Abstract
A growing number of in vivo experiments shows that high frequency bursts of action potentials can be recorded in thalamocortical neurons of awake animals. The mechanism underlying these bursts, however, remains controversial, because they have been proposed to depend on T-type Ca2+ channels that are inactivated at the depolarized membrane potentials usually associated with the awake state. Here, we show that the transient potentiation of the T current amplitude, which is induced by neuronal depolarization, drastically increases the probability of occurrence and the temporal precision of T-channel-dependent high frequency bursts. The data, therefore, provides the first biophysical mechanism that might account for the generation of these high frequency bursts of action potentials in the awake state. Remarkably, this regulation finely tunes the response of thalamocortical neurons to the corticofugal excitatory and intrathalamic inhibitory afferents but not to sensory inputs.
Keywords: corticothalamic, lemniscal, low-threshold calcium spike, nucleus reticularis thalami
An increasing number of in vivo extracellular recordings have shown that both single action potential (AP) and high-frequency bursts of APs are elicited in thalamocortical (TC) neurons of awake animals (1–5). Although these high frequency bursts represent a small fraction of the whole TC neuron output, they preferentially occur in response to stimuli of peculiar significance, such as natural scenes (6, 7), and may therefore participate to thalamic sensory processing (8). These extracellularly recorded bursts are preceded by a period of silence lasting at least 50–100 ms, and consist of three or more APs with an interspike interval ≤4 ms that increases as the burst progresses (1–5). This temporal structure is highly reminiscent of the well known firing pattern evoked in TC neurons by the slow depolarizing waveform called the low-threshold spike (LTS) [three to five spikes at 250–400 Hz preceded by an silent interval >50 ms (9)]. Indeed, recent intracellular recordings obtained in lightly anesthetized cats has directly indicated that the high frequency bursts that occur during visual processing are underlaid by LTSs (10).
However, extensive in vitro characterization of the low-voltage activated T-type Ca2+ channels that underlie an LTS (11, 12) has clearly indicated that these channels are fully inactivated in the range of membrane potentials believed to be associated with the wake state (13) and require substantial and prolonged hyperpolarization to de-inactivate [800-ms hyperpolarization to −100 mV to fully recover from inactivation (14)]. Therefore, the mechanisms that allow the expression of a physiologically significant T current (IT) during wakefulness remain controversial (13). Three possibilities exist that may reconcile the in vivo and in vitro data: (i) the high frequency bursts observed in vivo are not generated by IT, (ii) the voltage dependence of T channel inactivation is different in the awake state from that in vitro because of an as yet unknown modulation by transmitters or other exogenous substances, and (iii) some regulation boosts the amplitude of the IT generated at depolarized membrane potentials.
Recently, we demonstrated that, in sensory TC neurons, IT amplitude can be transiently potentiated by a phosphorylation mechanism, which exclusively occurs when the channels are inactivated, i.e., after a period of neuronal depolarization (14). Here, we systematically studied in vitro how this IT potentiation affects high-frequency burst output of TC neurons, using both direct current injection and synaptic potentials. We show that the IT regulation drastically enhances the probability of LTSs generation and increases the temporal precision of the associated APs firing, when few T channels are available for activation. Remarkably, after periods of depolarization similar to those occurring in the wake states, IT potentiation directly shapes the TC neuron response to the activation of the feedback corticothalamic excitatory and intrathalamic inhibitory inputs but not to feed-forward sensory afferents.
Results
Kinetics and Temporal Precision of the Burst Are Determined by IT Potentiation.
We will first summarize the relationships of IT potentiation with the voltage dependence and kinetics of the T channel inactivation, because these features are essential for a full understanding of the experimental protocol used in this study. As described in ref. 14, IT potentiation involves a nonclassical ATP-dependant mechanism that “phosphorylates” the channels according to their conformation. The phosphorylation occurs when the channels are inactivated and is slowly removed when they recover from inactivation and remain in closed states. Therefore, this mechanism displays the same voltage dependence as the steady-state inactivation of the channel, and the fraction of phosphorylated channels is directly under the control of the membrane potential [supporting information (SI) Fig. S1]. At depolarized potentials, the T channels are both inactivated and phosphorylated, and, upon hyperpolarization, the balance between the kinetics of the dephosphorylation and the de-inactivation determines the amplitude of the current that can be evoked. (Fig. S1). Therefore, to assess how IT potentiation affects the LTS, we used conditioning prepulses of various durations and/or amplitudes to control both the inactivation and potentiated states of the T channel population. Because these conditioning prepulses differently activate the h current (Ih), isolation of the effect of IT potentiation on the LTS generation was obtained by adding the Ih blocker ZD7288 (50 μM) to the perfusion medium. The conditioning prepulses were performed in voltage-clamp mode to ensure an accurate and reproducible control of the channel state. The amplifier was then switched to current-clamp mode to allow the membrane to repolarize and generate a LTS.
