaved during apoptosis.
Discussion
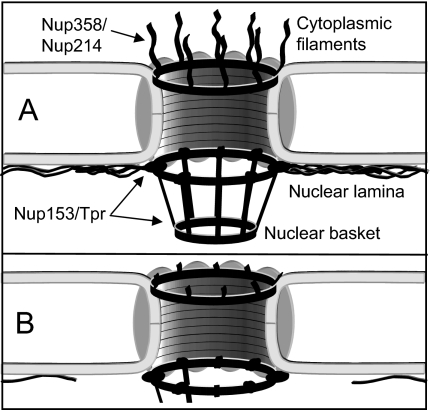
The present study demonstrates that apoptosis deprives the nuclear envelope of prominent structural and functional features: NPC cytoplasmic filaments and basket as well as the nuclear lamina, as schematically depicted in Fig. 6. This assault on the NE disfigures both its nucleoplasmic and cytoplasmic faces and leads to NE softening. According to the gene gating hypothesis postulated by Blobel (26), NPCs and the nuclear lamina contribute significantly to the maintenance of the three-dimensional structure of the genome (26). With respect to this hypothesis and the findings of the present study, it is presumed that the physiologically essential cross-talk between the chromatin and the NE is disrupted on degradation of the nuclear lamina and the nuclear basket during apoptosis. As the NE was found to soften and become delicate on degradation of the nuclear lamina, we presume that the nuclear lamina is of paramount importance for conferring not only structural but also mechanical stability to the NE, and thereby the cell nucleus as a whole. Moreover, the degradation of the nuclear lamina and the nuclear basket during apoptosis not only cuts off the cross-talk between the NE and the chromatin, but might also serve to facilitate the budding of micronuclei at late stages of apoptosis, as assumed by Lazebnik et al. (10). As mentioned above, the disfiguration of the NE structure was not limited to its nucleoplasmic face. Degradation of the nuclear basket was paralleled by degradation of the cytoplasmic filaments of the NPCs. Given the importance of the cytoplasmic filaments and the basket of the NPCs for selective nucleocytoplasmic transport (27–29), we predict that the bidirectional active transport across the NE is at some point shut down, as part of the nuclear execution program. In fact, Ferrando-May et al. (7), have shown an accumulation of mRNA in the nucleus during apoptosis, which could be explained by defects in the nuclear basket, comparable to those observed in the present study. By contrast, the passive permeability of apoptotic nuclei increases during apoptosis (7, 30). Since the overall structure of the NE remains intact, as seen in Fig. 1 and consistent with the observations of Wyllie et al. (31) as well as Lazebnik et al. (10), this increased permeability is likely due to structural alterations of the NPCs. AFM operated in fluid could help to elucidate the nature of these alterations in the near future.
Fig. 6.
Schematic model displaying apotosis-induced disfiguration of both NE faces. (A) Intact NE bearing prominent structural and functional features, cytoplasmic NPC filaments, nuclear basket, and nuclear lamina. (B) NE committed to apoptosis and thus deprived of the features mentioned above. Due to the degradation of the lamina and the C terminus of the key nuclear basket component Nup153, the connection between NPC and NE is perturbed.
Conclusions
All in all, the present study shows that apoptosis leads to a degradation of NPC components essential to the physiologically critical selective nucleocytoplasmic transport. Parallel degradation of the nuclear lamina softens the NE, perturbs the overall architecture of the cell nucleus, and severely destabilizes the cell nucleus. This is likely to be a prerequisite for the packaging of the cell nucleus into nuclear fragments.
Materials and Methods
Preparation of Oocytes, Nuclei, and Nuclear Envelopes.
The preparation of oocytes, nuclei, and nuclear envelopes was performed according to Kramer et al. (14). Samples that were used for force volume measurements were kept unfixed. The other samples were fixed using 2% glutaraldehyde and 1% paraformaldehyde.
Microinjection of Cytochrome c.
Typically, 50 nl aqueous solution of cytochrome c (Sigma) were microinjected into each oocyte by using a microinjector (OocytePipet; Drummond), resulting in a final concentration of 10 μM. After injection of cytochrome c, oocytes were incubated at 18°C for the indicated times.
Assay of DEVDase Activity in Oocyte Cell-Free Extracts.
The assay of DEVDase activity was performed according to Bhuyan et al. (4).
Antibodies.
