Abstract
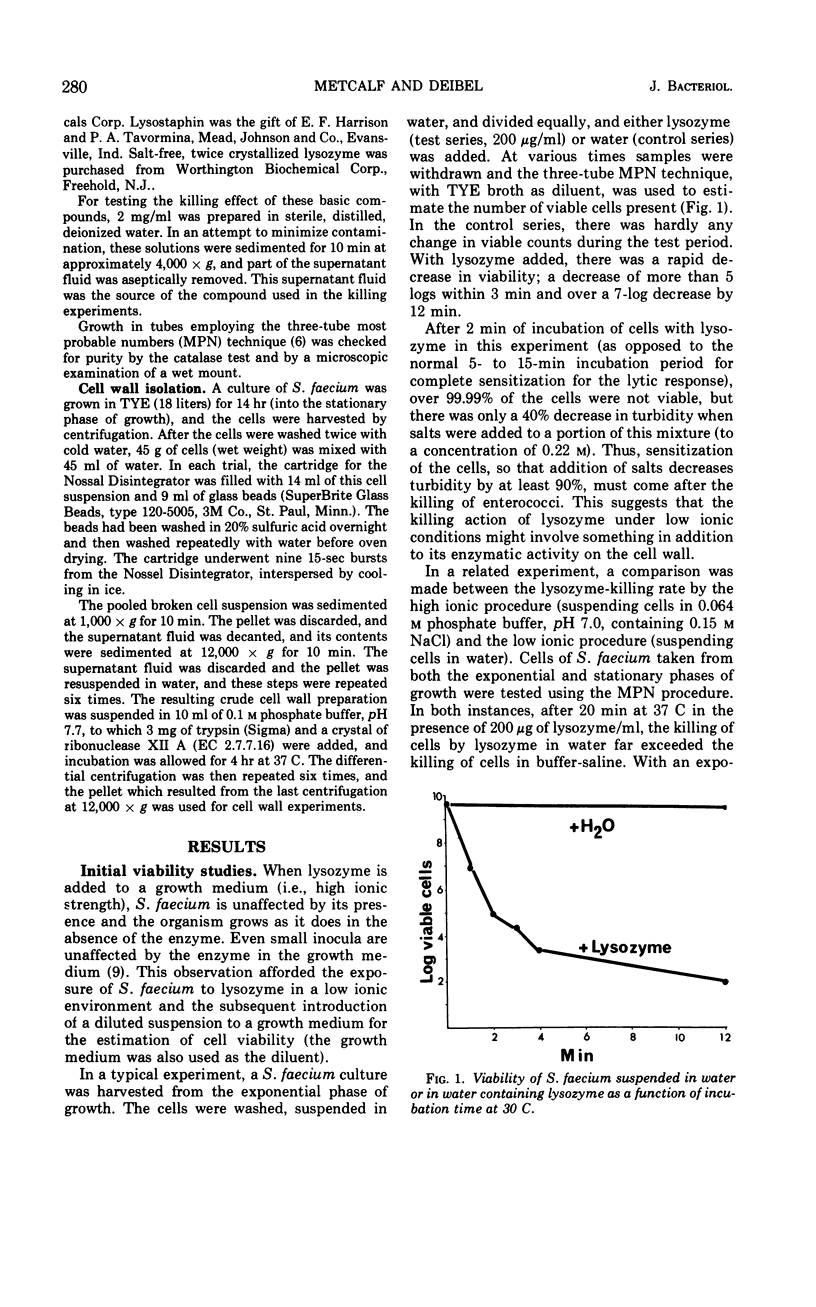
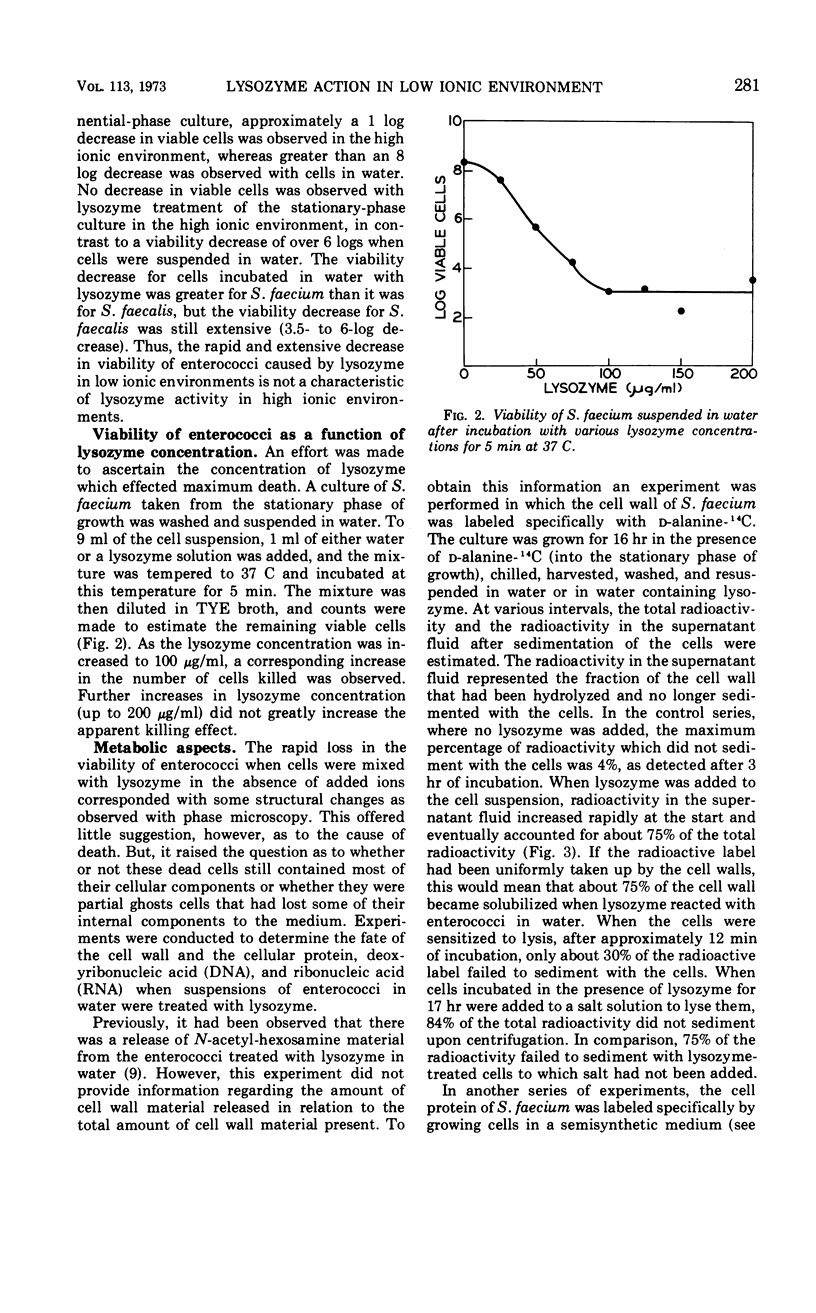
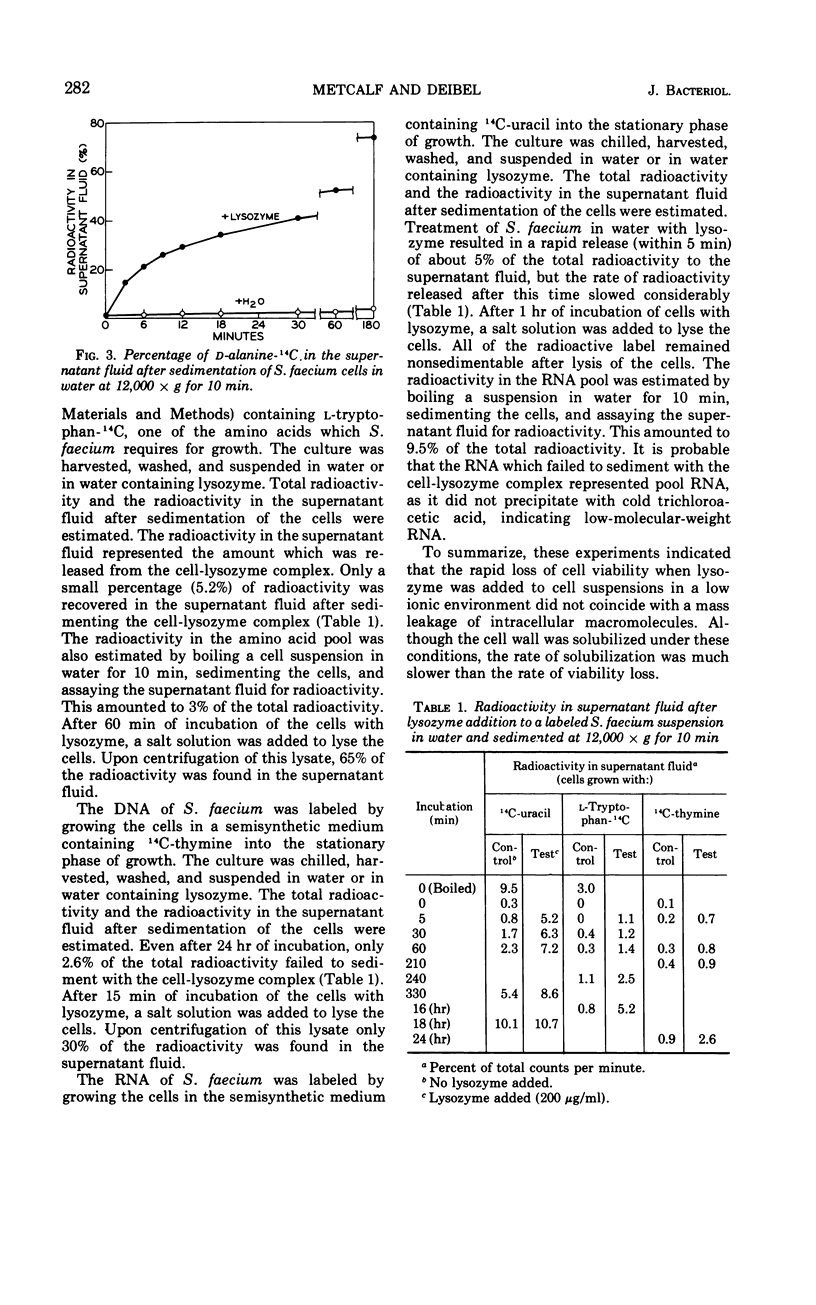
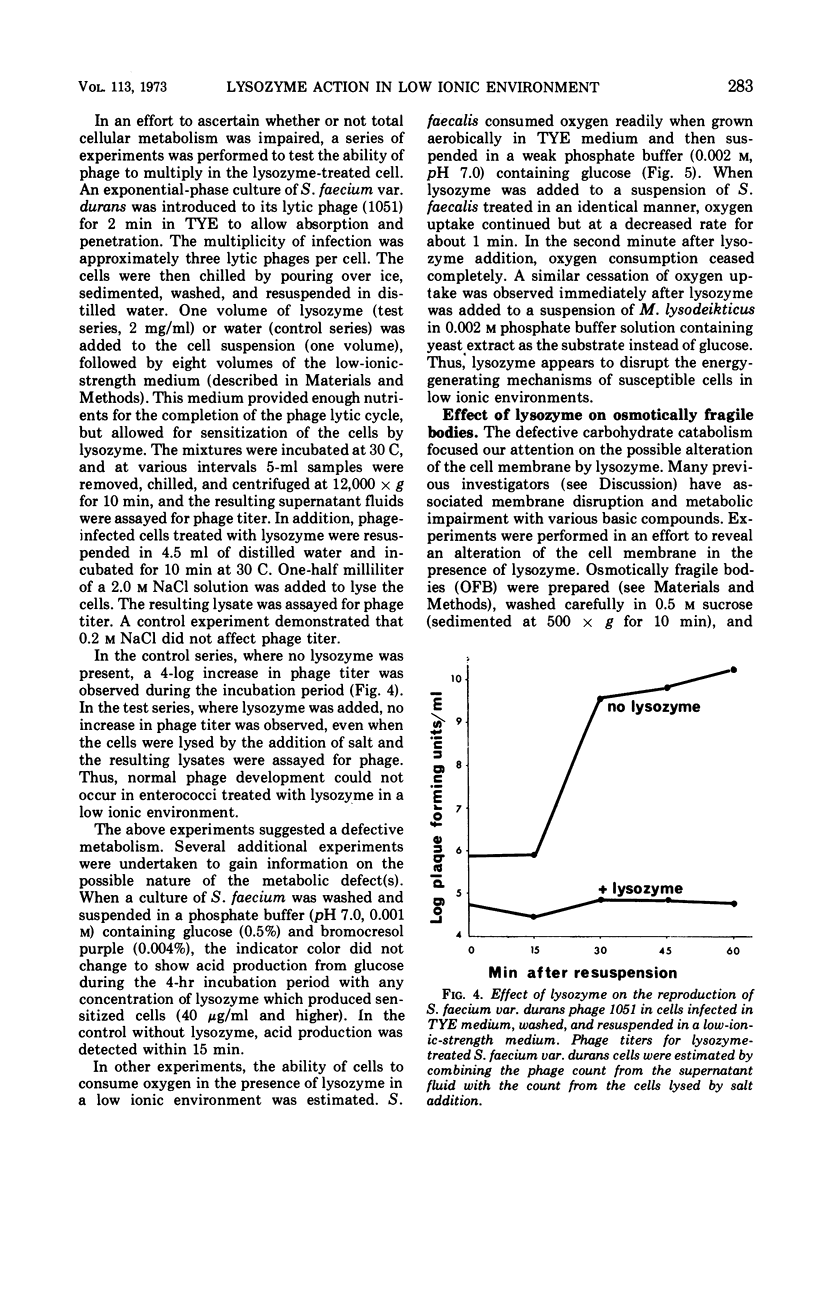
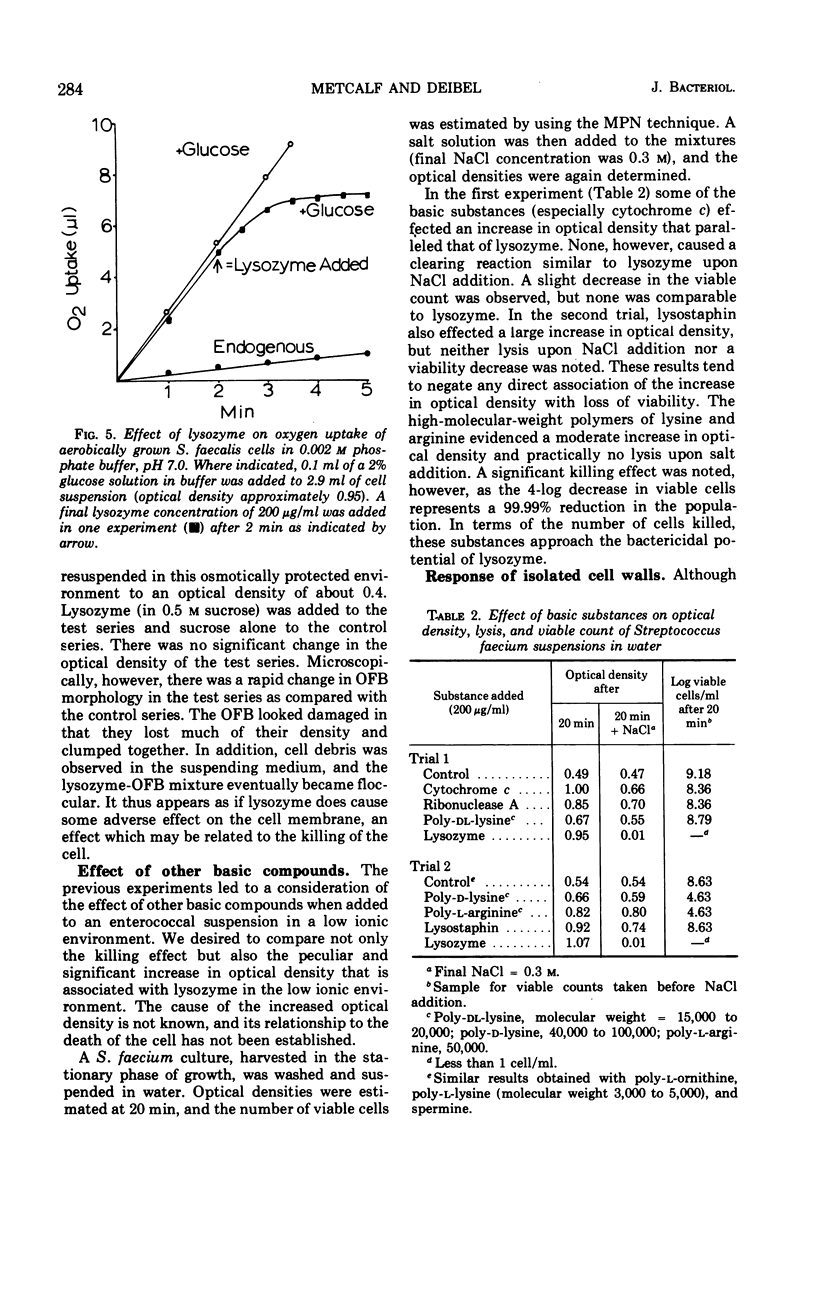
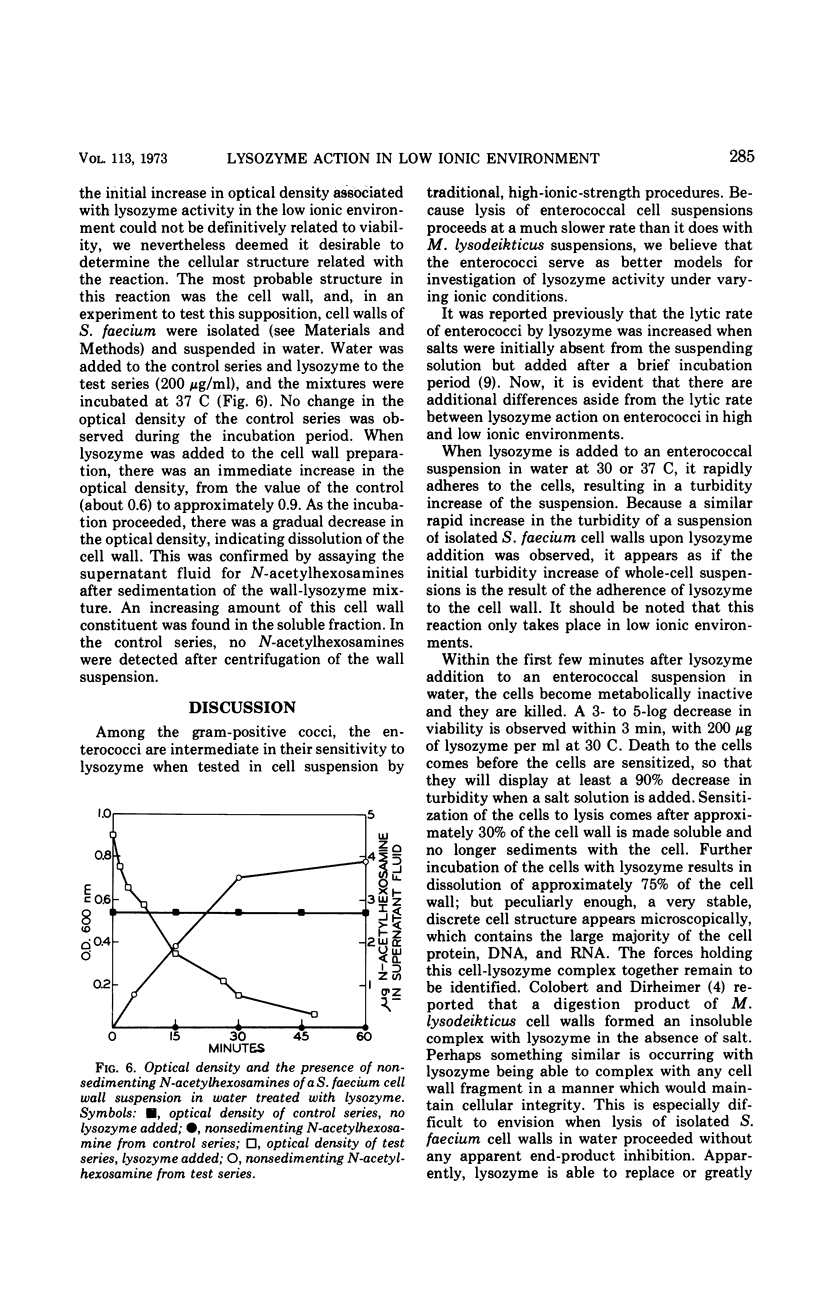
The action of lysozyme on the enterococcal cell differed markedly as a function of the ionic strength of the environment. In high ionic environments (I ≃ 0.3), the traditionally slow lytic response and decrease in viability were noted. In a low ionic environment the majority of the cell wall was hydrolyzed, but cellular integrity was preserved