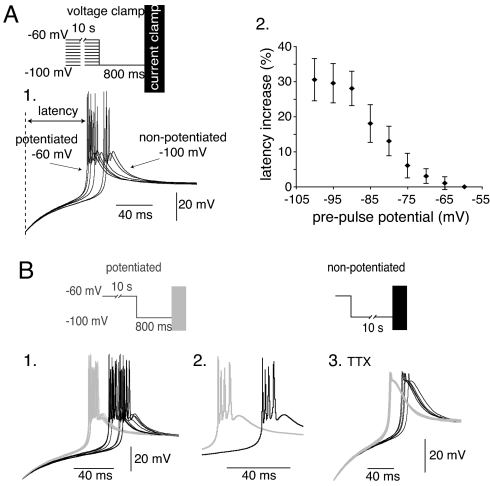
We first compared LTSs generated upon repolarization after a transient hyperpolarization that induced the recovery from inactivation of the whole T channel population. The phosphorylated states of the channels were controlled by using conditioning 10-s prepulses at various potentials (from −100 to −60 mV). The latency of LTSs generated by using the more depolarized prepulses, i.e., underlied by a larger proportion of T channels in a potentiated state, is systematically shorter than that of LTSs generated by using more hyperpolarized prepulses (Fig. 1A). Quantification shows that the time laps between the repolarization onset and the first AP follows the same voltage dependence as IT potentiation (compare with Fig. S1), and that the latency of the LTS onset decreases by 30% when the channel population is fully phosphorylated (Fig. 1A2). Controlling the potentiated state of the T channels with hyperpolarizing prepulses at −100 mV of various durations (0.8 to 10 s at −100 mV) produced similar results (Fig. S2). To confirm that these effects were due to the modulation of IT amplitude, the same protocols were repeated in presence of Ni2+. As shown in Fig. S3, no modification in the repolarizing rate and amplitude variation of the membrane potential was observed after long or short hyperpolarizing prepulses when IT was fully blocked (n = 3). Therefore, this set of experiments clearly demonstrates that the generation of APs by an LTS occurs with a shorter delay when the T channels are in the potentiated state.
Fig. 1.
IT potentiation controls the kinetics and the temporal precision of the LTS. (A) Prepulses, ranging from −60 to −100 mV, were applied to bring different fractions of T channels in the potentiated state. LTSs were thereafter evoked in the current-clamp mode at the offset of a 800-ms hyperpolarization to −100 mV that induces a complete recovery from inactivation of the T channels. (1) As illustrated by the superimposed LTSs evoked in a TC neuron submitted to prepulses at −60, −70, −75, −80, −90, and −100 mV, the shortest latency of the LTS onset (delay between the repolarization onset and the first AP) is observed after the more depolarized prepulse that induces a maximal IT potentiation. (2) The graph presents the mean increase in latency as a function of the prepulse potential (n = 6). (B) (1) Superimposed LTSs evoked in a TC neuron, using either 0.8-s (10 gray traces) or 10-s (10 black traces) hyperpolarization at −100 mV that bring the whole T channel population in a potentiated or nonpotentiated state, respectively. Note the higher temporal precision of the LTSs when T channels are potentiated. (2) Traces presented at a faster time base illustrate the similar number and frequency of APs in both conditions. (3) The 10 superimposed traces, obtained in presence of 1 μM TTX, illustrate the lack of effect of IT potentiation on the amplitude of the LTSs generated when the whole T channel population is available for activation.
Interestingly, superimposition of LTSs evoked in the same cell in conditions of maximal and minimal IT potentiation (conditioning prepulses to −100 mV of 0.8 and 10 s, respectively) also revealed a drastic reduction in the variability of the LTSs latency when the T channels were in the potentiated state (Fig. 1B). Similar analyses performed in 12 cells indicate that IT potentiation induces a 41 ± 10% reduction of the standard deviation of the first AP latency (11.5 and 6.7 in nonpotentiated and potentiated states, respectively). Therefore, generation of APs by an LTS occurs not only with a shorter delay but also with a greater temporal precision when T channels are in the potentiated state. As illustrated in Fig. 1B2, however, the number of APs generated by the LTSs (1–5 APs, n = 21) and their firing frequency (250–350 Hz, n = 13) was surprisingly not modified (Student paired t test, P > 0.4), suggesting that IT potentiation does not affect the LTS amplitude and duration in conditions where the whole T channel population has recovered from inactivation. This lack of effect was confirmed by recordings obtained in the presence of TTX, which show similar amplitude of LTSs evoked after 0.8 and 10 s conditioning prepulses to −100 mV (mean values of the maximal membrane potential reached during the LTS: −42.0 ± 6.5 and −42.5 ± 6.5 mV for 0.8- and 10-s prepulses, respectively, n = 12) (Fig. 1B3).
Interplay Between IT and K+ Currents Constrains the Effects of the T Current Potentiation.
