Abstract
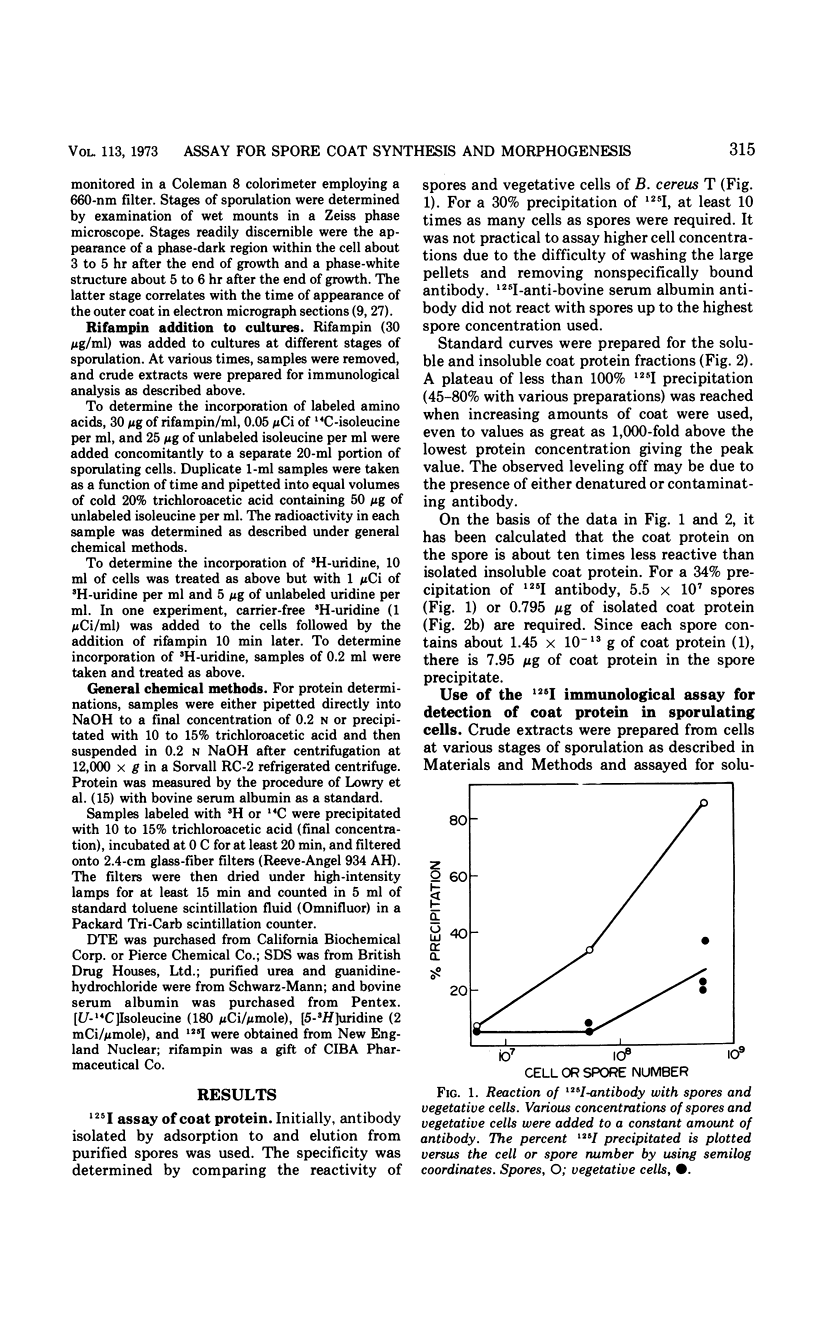
A quantitative assay employing 125I-labeled antibody has been developed for Bacillus cereus T spore coat protein. Populations of antibody molecules with various affinities for inner or outer coat can be prepared by selective adsorption to and elution from different coat preparations. Adsorption to and elution from intact spores results in an antibody preparation at least 15 times more reactive to outer coat. This antibody is useful for measuring the time and extent of spore coat maturation, i.e., outer spore coat formation. Rifampin inhibits the increase in content of this coat antigen.