and almost all cellular protein, deoxyribonucleic acid and ribonucleic acid remained with the lysozyme-cell complex. However, under these conditions, lysozyme inactivated energy-yielding metabolism, and a rapid extensive loss of viability was observed. Some other basic compounds without lytic activity on the cell wall also effected a substantial reduction in viability. The data suggest that lysozyme acts on the cell membrane to effect disruption of cellular metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S. The effects of ribonuclease and deoxyribonuclease on bacteriophage formation in protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1955 Dec;18(4):531–534. doi: 10.1016/0006-3002(55)90146-3. [DOI] [PubMed] [Google Scholar]
- BURGER W. C., STAHMANN M. A. The agglutination and growth inhibition of bacteria by lysine polypeptides. Arch Biochem Biophys. 1952 Jul;39(1):27–36. doi: 10.1016/0003-9861(52)90257-9. [DOI] [PubMed] [Google Scholar]
- Bachrach U., Weinstein A. Effect of aliphatic polyamines on growth and macromolecular syntheses in bacteria. J Gen Microbiol. 1970 Feb;60(2):159–165. doi: 10.1099/00221287-60-2-159. [DOI] [PubMed] [Google Scholar]
- COLOBERT L., DIRHEIMER G. [Action of lysozyme on a glycopeptidic substrate isolated from Micrococcus lysodeikticus]. Biochim Biophys Acta. 1961 Dec 23;54:455–468. doi: 10.1016/0006-3002(61)90085-3. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., NIVEN C. F., Jr Reciprocal replacement of oleic acid and CO2 in the nutrition of the minute streptococci and Lactobacillus leichmannii. J Bacteriol. 1955 Aug;70(2):134–140. doi: 10.1128/jb.70.2.134-140.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. E., Bachrach U. Inhibition of protein synthesis by spermine in growing cells of Staphylococcus aureus. J Bacteriol. 1966 Jul;92(1):49–55. doi: 10.1128/jb.92.1.49-55.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf R. H., Deibel R. H. Differential lytic response of enterococci associated with addition order of lysozyme and anions. J Bacteriol. 1969 Sep;99(3):674–680. doi: 10.1128/jb.99.3.674-680.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf R. H., Deibel R. H. Growth of Streptococcus faecium in the presence of lysozyme. Infect Immun. 1972 Aug;6(2):178–183. doi: 10.1128/iai.6.2.178-183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Svihla G., Dainko J. L., Schlenk F. Ultraviolet micrography of penetration of exogenous cytochrome c into the yeast cell. J Bacteriol. 1969 Oct;100(1):498–504. doi: 10.1128/jb.100.1.498-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. L., Shively J. M. Lethal action of ribonuclease for thermophilic bacilli. J Bacteriol. 1966 Feb;91(2):673–676. doi: 10.1128/jb.91.2.673-676.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yphantis D. A., Dainko J. L., Schlenk F. Effect of some proteins on the yeast cell membrane. J Bacteriol. 1967 Nov;94(5):1509–1515. doi: 10.1128/jb.94.5.1509-1515.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