The antibody against the N terminus of Nup153 (32) was a kind gift from Ian Mattaj (EMBL, Heidelberg, Germany). The anti-Nup358 antibody (33) was generously provided by Mary Dasso (Laboratory of Gene Regulation and Development, NICHD/NIH, Bethesda, MD). We are obliged to Douglass Forbes (University of California, San Diego, CA) for providing the anti-Tpr antibody (34). The anti-Lamin B antibody (X223) was purchased from Progen, and the Mab414 was from Covance. Peroxidase-labeled secondary antibodies were purchased from Dianova.
Western Blot Analysis.
Samples containing nuclei were prepared for each point in time (60 min, 150 min, and 240 min) after injection of cytochrome c. Controls (0 min) were injected with an equal volume of solvent. Samples were resolved on 10% SDS/PAGE gels according to Ludwig et al. (35)
AFM.
The application of AFM to NEs has been described in detail elsewhere (36). OTR4 AFM tips (Olympus) were used. The images were recorded in tapping mode, with 512 lines per screen, at a scan rate of 1.5 Hz.
Data Analysis and Image Processing.
AFM images were analyzed by using SPIP software (Image Metrology)
Force Curves, Force Volume Measurements, and Stiffness Maps.
The principle of force volume measurement was described elsewhere (37). The settings for the force volume measurements were as follows: Number of samples was 128 × 128 × 128 (x,y,z), scan rate was 9 Hz (force curves/sec), and the trigger threshold was set to 310 pN. Soft cantilevers with a spring constant of 0.01 N/m were used (MSCT; Veeco). All curves of a force volume measurement were analyzed automatically using the free software PUNIAS (http://site.voila.fr/punias/).
Statistical Analyses.
Data are presented as the mean ± standard error of the mean (SEM). In each experimental series (solvent and cytochrome c), 10 nuclei were isolated. Statistical significance of mean values was tested with the unpaired Student's t test. P value was always 0.001 or less.
Supplementary Material
Acknowledgments.
We thank Ian Mattaj, Douglas Forbes, and Mary Dasso for the antibodies and Volker Cordes for constructive discussion. This study was supported by “Innovative Medizinische Forschung” Grants SH-110315, SH-520404, and SH-120613 and by “Deutsche Forschungsgemeinschaft (Graduate School Molecular Basis of Dynamic Cellular Processes)” Grants SFB629 and OB 63/16-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801967105/DCSupplemental.
References
- 1.Ashkenazi A, Dixit V-M. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A, O'Connor L, Dixit V-M. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Adrain C, Martin S-J. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–397. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 4.Bhuyan A-K, Varshney A, Mathew M-K. Resting membrane potential as a marker of apoptosis: Studies on Xenopus oocytes microinjected with cytochrome c. Cell Death Differ. 2001;8:63–69. doi: 10.1038/sj.cdd.4400773. [DOI] [PubMed] [Google Scholar]
- 5.Green D-R, Reed J-C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 6.Buendia B, Courvalin J-C, Collas P. Dynamics of the nuclear envelope at mitosis and during apoptosis. Cell Mol Life Sci. 2001;58:1781–1789. doi: 10.1007/PL00000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrando-May E, et al. Caspases mediate nucleoporin cleavage, but not early redistribution of nuclear transport factors and modulation of nuclear permeability in apoptosis. Cell Death Differ. 2001;8:495–505. doi: 10.1038/sj.cdd.4400837. [DOI] [PubMed] [Google Scholar]
- 8.Kihlmark M, Imreh G, Hallberg E. Sequential degradation of proteins from the nuclear envelope during apoptosis. J Cell Sci. 2001;114:3643–3653. doi: 10.1242/jcs.114.20.3643. [DOI] [PubMed] [Google Scholar]
- 9.Patre M, Tabbert A, Hermann D, Walczak H, Rackwitz H-R, Cordes V-C, Ferrando-May E. Caspases target only two architectural components within the core structure of the nuclear pore complex. J Biol Chem. 2006;281:1296–1304. doi: 10.1074/jbc.M511717200. [DOI] [PubMed] [Google Scholar]
- 10.Lazebnik Y-A, Cole S, Cooke C-A, Nelson W-G, Earnshaw W-C. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: A model system for analysis of the active phase of apoptosis. J Cell Biol. 1993;123:7–22. doi: 10.1083/jcb.123.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruenbaum Y, Margalit A, Goldman R-D, Shumaker D-K, Wilson K-L. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- 12.Stewart C-L, Roux K-J, Burke B. Blurring the boundary: The nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 13.Gerace L, Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- 14.Kramer A, Ludwig Y, Shahin V, Oberleithner H. A pathway separate from the central channel through the nuclear pore complex for inorganic ions and small macromolecules. J Biol Chem. 2007;282:31437–31443. doi: 10.1074/jbc.M703720200. [DOI] [PubMed] [Google Scholar]