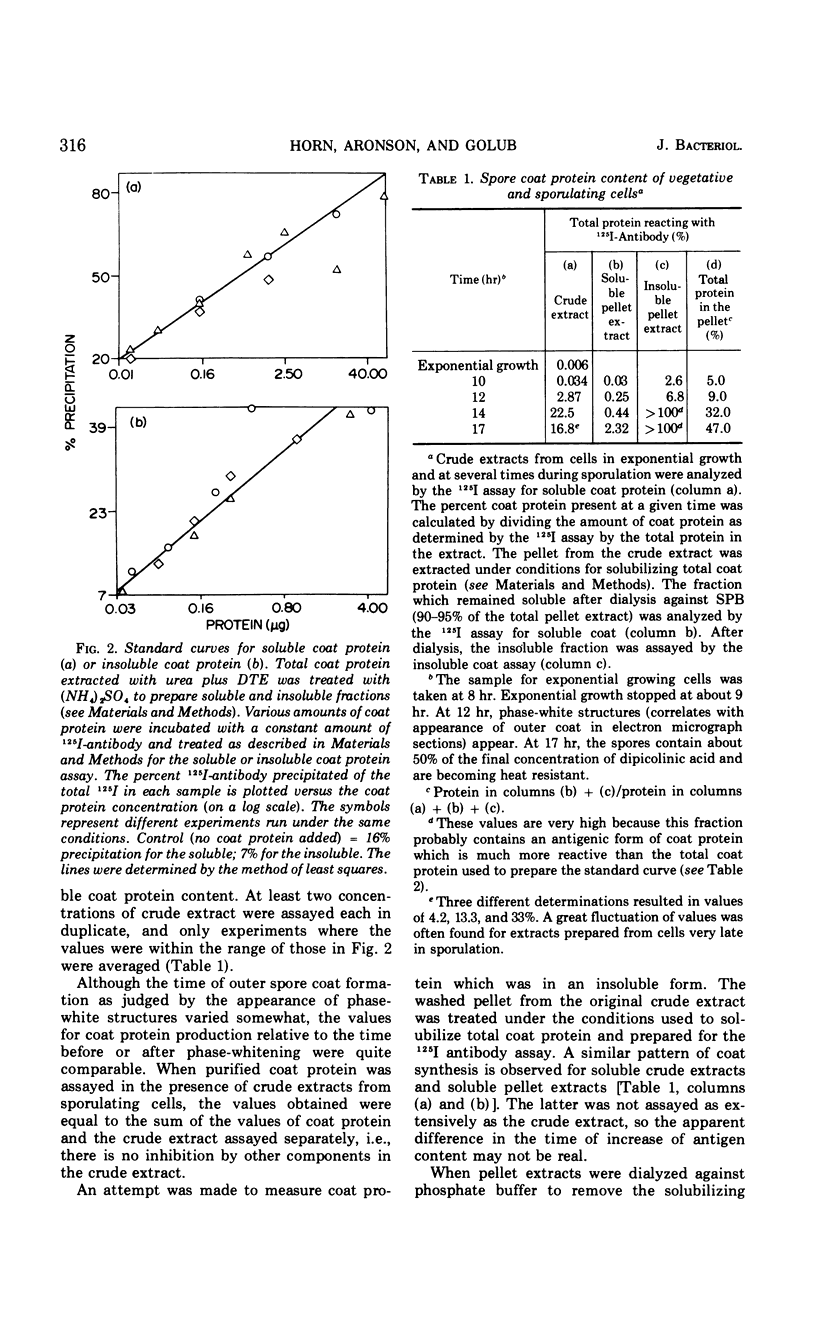
Full text
PDF

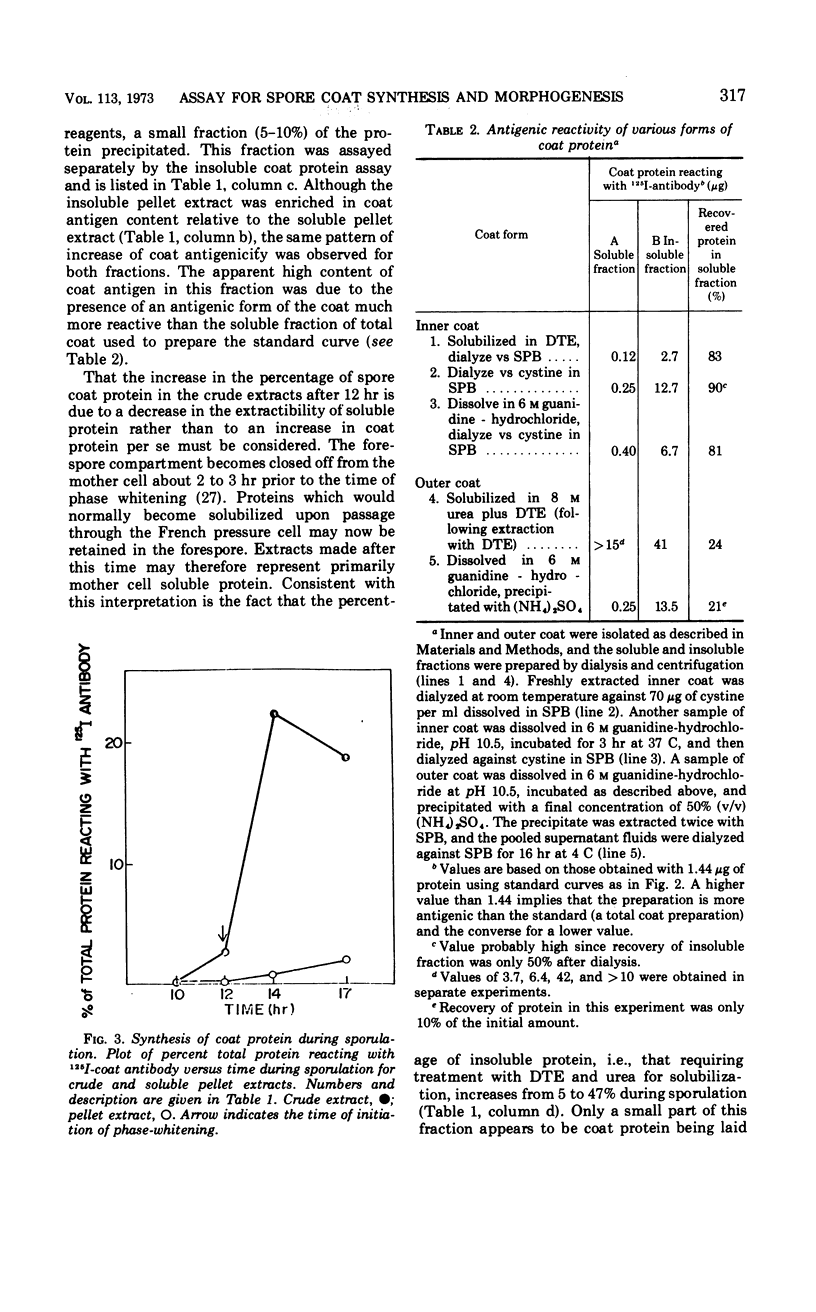

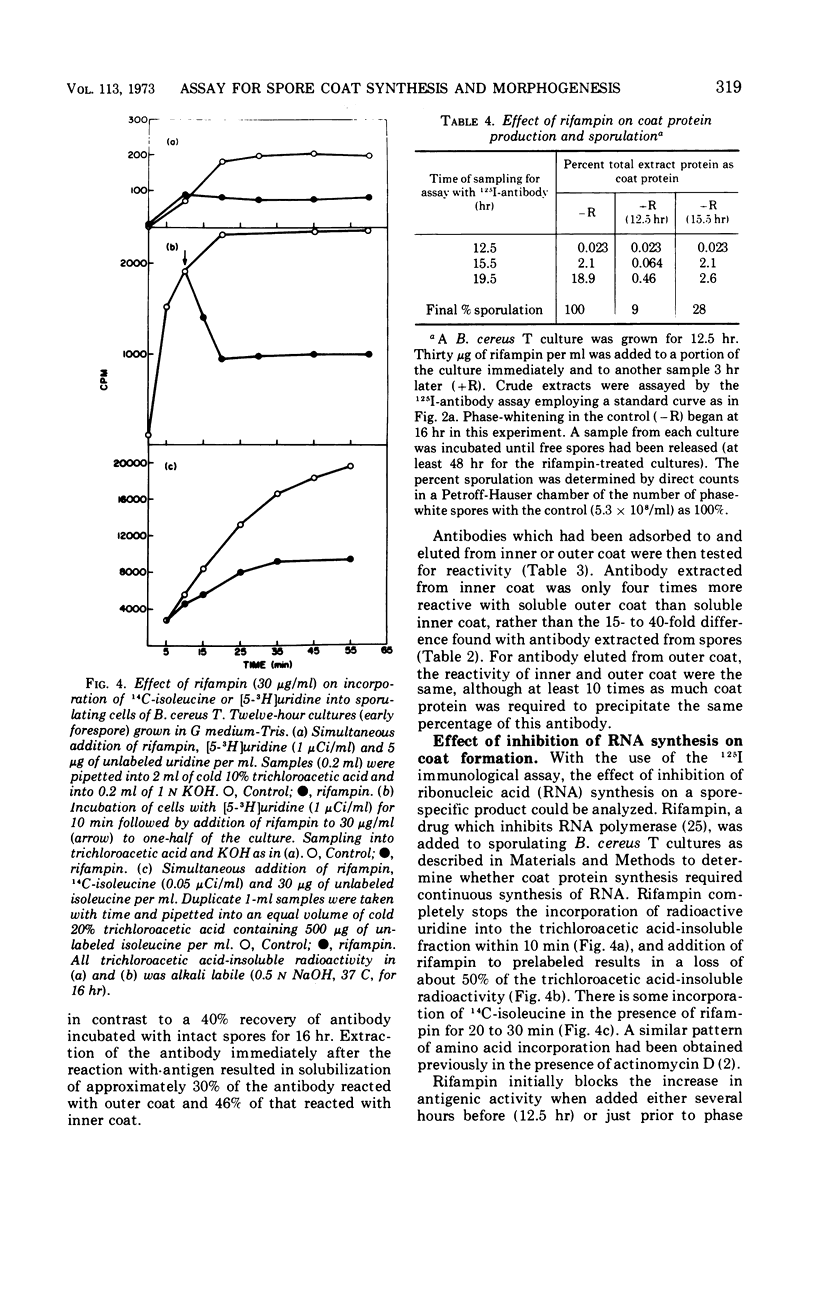


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I., ROSASDELVALLE M. RNA AND PROTEIN SYNTHESIS REQUIRED FOR BACTERIAL SPORE FORMATION. Biochim Biophys Acta. 1964 Jun 22;87:267–276. doi: 10.1016/0926-6550(64)90222-1. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Reconstitution of bacterial spore coat layers in vitro. J Bacteriol. 1971 Oct;108(1):571–578. doi: 10.1128/jb.108.1.571-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- GOLLAKOTA K. G., HALVORSON H. O. Biochemical changes occurring during sporulation of Bacillus cereus. Inhibition of sporulation by alpha-picolinic acid. J Bacteriol. 1960 Jan;79:1–8. doi: 10.1128/jb.79.1.1-8.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines S., Currie J. A., Tully J. G. Induction of immunological unresponsiveness to Vi antigen. Ann Inst Pasteur (Paris) 1966 Jan;110(1):60–68. [PubMed] [Google Scholar]
- Hashimoo T., Conti S. F. Ultrastructural changes associated with activation and germination of Bacillus cereus T spores. J Bacteriol. 1971 Jan;105(1):361–368. doi: 10.1128/jb.105.1.361-368.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. The composition and structure of bacterial spores. J Cell Biol. 1963 Mar;16:579–592. doi: 10.1083/jcb.16.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D. Symposium on bacterial spores: I. Cytology of spore formation and germination. J Appl Bacteriol. 1970 Mar;33(1):1–12. doi: 10.1111/j.1365-2672.1970.tb05229.x. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]


