Abstract
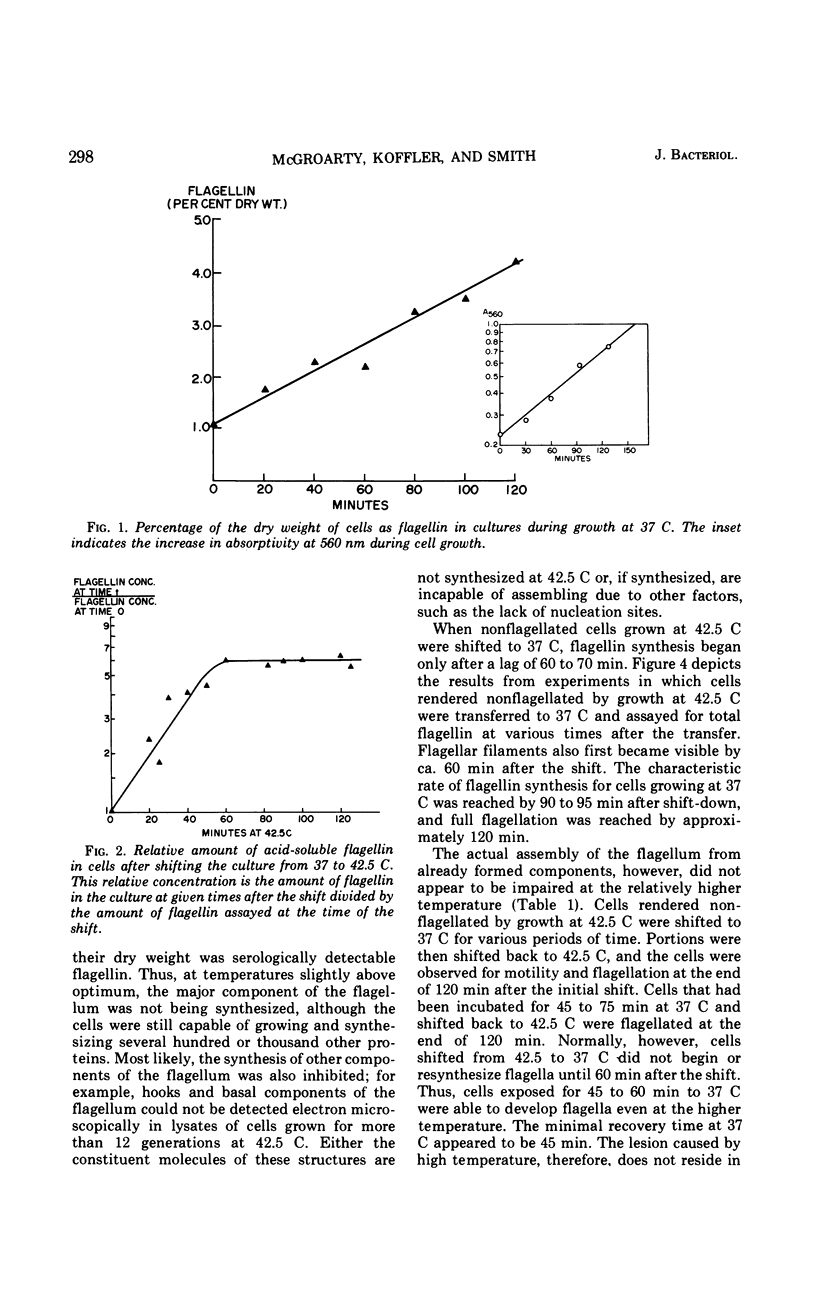
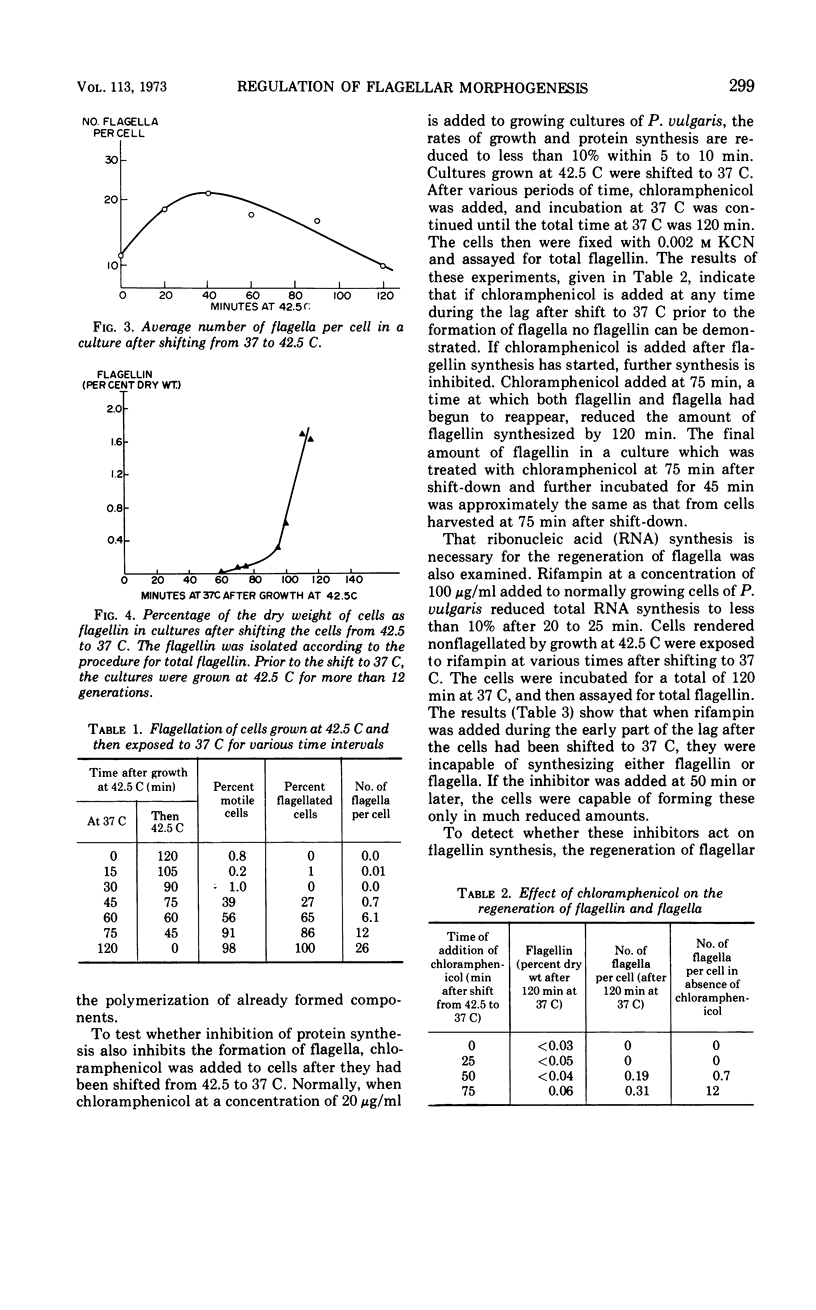
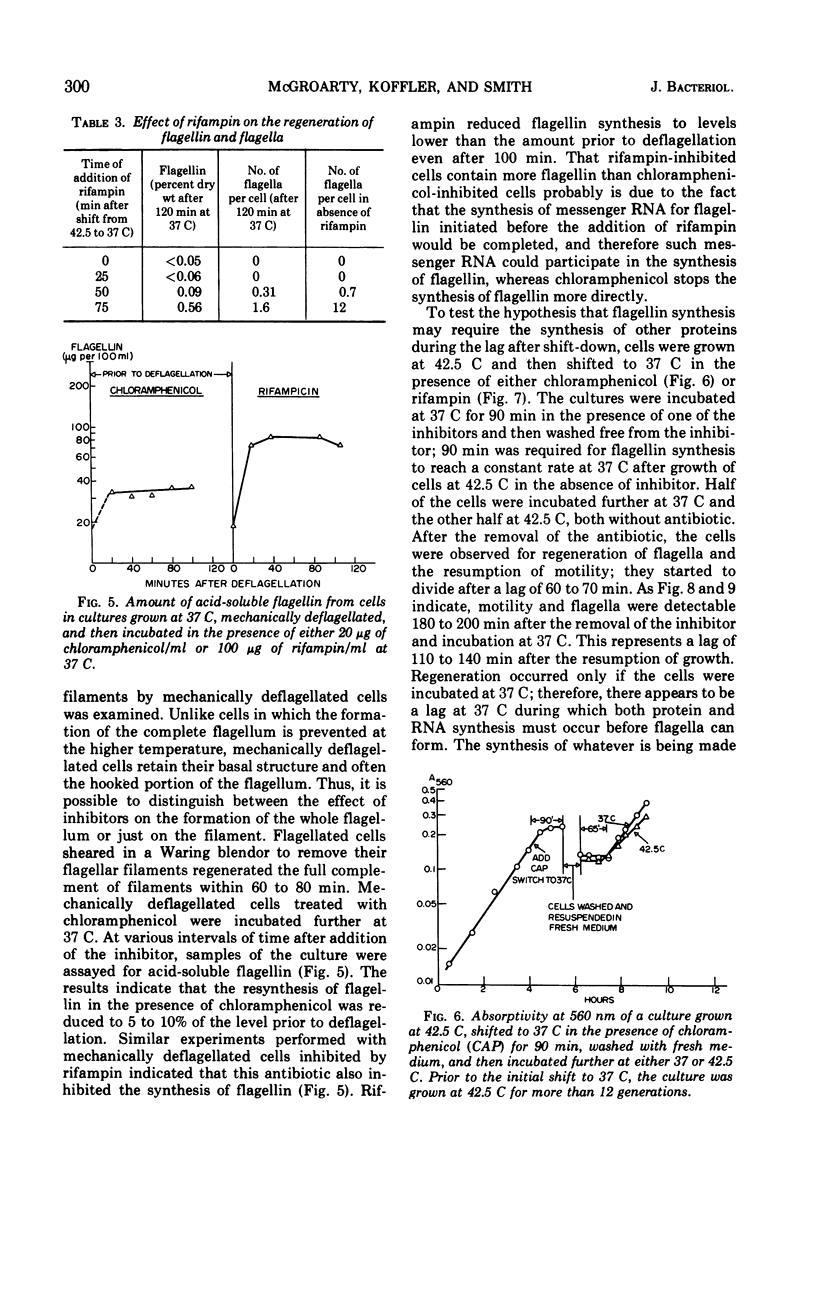
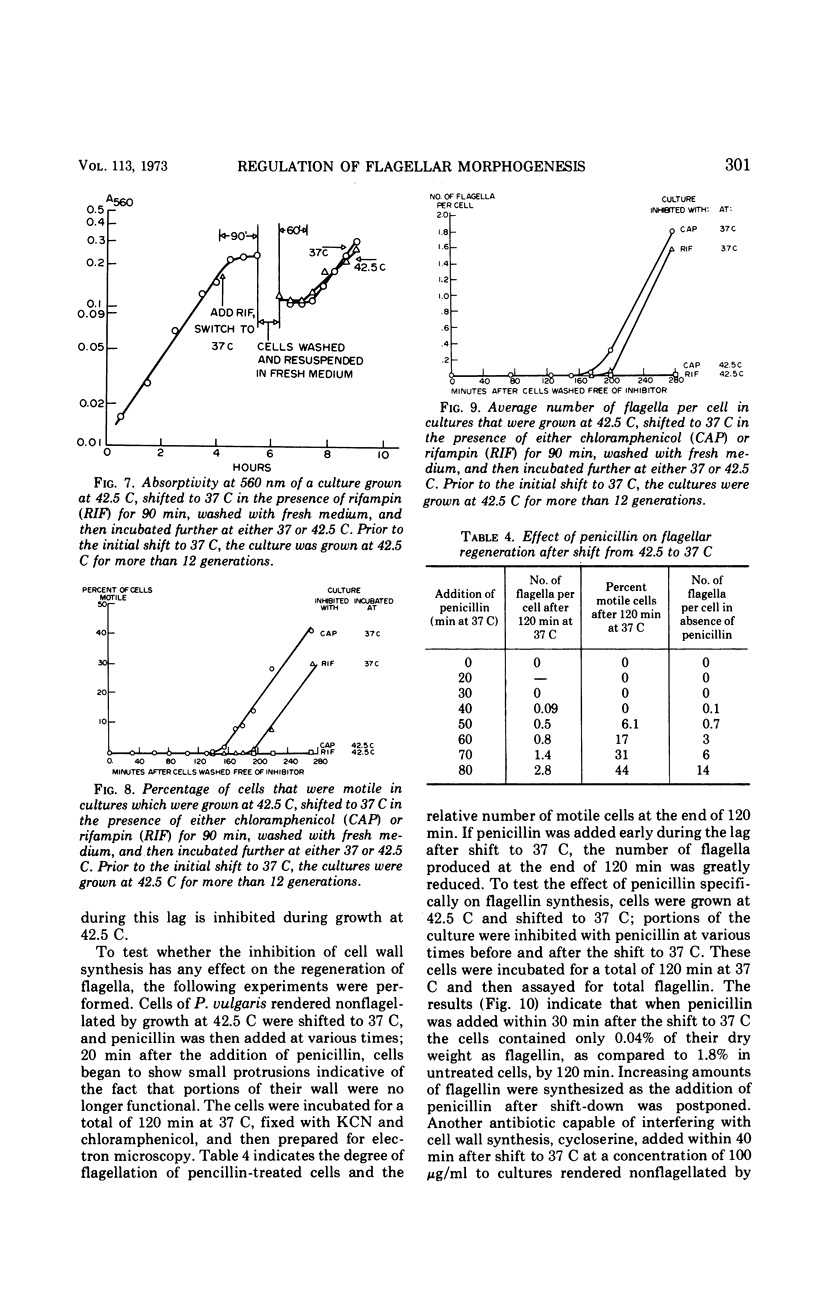
When cells of Proteus vulgaris were transferred from 37 to 42 C, a temperature at which they continue to grow almost optimally, they ceased to form flagella after approximately one generation time. This failure was due to a lesion in the flagellin-synthesizing process rather than the inability of these cells to assemble the organelle from constituents once formed. After transfer back to 37 C, these cells regained their ability to synthesize flagellin and form flagella, after one generation. When added during this period, chloramphenicol, rifampin, or penicillin prevented the synthesis of flagellin. The