- 15.Oberleithner H, et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–16286. [Google Scholar]
- 16.Schafer C, et al. Aldosterone signaling pathway across the nuclear envelope. Proc Natl Acad Sci USA. 2002;99:7154–7159. doi: 10.1073/pnas.092140799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Clapham D-E. Conformational changes of the in situ nuclear pore complex. Biophys J. 1999;77:241–247. doi: 10.1016/S0006-3495(99)76885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluck R-M, Bossy-Wetzel E, Green D-R, Newmeyer D-D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 19.Kuwana T, et al. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 20.Ball J-R, Ullman K-S. Versatility at the nuclear pore complex: Lessons learned from the nucleoporin Nup153. Chromosoma. 2005;114:319–330. doi: 10.1007/s00412-005-0019-3. [DOI] [PubMed] [Google Scholar]
- 21.Buendia B, Santa-Maria A, Courvalin J-C. Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J Cell Sci. 1999;112:1743–1753. doi: 10.1242/jcs.112.11.1743. [DOI] [PubMed] [Google Scholar]
- 22.Fahrenkrog B, et al. Domain-specific antibodies reveal multiple-site topology of Nup153 within the nuclear pore complex. J Struct Biol. 2002;140:254–267. doi: 10.1016/s1047-8477(02)00524-5. [DOI] [PubMed] [Google Scholar]
- 23.Krull S, Thyberg J, Bjorkroth B, Rackwitz H-R, Cordes V-C. Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol Biol Cell. 2004;15:4261–4277. doi: 10.1091/mbc.E04-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pante N, Bastos R, McMorrow I, Burke B, Aebi U. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J Cell Biol. 1994;126:603–617. doi: 10.1083/jcb.126.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernad R, van der Velde H, Fornerod M, Pickersgill H. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol. 2004;24:2373–2384. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blobel G. Gene gating: A hypothesis. Proc Natl Acad Sci USA. 1985;82:8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiseleva E, Goldberg M-W, Allen T-D, Akey C-W. Active nuclear pore complexes in Chironomus: Visualization of transporter configurations related to mRNP export. J Cell Sci. 1998;111(Pt 2):223–236. doi: 10.1242/jcs.111.2.223. [DOI] [PubMed] [Google Scholar]
- 28.Nigg E-A. Nucleocytoplasmic transport: Signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 29.Pante N, Aebi U. Sequential binding of import ligands to distinct nucleopore regions during their nuclear import. Science. 1996;273:1729–1732. doi: 10.1126/science.273.5282.1729. [DOI] [PubMed] [Google Scholar]
- 30.Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151:951–959. doi: 10.1083/jcb.151.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyllie A-H, Kerr J-F, Currie A-R. Cell death: The significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 32.Walther T-C, et al. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J. 2001;20:5703–5714. doi: 10.1093/emboj/20.20.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph J, Liu S-T, Jablonski S-A, Yen T-J, Dasso M. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr Biol. 2004;14:611–617. doi: 10.1016/j.cub.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Shah S, Tugendreich S, Forbes D. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J Cell Biol. 1998;141:31–49. doi: 10.1083/jcb.141.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig Y, et al. Hot spot formation in the nuclear envelope of oocytes in response to steroids. Cell Physiol Biochem. 2006;17:181–192. doi: 10.1159/000094123. [DOI] [PubMed] [Google Scholar]
- 36.Kramer A, Liashkovich I, Ludwig Y, Shahin V. Atomic force microscopy visualises a hydrophobic meshwork in the central channel of the nuclear pore. Pflugers Arch. 2008;456:155–162. doi: 10.1007/s00424-007-0396-y. [DOI] [PubMed] [Google Scholar]
- 37.Heinz W-F, Hoh J-H. Spatially resolved force spectroscopy of biological surfaces using the atomic force microscope. Trends Biotechnol. 1999;17:143–150. doi: 10.1016/s0167-7799(99)01304-9. [DOI] [PubMed] [Google Scholar]
- 38.Corbett A-H, Krebber H. Hot trends erupting in the nuclear transport field. Workshop on mechanisms of nuclear transport. EMBO Rep. 2004;5:453–458. doi: 10.1038/sj.embor.7400155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.