Abstract
Shear stress of the blood at the vessel wall plays an important role in many processes in the cardiovascular system primarily focused on the regulation of vessel lumen and wall dimensions. There is ample evidence that atherosclerotic plaques are generated at low shear stress regions in the cardiovascular system, while high shear stress regions are protected. In the course of plaque progression, advanced plaques start to encroach into the lumen, and thereby start to experience high shear stress at the endothelium. Until now the consequences of high shear stress working at the endothelium of an advanced plaque are unknown. As high shear stress influences tissue regression, we hypothesised that high shear stress can destabilise the plaque by cap weakening leading to ulceration. We investigated this hypothesis in a magnetic resonance imaging (MRI) dataset of a 67-year-old woman with a plaque in the carotid artery at baseline and an ulcer at ten-month follow-up. The lumen, plaque components (lipid/necrotic core, intraplaque haemorrhage) and ulcer were reconstructed three dimensionally and the geometry at baseline was used for shear stress calculation using computational fluid dynamics. Correlation of the change in plaque composition with the shear stress at baseline showed that the ulcer was generated exclusively at the high shear stress location. In this serial MRI study we found plaque ulceration at the high shear stress location of a protruding plaque in the carotid artery. Our data suggest that high shear stress influences plaque vulnerability and therefore may become a potential parameter for predicting future events. (Neth Heart J 2008;16:280-3.)
Keywords: shear stress, plaque vulnerability, magnetic resonance imaging
Cerebrovascular events are frequently caused by rupture of a vulnerable plaque in the carotid arteries. Rupture-prone, or vulnerable, plaques are characterised by their specific morphology and composition: a large lipid pool covered by a thin fibrous cap infiltrated by macrophages,1 and expansive remodelling.2,3 The strength of the cap of a vulnerable plaque is determined by the balance between cap-consolidating matrix synthesis by smooth muscle cells (SMC) and enzymatic matrix degradation induced by macrophages.4 Studies on the spatial distribution of plaque components showed that the concentration of macrophages is higher,5,6 the density of SMC is lower and metalloproteinase (MMP-9) activity is higher at the upstream side of the plaque compared with the downstream side,6 suggesting that plaque vulnerability is associated with the direction of the flow. Indeed, plaque rupture was observed to occur most frequently at the upstream part of a lumen intruding-plaque (figure 4).6-8 Until now, it is unclear which factors influence the higher vulnerability at the upstream side of a lumen-intruding plaque. In this paper we will focus on the blood flow induced wall shear stress, which is high at the upstream, and low at the downstream side of a lumen-intruding plaque. There is ample evidence that shear stress affects endothelial metabolism, such that high shear stress induces antiproliferative,9 anti-inflammatory,10,11 and antithrombotic actions,12 whereas low shear stress stimulates proatherogenic processes.9 Indeed, in the presence of atherosclerotic risk factors, plaques, including plaques with a vulnerable phenotype in mice, are more likely to develop at low shear stress sites.13-16 However, when compensatory remodelling fails7 or following intraplaque haemorrhage,17 lumen narrowing is observed and shear stress increases at the upstream side of the plaque (figure 1).14 The question rises as to what the influence is of the increased shear stress on the endothelium of lumen-intruding plaques. There is abundant evidence that the endothelium responds to high shear stress such that it induces an antiproliferative action9 which may lead to cap thinning. For that reason, we hypothesised that high shear stress at the upstream side of the plaque has a biological effect on the fibrous cap and therefore enhances plaque vulnerability.18 We present a patient, in which we demonstrate the relation between high shear stress and plaque rupture in a ten-month follow-up period.
Figure 4.
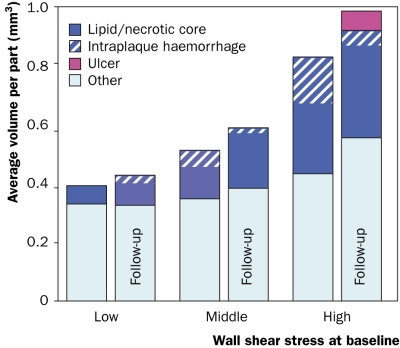
Vessel wall composition at baseline and ten-month followup as function of baseline wall shear stress.
Figure 1.
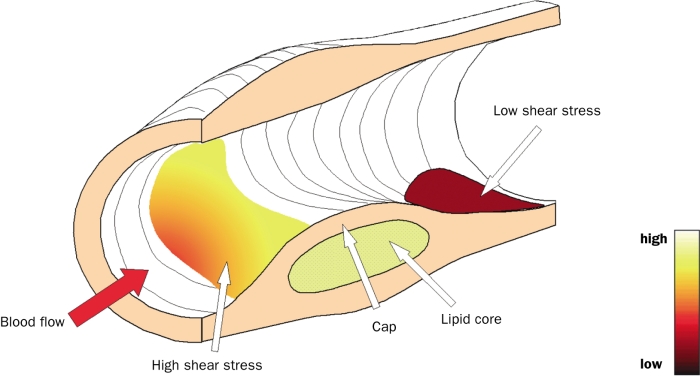
Drawing of a lumen-intruding plaque with shear stress projected at the lumen (colour code).