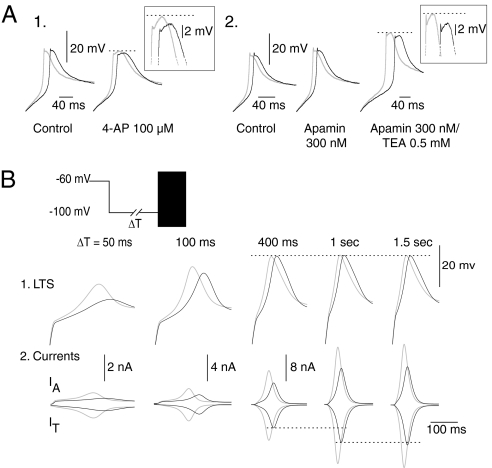
The peak potential reached by an LTS is mainly controlled by the depolarizing IT and the repolarizing K+ currents present in TC neurons (15). Using a pharmacological approach, we investigated how activation of the K+ currents may explain why an enhanced IT does not systematically increase the amplitude of the evoked LTS. After the block of the Ca2+-activated K+ conductances (IKCa2+) with low doses of 4-AP (0.1 mM) and TEA (0.5 mM), IT potentiation does have a small but clear effect on the LTS amplitude (Fig. 2A), suggesting that the larger Ca2+ influx due to IT potentiation recruits more IKCa2+ channels that, in turn, limit the effect of the increase in IT on the LTS amplitude. The lack of effect of apamin (300 nM) (Fig. 2A2) suggests that SK channels are not involved in the control of the LTS amplitude. Thereafter, we investigated the impact of the transient K+ type A current (IA) that, because of its voltage range of activation and its fast kinetics, is considered a major contributor to the LTS waveform (16). However, because the block of IA requires high doses of 4-AP that in turn induce the generation of high-threshold calcium spikes (data not shown), we performed a theoretical analysis based on a mathematical model. The model combines the IT, IA, Ih, and leakage current, whereas the currents involved in the generation of the AP were not included. Using brief transient hyperpolarizations that de-inactivate different fractions of T channels to evoke an LTS, our model shows that the depolarizing level of the LTS reaches its maximum with 400-ms hyperpolarizing prepulses, although IT amplitude continuously increases with up to 1-s hyperpolarizing prepulses when full recovery from inactivation is achieved (Fig. 2B, black traces). The unexpected limit in the LTS amplitude is an indirect consequence of the acceleration in the LTS rise time associated with the larger IT. Indeed, this acceleration induces an increase in IA, the amplitude of which is especially sensitive to the rate of depolarization because of its activation and inactivation kinetics. Therefore, for hyperpolarizations longer than a few hundred milliseconds, a balance between the increase in IT and IA is reached, which precludes any modification of the LTS amplitude. When this balance is reached, multiplying IT amplitude by 1.5 to mimic the effect of the potentiation of the channels does not affect the LTS amplitude (Fig. 2B, gray traces). However, the model predicts that when submaximal LTSs are generated after hyperpolarizations shorter than a few hundred milliseconds, IT potentiation should drastically control both the amplitude and kinetics of the LTS (Fig. 2B).
Fig. 2.
The interplay between T and K+ currents limits the effects of IT potentiation. (A) LTSs were evoked in presence of TTX, using the same protocol as in Fig. 1B. (1 and 2) Applications of low doses of 4-AP (100 μM) or TEA (0.5 mM), but not of apamin (300 nM), reveal a small but clear increase in the amplitude of the LTS evoked with potentiated channels suggesting that non-SK-type IKCa2+ limit the effect of IT potentiation in control conditions. (B) Modeling study of the interplay between IT and IA. LTSs evoked at the offset of transient hyperpolarizations of increasing duration (ΔT) are presented in 1, and corresponding IT and IA are shown in 2. Black and gray traces were obtained when simulating nonpotentiated or potentiated T channels, respectively. Simulation shows that the amplitude of the LTSs generated with hyperpolarizations >400 ms reaches a maximum that is insensitive to any enhancement of IT because this increase is matched by a parallel increase in IA.
Effect of IT Potentiation Is Maximal When a Small Number of T Channels Are Available or Activated.