regeneration of the organelle at 37 C, then, requires growth for one generation, a period during which not only ribonucleic acid and protein synthesis, but also the presence of an intact cell envelope or concurrent synthesis of cell wall, are required.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., KOFFLER H. IN VITRO FORMATION OF FLAGELLA-LIKE FILAMENTS AND OTHER STRUCTURES FROM FLAGELLIN. J Mol Biol. 1964 Jul;9:168–185. doi: 10.1016/s0022-2836(64)80098-x. [DOI] [PubMed] [Google Scholar]
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967 Feb;46(2):175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- BISSET K. A., PEASE P. The distribution of flagella in dividing bacteria. J Gen Microbiol. 1957 Apr;16(2):382–384. doi: 10.1099/00221287-16-2-382. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dobrogosz W. J., Hamilton P. B. The role of cyclic AMP in chemotaxis in Escherichia coli. Biochem Biophys Res Commun. 1971 Jan 22;42(2):202–207. doi: 10.1016/0006-291x(71)90088-x. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of inhibitors on the formation of flagella by Salmonella typhimurium. J Gen Microbiol. 1960 Dec;23:519–538. doi: 10.1099/00221287-23-3-519. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRISON R. B. The effect of temperature and chloramphenicol on the development of flagella and motility in a strain of Escherichia coli. J Pathol Bacteriol. 1961 Jul;82:189–192. doi: 10.1002/path.1700820123. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- PRESTON N. W., MAITLAND H. B. The influence of temperature on the motility of Pasteurella pseudotuberculosis. J Gen Microbiol. 1952 Aug;7(1-2):117–128. doi: 10.1099/00221287-7-1-2-117. [DOI] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. An environmentally-induced transition from the flagellated to the non-flagellated state in Salmonella typhimurium: the fate of parental flagella at cell division. J Gen Microbiol. 1962 Jun;28:257–270. doi: 10.1099/00221287-28-2-257. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Koffler H. Bacterial flagella. Adv Microb Physiol. 1971;6:219–339. doi: 10.1016/s0065-2911(08)60070-3. [DOI] [PubMed] [Google Scholar]
- Vaituzis Z., Doetsch R. N. Flagella of Escherichia coli spheroplasts. J Bacteriol. 1966 May;91(5):2103–2104. doi: 10.1128/jb.91.5.2103-2104.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