Materials and Methods
Patient
A 67-year-old individual, who was found to have moderate carotid stenosis by duplex ultrasonography, was scanned using serial carotid magnetic resonance imaging (MRI). The patient’s baseline MRI showed a plaque in the right carotid artery, while at the ten-month follow-up MRI a plaque rupture with an ulcer was observed.19 The institutional review committee approved the study and the patient gave informed consent.
MRI
MRI of the carotid arteries was performed at baseline and at ten-month follow-up. The high-resolution, multisequence MRI protocol included four sequences: 3D time of flight (TOF), T1, T2 and proton density (PD) weightings. The resolution was 0.3x0.3x2 mm. The image segmentation was based on the signal intensities relative to the adjacent sternocleidomastoid muscle. A validated scheme of hyper-, isoand hypo-intense signal intensities from the TOF, T1, T2 and PD images was used to identify the lumen, lipid/necrotic core, intraplaque haemorrhage and ulcer.20
Computational fluid dynamics
Using the baseline lumen and wall contours, a 3D reconstruction of the carotid bifurcation including the wall components was performed. The 3D reconstruction of the lumen geometry was imported into GAMBIT (Fluent Inc., Canonsburg, Pennsylvania, USA) from which a 3D meshed volume was created. At the entrance and exit of the carotid bifurcation circular segments were added to minimise the influence of the boundary conditions. A parabolic inflow profile with a peak velocity of 0.6 m/s was chosen as inflow condition to result in physiological shear stress values (1.2 Pa) in the common carotid artery. Flow velocities and shear stress distribution was calculated using FIDAP (Fluent Inc., Canonsburg, Pennsylvania, USA) applying free outflow for the internal and external arteries; there was no slip at the wall. Blood was simulated as an incompressible Newtonian fluid with a viscosity of 3.5 mPa.s and a density of 1050 kg/m3.
Analysis
MRI cross-sections were matched at baseline and follow-up using the location of the bifurcation as marker. Only the slices that contained plaque at baseline and/or follow-up were selected for further analysis. For each slice the wall was divided into 256 parts, such that each baseline lumen contour was divided into 256 equidistant sections. In each part the average baseline shear stress and the baseline wall component volumes (i.e. area times slice thickness) were calculated using in-house created software. Subsequently, in each part the volumes of the wall components at follow-up were determined.
Results
At baseline, the lipid/necrotic core volume was 308 mm3, from which 34% consisted of intraplaque haemorrhage, and increased to 335 mm3 with 16% intraplaque haemorrhage during the ten-month follow-up period. Figure 2 shows the 3D reconstruction of the lumen and wall components at follow-up. The average shear stress at baseline in the carotid bifurcation was 3.2±2.0 Pa and the site of ulceration was observed at the highest shear stress (figures 2 and 3). To quantify this observation, the lumen surface was divided into three groups based on the local shear stress (low, middle, and high). For each tertile the average volume of wall components at baseline and follow-up was computed. The total volume and the lipid/necrotic volume increased both with shear stress and time and the ulcer at follow-up was found in the highest shear stress tertile (figure 4).
Figure 2.
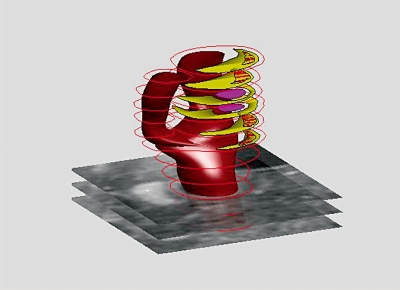
3D reconstruction of lumen and wall of a human carotid artery at ten-month follow-up based on MRI (pink: ulcer, yellow: glipid necrotic core, yellow/red: intraplaque haemorrhage).
Figure 3.
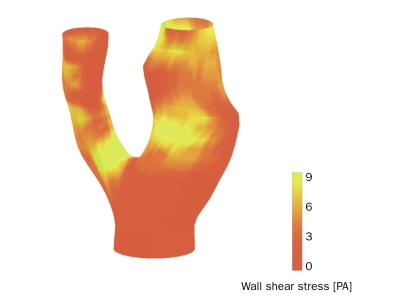
3D reconstruction of baseline lumen geometry of a human carotid artery with shear stress distribution mapped in colour code.
Discussion
Application of serial MRI in combination with computational fluid dynamics in the carotid artery of a single patient showed that high shear stress at baseline and subsequent ulceration ten months later are co-localised. Data collection of this patient is quite unique, as not many studies include a baseline and follow-up MRI and not many patients happen to experience ulceration in such a small time window.