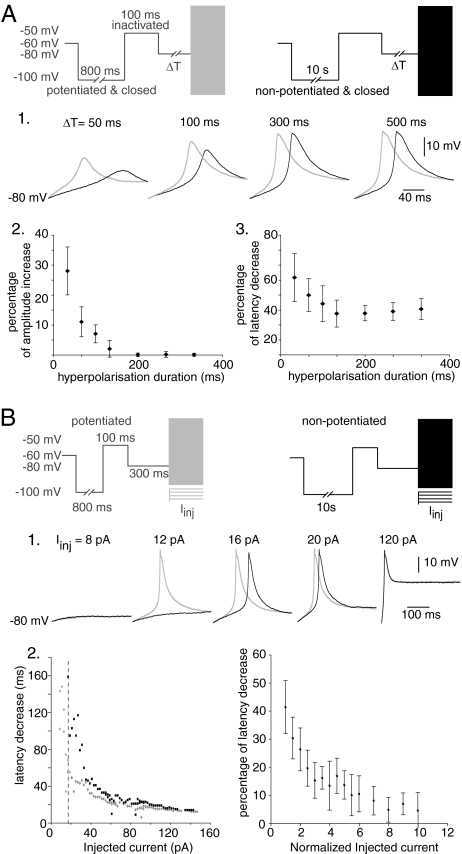
To test this prediction, rebound LTSs were evoked by short (50–500 ms) hyperpolarizations to −80 mV, thus allowing only part of the channel population to recover from inactivation. These protocols were preceded by conditioning prepulses to −100 mV of either short (0.8-s) or long (10-s) duration that led the whole T channel population into potentiated or nonpotentiated closed states, respectively, followed by a depolarization to −50 mV that was sufficiently long to induce a complete inactivation of the channels without affecting their potentiated/nonpotentiated states [τinact = 15 ms; τpotentiation-on = 384 ms; τpotentiation-off = 2.6 s (14)]. As expected from the simulation, the effect of the potentiation is maximal when only a small proportion of the T channel population is available to generate the LTS (Fig. 3A1). Indeed, the channel potentiation clearly increases the amplitude of LTS evoked after hyperpolarizations <200 ms, and this effect is associated to a drastic acceleration in the onset kinetics. Therefore, as shown in Fig. 3A 2 and 3, the effects on both the LTSs amplitude and kinetics of IT potentiation are inversely related to the duration of the preceding hyperpolarization, i.e., the amount of T channels available for activation, reaching a minimum ≈200 ms.
Fig. 3.
The effects of IT potentiation depend on both the number of activated channels and on the properties of the activating stimulus. (A) In the presence of TTX, conditioning prepulses to −100 mV were used to set the whole T channel population either in a potentiated (800 ms; gray traces) or nonpotentiated closed state (10 s; black traces). Channels were thereafter inactivated at −50 mV for 100 ms. 1. Rebound LTSs were evoked on the offset of variable transient hyperpolarizations to −80 mV (ΔT) that induce partial recovery from inactivation. When submaximal LTSs were evoked with hyperpolarizations <200 ms, IT potentiation effect was maximal and affected both the amplitude and the kinetics of the LTSs. The graphs in 2 and 3 present the mean effects of IT potentiation on the amplitude and kinetic of the LTSs (n = 6 cells) as a function of the duration of the transient hyperpolarization. (B) (1) Using a protocol similar to that in A, LTSs were evoked after a 300-ms hyperpolarization at −80 mV by current injection (Iinj) of variable intensities. Traces show that IT potentiation effect on the LTSs onset kinetics decreased when the intensity of Iinj was increased and finally disappeared for strong initial depolarisation (Iinj = 120 pA). Conversely, when a minimal current injection (Iinj = 12 pA) was used, an LTS was only evoked when T channels were in the potentiated states. (2) In this neuron, the graph at Left presenting the LTSs latency as a function of Iinj shows that with injections smaller than 16 pA (vertical line), LTSs were only evoked when T channels were potentiated. For seven cells, the mean decrease in latency (normalized to the latency duration of the LTS evoked with nonpotentiated T channels) is plotted at Right as a function of Iinj (normalized to the minimal value that evoked an LTS in both channel conditions).
Because the impact of the potentiation appears more pronounced in conditions where a small IT is evoked, we hypothesize that, regardless of the amount of T channels available for activation, IT potentiation should more strongly affect the neuron excitability when a weak depolarization opens few channels and evokes a small initial current. To test this hypothesis, LTSs were evoked by depolarizing a TC neuron to different potentials. Before these depolarization, 300-ms hyperpolarization to −80 mV, mimicking the hyperpolarization that physiologically occur in TC neurons (17), were applied to induce recovery from inactivation of a large fraction of the T channel population. As shown in Fig. 3B, when the LTS was triggered by a large current injection that induced a massive opening of the T channel population, no effect of the potentiated/nonpotentiated states of the channels was observed on the LTS generation (Fig. 3B1, 120 pA). However, when the strength of the current injections was progressively reduced, the impact of the potentiation on the LTS onset kinetics was dramatically increased. Finally, with minimal current injections (Fig. 3B1, 12 pA), LTSs were only generated when the T channels were potentiated. These results show that when an initial depolarization only recruits a few channels, IT potentiation occurring in this small number of channels drastically affects both the probability to trigger an LTS and the duration of the first slow-depolarizing phase of the LTS. However, when the number of channels that are opened by the initial depolarization is large enough to suppress this slow-depolarizing phase, IT potentiation has no effect on the LTS generation.
Therefore, IT potentiation directly determines the triggering threshold of the LTS, i.e., the minimal depolarization required to evoke a full blown LTS. Moreover, it controls both the amplitude and kinetics of the LTS, and these effects do not only depend on the number of activated channels but also on the characteristics of the stimulus activating the channels. Because a larger modulation is observed in conditions where only a few channels are available for activation and/or after weak depolarizations, IT potentiation seems ideally suited to tightly control the physiological responses of the TC neurons to small postsynaptic potentials, both inhibitory and excitatory.
