Abstract
A convenient synthesis of highly functionalized, α,α-disubstituted amino acid amide derivatives has been accomplished by using cyclic and acyclic ketones as the carbonyl inputs in the Ugi multicomponent reaction. An application of this extension of the Ugi reaction to the synthesis of α,α-divinyl amino acids that may be cyclized via ring-closing metathesis to provide highly substituted pyrrolidines is described.
Keywords: Ugi reaction, Multi-component reaction, ring-closing metathesis
The design and development of convergent strategies to synthesize diverse arrays of drug-like compounds for biological screening is an important objective in contemporary chemical biology and medicinal chemistry. In this context, the use of multicomponent reactions (MCRs),i followed by post-condensation modifications via various ring-forming reactions has proven to be a powerful tool as such processes can rapidly lead to the generation of libraries of functionalized compounds with diverse heterocyclic scaffolds. Indeed, our group has long had an interest in discovering new MCRs as well as expanding the scope of existing ones to rapidly access both alkaloid and drug-like motifs.ii,iii
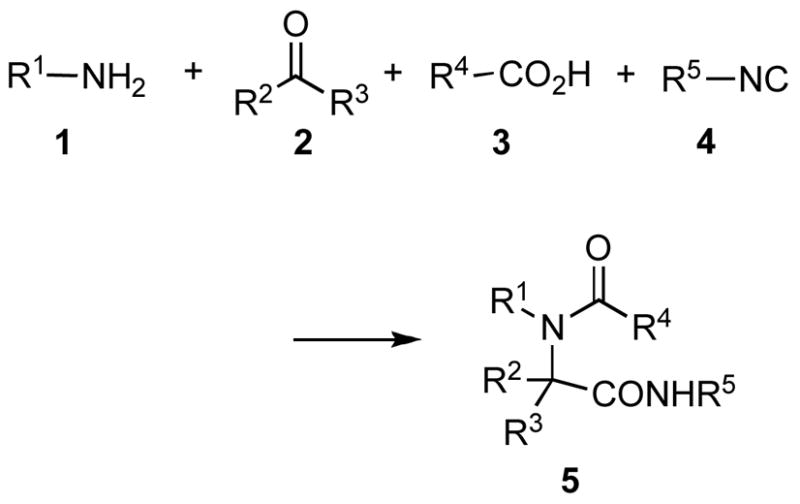
The Ugi reaction is arguably one of the most important MCRs, and it is widely used in the pharmaceutical industry for preparing collections of compounds.iv This powerful reaction involves a one-pot condensation of an amine 1, a carbonyl compound 2, a carboxylic acid 3, and an isocyanide 4 to provide a substituted peptide-like product 5 (Scheme 1). Although the Ugi MCR has proven to be quite versatile, there are some limitations that become apparent upon examination of the literature. For example, the reaction is widely applicable to a variety of readily available amines, carboxylic acids and aldehydes, but commercial access to isocyanides is more restricted than for the other three components. Moreover, it is often desirable to convert the initially formed amide into carboxyl derivatives and other functional groups. The latter issue has been nicely addressed by the invention of a number of so-called convertible isocyanides that can be elaborated after the condensation into various functional groups.v Aldehydes are widely used as components, but there are relatively few examples of the use of ketones as inputs. For example, Ugi reactions with simple ketones such as acetone, cyclopentanone and cyclohexanone are known to proceed in high yields in one-pot reactions.vi N-Methyl and N-benzyl piperidones have also been employed as ketone components to give good yields of products,vii and a library of spiro keto piperazines has been synthesized utilizing N-alkyl and N-aryl piperidones as the ketone components in an Ugi MCR.viii However, use of acyclic dialkyl and diaryl ketones typically requires preformation of the imine intermediate in a separate step, and the yields of the Ugi adducts tend to be modest.ix
Scheme 1.