Knowledge is lacking about the mechanisms which make the vulnerable plaque susceptible to rupture. In a review a number of biological pathways were proposed, which could explain the important role of high shear stress in destabilisation of the vulnerable plaque.18 In this case study the weakest location appeared at the upstream highest shear stress region of the plaque. This agrees well with observations that high shear stress is co-localised with high strain spots in human coronary arteries.19 Presence of high strain spots at plaques have been shown to be indicative for the presence of thin-cap fibroatheroma with a sensitivity and specificity of 88 and 89%, respectively.21 Moreover, patients with acute coronary syndromes had a higher number of high strain spots than stable patients.22
Intraplaque haemorrhage is known to be involved in plaque progression and cerebral events. The observed intraplaque haemorrhage at baseline could have accelerated the destabilisation of the plaque; however, in this case the site of rupture was precisely at the highest shear stress region. More patients will be required to confirm this preliminary finding that high shear stress is involved in plaque rupture and to prove the value of this technology in risk prediction.
Acknowledgements
The financial support for H.C. Groen of the Interuniversity Cardiology Institute of the Netherlands is greatly acknowledged. We would like to thank both Dr Liu and Dr Chu for the MRI data.
References
- 1.Moreno P, Falk E, Palacios I, et al. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation 1994;90:775-8. [DOI] [PubMed] [Google Scholar]
- 2.Pasterkamp G, Schoneveld AH, van der Wal AC, et al. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol 1998/9;32:655-62. [DOI] [PubMed] [Google Scholar]
- 3.Burke AP, Farb A, Malcom GT, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276-82. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Geng YJ, Aikawa M, et al. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996;7:330-5. [DOI] [PubMed] [Google Scholar]
- 5.Dirksen MT, van der Wal AC, van den Berg FM, et al. Distribution of inflammatory cells in atherosclerotic plaques relates to the direction of flow. Circulation 1998;98:2000-3. [DOI] [PubMed] [Google Scholar]
- 6.Krams R, Cheng C, Helderman F, et al. Shear stress is associated with markers of plaque vulnerability and MMP-9 activity. Eurointervention. 2006;2:250-6. [PubMed] [Google Scholar]
- 7.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371-5. [DOI] [PubMed] [Google Scholar]
- 8.Masawa N, Yoshida Y, Yamada T, et al. Three-dimensional analysis of human carotid atherosclerotic ulcer associated with recent thrombotic occlusion. Pathol Int 1994;44:745-52. [DOI] [PubMed] [Google Scholar]
- 9.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035-42. [DOI] [PubMed] [Google Scholar]
- 10.Yamawaki H, Lehoux S, Berk BC. Chronic physiological shear stress inhibits tumor necrosis factor-induced proinflammatory responses in rabbit aorta perfused ex vivo. Circulation 2003;108:1619-25. [DOI] [PubMed] [Google Scholar]
- 11.Ando J, Tsuboi H, Korenaga R, et al. Down-regulation of vascular adhesion molecule-1 by fluid shear stress in cultured mouse endothelial cells. Ann N Y Acad Sci 1995;748:148-56; discussion [DOI] [PubMed] [Google Scholar]
- 12.Malek AM, Jackman R, Rosenberg RD, et al. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res 1994;74:852-60. [DOI] [PubMed] [Google Scholar]
- 13.Krams R, Wentzel J, Oomen J, et al. Evaluation of endothelial shear stress and 3D geometry as factors determining the development of atherosclerosis and remodeling in human coronary arteries in vivo. Combining 3D reconstruction from angiography and IVUS (ANGUS) with computational fluid dynamics. Arterioscler Thromb Vasc Biol 1997;17:2061-5. [DOI] [PubMed] [Google Scholar]
- 14.Wentzel JJ, Janssen E, Vos J, et al. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 2003;108:17-23. [DOI] [PubMed] [Google Scholar]
- 15.Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006;113:2744-53. [DOI] [PubMed] [Google Scholar]
- 16.Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the Localization of High-Risk Coronary Atherosclerotic Plaques on the Basis of Low Endothelial Shear Stress. An Intravascular Ultrasound and Histopathology Natural History Study. Circulation 2008;26:993-1002. [DOI] [PubMed] [Google Scholar]
- 17.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316-25. [DOI] [PubMed] [Google Scholar]
- 18.Slager CJ, Wentzel JJ, Gijsen FJ, et al. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat Clin Pract Cardiovasc Med 2005;2:456-64. [DOI] [PubMed] [Google Scholar]
- 19.Gijsen F, Wentzel J, Mastik F, et al. Shear stress predicts distribution of high strain spots on plaques in human coronary arteries. Eur Heart J 2006;27 (Suppl I):583-3. [Google Scholar]
- 20.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25:234-9. [DOI] [PubMed] [Google Scholar]
- 21.Schaar JA, de Korte CL, Mastik F, et al. Characterizing vulnerable plaque features with intravascular elastography. Circulation 2003;25:2636-41. [DOI] [PubMed] [Google Scholar]
- 22.Schaar JA, Regar E, Mastik F, et al. Incidence of high-strain patterns in human coronary arteries: assessment with three-dimensional intravascular palpography and correlation with clinical presentation. Circulation 2004;109:2716-9. [DOI] [PubMed] [Google Scholar]