IT Potentiation Finely Tunes the TC Response to the Excitatory Cortical Feedback and Inhibitory GABAergic Afferents but Not to Sensory Inputs.
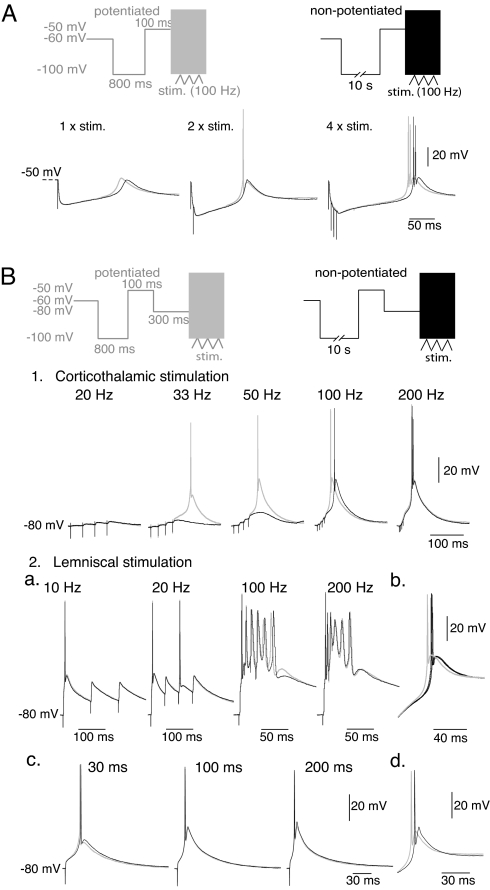
In TC neurons, short hyperpolarizations are typically induced by the brief bursts of GABAergic postsynaptic potentials originating from the NRT (17). Using similar protocols as those used above to maintain the T channel population either in the potentiated or the nonpotentiated states, local electrical stimulation of the afferent NRT fibers was performed to elicit composite GABAA/B inhibitory postsynaptic potentials (IPSPs) of different durations and amplitudes (17). When large-amplitude long-duration (>150 ms) IPSPs are evoked, IT potentiation has a drastic effect on the latency of the burst of APs but does not modify their number and frequency (Fig. 4A, four stimuli at 100 Hz). However, when large-amplitude IPSPs with shorter durations or smaller-amplitude IPSPs are generated by reducing the number of stimuli, IT potentiation boosts the amplitude of the rebound LTS and increases its probability to fire APs (Fig. 4A, one and two stimuli, respectively, n = 12). To check whether Ih activation counteracts the effect of IT potentiation on the rebound LTS, similar protocols were performed in the absence of ZD7288 or using the dynamic-clamp technique to introduce a simulated Ih conductance. As shown in Fig. S4A, similar results were obtained in the absence and in the presence of Ih. We conclude that IT potentiation that occurs at depolarized membrane potential actually facilitates the TC bursting activity driven by the NRT input. These effects are tightly dependent on the IPSPs temporal summation and therefore, IT potentiation participates to the fine tuning of the TC response to high frequency NRT neuron firing.
Fig. 4.
IT potentiation defines the integrative properties of the NRT input and cortical feedback but does not control the timing of the sensory input response. (A) After conditioning prepulses similar to those in Fig. 3A, LTSs were evoked by local stimuli of the GABAergic NRT inputs. Traces show typical rebound LTSs evoked with potentiated (gray traces) and nonpotentiated (black traces) T channels in a TC neuron successively submitted to one, two, and four local stimulations at 100 Hz. (B) After conditioning prepulses similar to those in Fig. 3A, LTSs were evoked by stimulations of either the corticothalamic (1) or the lemniscal pathway (2) at various frequencies (indicated above the corresponding traces). Stimulations were performed after a 300-ms hyperpolarization to −80 mV to induce the recovery from inactivation of a large fraction of the T channel population. (1) For stimulation frequencies of the corticothalamic pathway <100 Hz, IT potentiation has a drastic effect on the LTS probability and amplitude. With higher stimulation frequencies, LTSs of maximal amplitude are always generated and IT potentiation only accelerates the onset kinetics of the LTSs. However, this effect is highly frequency-dependent, and at the higher frequency used (200 Hz) almost no difference in the timing of the LTSs is observed. (2) (a and b) Responses to stimulations of the lemniscal pathway are insensitive to the potentiated/nonpotentiated states of the T channel population whatever the stimulation frequency, although the potentiation of the IT was present in this neuron (b) (protocols similar as those in Fig. 1B). (c) The lack of effect of IT potentiation on the triggered LTSs was also observed when stimulations were performed after 30-ms, 100-ms, and 200-ms hyperpolarizations to −80 mV to reduce the fraction of T channels that can be activated by the sensory EPSPs. (d) Same control protocol as in b.
LTSs triggered by glutamatergic excitatory postsynaptic potentials (EPSPs) also occur during tonic or transient hyperpolarization (17). The two main excitatory pathways that converge on TC neurons, i.e., the sensory input and the corticothalamic feedback, generate very different glutamatergic EPSPs (18). In our conditions, minimal stimulation of the internal capsula to activate the corticothalamic fibers elicited typical corticothalamic EPSPs of small amplitude characterized by a slow rising phase and the presence of a robust frequency-dependent facilitation (19). To analyze the effect of IT potentiation, trains of two to six stimulations were applied at frequencies ranging from 10 to 200 Hz. Such stimulations were delivered after a 300-ms period at −80 mV after conditioning prepulses similar to those presented in Fig. 3 to induce either the potentiation or the depotentiation of the T channels. At low frequencies of stimulation (20–50 Hz, n = 6) temporal summation of the EPSPs generates a slow depolarizing waveform and IT potentiation clearly sets the LTS triggering-threshold in response to this input. In this range of frequencies, LTSs could be evoked either at a lower stimulating frequency or with fewer stimuli at a given frequency when the T channels were in the potentiated rather than in the nonpotentiated states. For example, in Fig. 4B1, the summation of the 4 EPSPs evoked at 33 Hz exclusively triggered a LTS when the T channels were in the potentiated state. With slightly higher stimulation frequency (50 Hz) (Fig. 4B1), the LTS triggering-threshold is reached in both conditions but IT potentiation remarkably boosts the LTS amplitude and thus conditions the APs generation. At higher frequencies (50–200 Hz, n = 6; see 100 Hz in Fig. 4B1), an LTS of maximal amplitude is already evoked by 4–6 EPSPs in condition of nonpotentiated T channels, and the effect of the potentiation is restricted to the acceleration of the onset kinetics. This effect becomes minimal with the fastest depolarizing slope induced by the highest stimulating frequency (200 Hz) (Fig. 4B1) that massively recruits the T channels, as predicted by the current injection experiments (Fig. 3B). As shown in Fig. S4B1, similar results were obtained in the presence of Ih. We conclude that because corticothalamic EPSPs are small amplitude and slow rising synaptic events, the probability for these synaptic inputs to evoke a LTS, and the kinetics and the amplitude of the LTS, are finely controlled by IT potentiation according to the EPSPs number and frequency. Thus, IT potentiation finely tunes APs generation evoked by the corticothalamic feedback, controlling both TC neuron excitability and output timing.
In contrast, stimulations of lemniscal fibers never revealed any effect of the IT regulation on either the amplitude or the kinetics of the LTSs evoked by the sensory EPSPs, and therefore on the latency of the first AP (Fig. 4B2a and Fig. S4B2). In our recording conditions, a single minimal stimulation of the sensory lemniscal pathway always evokes large-amplitude and fast-rising EPSPs. As predicted by the current injection experiments (Fig. 3B), the massive opening of T channels by large-amplitude EPSP generates LTS that lacks the first slow depolarizing phase and is insensitive to IT potentiation (n = 11). As a control experiment, the effect of IT potentiation on the rebound LTS was tested in the same cells (Fig. 4B2b). In three cells, increasing the stimulation frequency (up to 200 Hz) clearly demonstrates that, when it occurs, the AP closely follows the lemniscal EPSP regardless of the potentiated/nonpotentiated states of the T channels (Fig. 4B2a). Furthermore, the sensitivity of the lemniscal response to IT potentiation was also tested in a condition where a reduced amount of channels had recovered from inactivation before the sensory EPSP was generated. Decreasing the duration of the hyperpolarizing prepulse from 300 to 30 ms in three cells never revealed an effect of IT potentiation on the LTS timing or associated firing evoked by lemniscal EPSPs (Fig. 4B2c). Thus, in our recording conditions the timing between the sensory input and associated AP is remarkably robust and unaffected by IT potentiation.
Discussion
The main finding of this study is that IT potentiation that occurs when T channels are inactivated is a strong candidate pathway for the occurrence of the LTS-dependent high-frequency bursts of APs that are observed in TC neurons of awake animals. In particular, IT potentiation: (i) dramatically increases the bursting probability in condition where LTS generation is especially unfavorable, i.e., when few T channels are available for activation; (ii) directly defines the LTS triggering-threshold, i.e., the minimal depolarization requires to evoke a full blown LTS; (iii) decreases the delay and enhances the temporal fidelity of the high-frequency burst response; and (iv) provides a fine tuning mechanism to corticothalamic EPSPs and NRT IPSPs but not to sensory EPSPs.
A simple intuitive process cannot predict the impact of IT potentiation on the LTS because the interplay between the depolarizing IT and the repolarizing K+ conductances tightly constrains the conditions where an increase in IT amplitude is mirrored by an increase in the LTS amplitude. As a consequence, modulation of the LTS amplitude, and consequently of the AP firing elicited by an LTS, occurs when a small fraction of the channel population has recovered from inactivation, as after brief physiological hyperpolarization due to inhibitory synaptic events (20). In addition, the impact of IT potentiation also depends on the triggering event. The small initial IT evoked by weak depolarization induces further depolarization that open additional T channels through a positive feedback mechanism. In this condition, potentiation of the current flowing through the progressively recruited channels drastically accelerates this slow-depolarizing phase and the LTS onset kinetics. Conversely, when LTSs are triggered by large depolarization that induce a massive opening of the T channel population, the first slow-depolarizing phase is suppressed and IT potentiation has almost no effect on the LTSs kinetics.
These basic properties fully explain the differences in TC bursting responses that we observed after activation of the lemniscal sensory input and the corticothalamic feedback. The modulation of the LTS amplitude observed after stimulations of the cortical afferents is nevertheless puzzling because stimulations were applied after a 300-ms hyperpolarization that should de-inactivate a sufficient amount of T channels to generate LTSs of maximal amplitude regardless of the potentiated/nonpotentiated state of the channels (see Fig. 3). However, during the slow ramp-like depolarization due to the summation of the corticothalamic EPSPs, an additional channel inactivation occurs (21) that may be sufficient to allow the control of the LTS amplitude and its associated firing.
Considering the specific expression of IT potentiation in the primary sensory TC neurons (14), it is tempting to suggest that it plays a key role in the processing of the sensory information. Indeed, in lightly anesthetized and awake animals, burst firing that effectively transmits peripheral information to the cortex is present in TC cells (1, 5). These bursts are always preceded by a period of silence lasting at least 50–100 ms (22) that suggest the occurrence of hyperpolarizing events required to deinactivate the T channels. As we show, using both current injection and stimulation of inhibitory inputs, such hyperpolarization duration would be fully within the range where IT potentiation tightly controls the LTS amplitude and, therefore, the bursting probability.
Because sensory EPSPs are fast rising and large synaptic events in vitro (18, 23), IT potentiation does not affect the bursting probability in response to lemniscal input in our recording conditions and this may seem at odd with its potential role in sensory processing. However, pure isolated sensory EPSPs should seldom occur in vivo because of the activation of multiple whiskers (24, 25), the recruitment of the feedback thalamic inhibitory and corticothalamic pathways, and the modulatory brainstem afferents (19). Therefore, although IT potentiation is unlikely to affect the burst directly generated by large sensory EPSPs, its effect on the integration of the sensory input may be more complex when one considers the full dynamic of the thalamocortical network during sensory processing. Indeed, the long and variable delay between the sensory stimuli and the occurrence of the burst recorded in vivo (1–5) does not allow to make firm conclusions about the nature of the synaptic inputs that trigger bursting in awake animal and numerous evidences suggest that activation of the corticothalamic feedback is essential for burst firing in TC neurons. Indeed, background synaptic events originating from the cortex change the transfer function of the TC neurons in lateral geniculate nucleus slices, mixing single APs and bursts at all membrane potentials (26). Furthermore, the bursting activity of the ventrobasal neurons in alert rats is mainly associated with the whisker twitching behavior that disappears during cortical inactivation (2), and focal pharmacological enhancement of the activity of visual cortex layer VI neurons induces an increase in the burst to tonic ratio of TC neurons (27). Therefore, the marked impact of IT potentiation on the generation of the LTS by the corticothalamic EPSPs could be a key element controlling the bursting activity during sensory processing.
Materials and Methods
Electrophysiology.
Horizontal thalamic slices containing the ventrobasal nucleus and the nucleus reticularis (300 μM) were prepared from Wistar rats (PN 14–19, details are presented in SI Text).
Slices were perfused at 32°C with a medium containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 25 mM glucose. Whole-cell patch recordings of TC neurons were made by using a Cairn Optopatch and Axograph software, Version 4.9. Filter and acquisition frequencies were set at 2 and 10 kHz, respectively. Neurons were first submitted to conditioning prepulses delivered in the voltage-clamp mode before instantaneously switching to the current-clamp mode.
Electrodes were filled with 140 mM methanesulphonate, 0.1 mM CaCl2, 5 MgCl2, 1 mM EGTA, 10 mM Hepes, 4 mM Na-ATP, and 15 mM phosphocreatine and 50 units/ml creatine phosphokinase (pH 7.3) 290 mOsm. Only data obtained from pipettes with access resistances of 9–13 MΩ with <30% change during the experiment were included. When necessary, 1 μM tetrodotoxin (TTX; Tocris) was added to the extracellular medium, and K+ conductances were blocked with Tetra-Ethyl-Amonium Chloride (TEA; Sigma) and 4-aminopyridine (4-AP; Sigma).
The stimulating electrode, consisting of a glass pipette filled with the extracellular medium, was positioned 25 to 60 μm from the recorded neuron to evoke IPSPs, in the medial lemniscus to evoke sensory EPSPs or in the internal capsule to evoke cortical EPSPs. Isolation of GABAA IPSPs and glutamatergic EPSPs was obtained by adding to the perfusion medium 50 μM DL-APV (Fluka) and 10 μM CNQX (ICN) or 1 μM SR95531 (Sigma), respectively. Quantitative data are given as mean ± SD.
Model.
Simulations were performed under the Neuron 5.8 environment (28).
The current balance equation is:
where Ileak is the leakage current, Ielec is the current injected, V is the membrane potential, and C = 290 pF is the total capacitance.
IT was simulated by using a multistate Markov model (29). Simulations were successively performed assuming the whole T channel population either in a potentiated or a nonpotentiated state. To established these conditions, the maximum Ca2+ permeability was alternatively set to 1.38 × 10−4 cm/s and 2.2 × 10−4 cm/s to generate a 1.5 difference between nonpotentiated and potentiated IT amplitude respectively in the Goldman–Hodgkin–Katz equation. A previously introduced Hodgkin–Huxley-like model (15, 21) was used to simulate IA (maximal conductance = 6.9 106 nS/cm2) and Ih (maximal conductance = 1 105 nS/cm2), and the leakage current was modeled by the sum of a Na+ (maximal conductance = 9.14 103 nS/cm2) and K+ (maximal conductance = 24 103 nS/cm2) current. A detailed description of the model is presented in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by ANR-06-NEURO and the Fondation pour la Recherche Médicale (T.B.). We thank Dr Le Masson for providing RT-Neuron.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801484105/DCSupplemental.
References
- 1.Bezdudnaya T, et al. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramcharan EJ, Cox CL, Zhan XJ, Sherman SM, Gnadt JW. Cellular mechanisms underlying activity patterns in the monkey thalamus during visual behavior. J Neurophysiol. 2000;84:1982–1987. doi: 10.1152/jn.2000.84.4.1982. [DOI] [PubMed] [Google Scholar]
- 4.Swadlow HA, Gusev AG. The impact of “bursting” thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- 5.Guido W, Weyand T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol. 1995;74:1782–1786. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- 6.Lesica NA, Stanley GB. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. J Neurosci. 2004;24:10731–10740. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning KS, Reinagel P. Visual control of burst priming in the anesthetized lateral geniculate nucleus. J Neurosci. 2005;25:3531–3538. doi: 10.1523/JNEUROSCI.4417-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinagel P. The inner life of bursts. Neuron. 2007;55:339–341. doi: 10.1016/j.neuron.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: A comparison between cortically projecting and reticularis neurones. J Physiol (London) 1986;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, et al. Feedforward excitation and inhibition evoke dual modes of firing in the cat's visual thalamus during naturalistic viewing. Neuron. 2007;55:465–478. doi: 10.1016/j.neuron.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 12.Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: An in vitro study. J Physiol. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 14.Leresche N, Hering J, Lambert RC. Paradoxical potentiation of neuronal T-type Ca2+ current by ATP at resting membrane potential. J Neurosci. 2004;24:5592–5602. doi: 10.1523/JNEUROSCI.1038-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 16.Pape HC, Budde T, Mager R, Kisvarday ZF. Prevention of Ca(2+)-mediated action potentials in GABAergic local circuit neurones of rat thalamus by a transient K+ current. J Physiol (London) 1994;478(Pt 3):403–422. doi: 10.1113/jphysiol.1994.sp020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steriade M, Jones EG, McCormick DA. THALAMUS. Oxford: Elsevier; 1997. [Google Scholar]
- 18.Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. J Physiol (London) 1998;510:829–843. doi: 10.1111/j.1469-7793.1998.829bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez C, Cox CL, Rinzel J, Sherman SM. Dynamics of low-threshold spike activation in relay neurons of the cat lateral geniculate nucleus. J Neurosci. 2001;21:1022–1032. doi: 10.1523/JNEUROSCI.21-03-01022.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu SM, Guido W, Sherman SM. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: Contributions of the low-threshold Ca2+ conductance. J Neurophysiol. 68:2185–2198. doi: 10.1152/jn.1992.68.6.2185. [DOI] [PubMed] [Google Scholar]
- 23.Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol. 2002;87:946–953. doi: 10.1152/jn.00426.2001. [DOI] [PubMed] [Google Scholar]
- 24.Ghazanfar AA, Nicolelis MA. Feature article: The structure and function of dynamic cortical and thalamic receptive fields Cereb Cortex. 2001;11:183–193. doi: 10.1093/cercor/11.3.183. [DOI] [PubMed] [Google Scholar]
- 25.Deschênes M, Timofeeva E, Lavallee P. The relay of high-frequency sensory signals in the Whisker-to-barreloid pathway. J Neurosci. 2003;23:6778–6787. doi: 10.1523/JNEUROSCI.23-17-06778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfart J, Debay D, Le Masson G, Destexhe A, Bal T. Synaptic background activity controls spike transfer from thalamus to cortex. Nat Neurosci. 2005;8:1760–1767. doi: 10.1038/nn1591. [DOI] [PubMed] [Google Scholar]
- 27.Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B. 2002;357:1739–1752. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 29.Lambert RC, Bessaïh T, Leresche N. Biophysical mechanisms underlying the paradoxical potentiation of the low-voltage activated calcium current in thalamocortical neurons: A modeling study. Thalamus Relat Syst. 2005;3:165–173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.