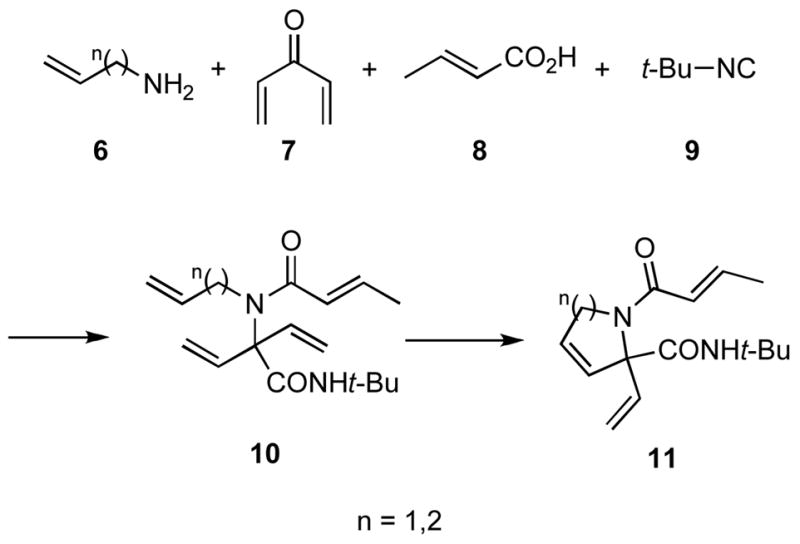
During the course of recent efforts to develop facile approaches to alkaloid natural products and other nitrogen heterocycles, we were attracted to the possibility of employing an Ugi MCR followed by a ring closing metathesis reaction (RCM).x In particular, we envisioned that an Ugi MCR using an unsaturated amine 6, a functional equivalent of divinyl ketone 7, which is known to be unstable,xi crotonic acid (8) and an isocyanide like 9 would lead to an adduct 10 that might be cyclized via an asymmetric ring closing metathesis (ARCM)xii to generate highly substituted pyrrolidine and piperidine derivatives 11 in enantiomerically pure form (Scheme 2). This plan presented a unique opportunity to explore the scope of the Ugi MCR reaction with ketones and to probe the feasibility of cyclizing tetraene substrates containing prochiral vinyl groups in a ARCM. Although tandem Ugi/RCM processes to access bicyclic lactams (6- and 7- membered rings),xiii 9-membered lactamsxiv and macrocyclic peptidesxv are known, the preparation of compounds related to 11 via Ugi/ARCM is not.xvi Herein we wish to report some of our findings on the use of ketones in Ugi MCRs and on the synthesis of α,α-divinyl peptidic compounds that serve as substrates for RCM.
Scheme 2.
Our initial focus was upon acyclic ketones that could be easily refunctionalized via elimination reactions to provide the requisite α,α-divinyl amino acid derivatives. We quickly discovered, however, that acyclic ketones, such as 12xvii and 13xviii did not provide any of the desired Ugi products in one-pot reaction with the benzylamine 14 and the acid and isocyanide components 8 and 9, respectively (Scheme 3). However, after some experimentation, we found that preforming the imine derived from 13 and 14 via Lewis acid catalysis (TiCl4)xix or azeotropic distillation followed by the addition of acid 8 and isocyanide 9 provided the adduct 16 in 30% yield. Because all attempts to preform the imine from 12 and 14 were unsuccessful, none of the Ugi adduct 15 could be prepared.
Scheme 3.
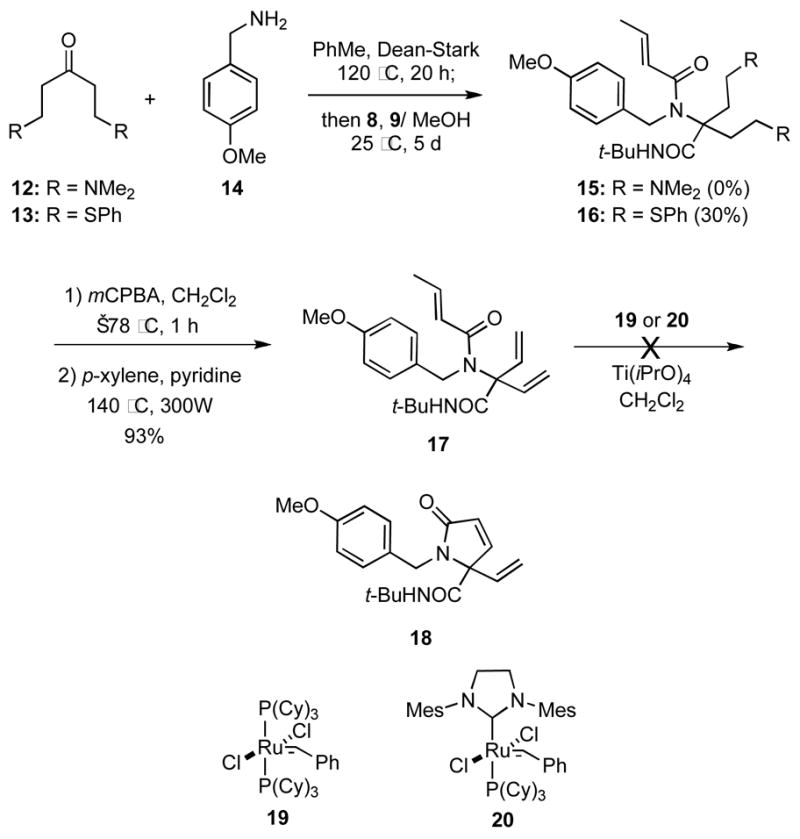
Compound 16 was readily transformed into the divinyl substrate 17 in excellent overall yield by S-oxidation with mCPBA to give an intermediate bis-sulfoxide that underwent facile elimination upon thermolysis. Although this elimination could be effected under conventional reflux conditions (18 h), it was considerably more facile in a microwave reactor (4 h). Unfortunately, in a series of exploratory experiments, we were unable to induce the RCM of 17 to give 18 using either Grubbs 1st or 2nd generation catalysts 19 and 20, respectively; only unreacted starting material was recovered. Reasoning that the amide carbonyl oxygen atom of compound 17 might form an unreactive 6-membered chelated structure with the metal carbene on one of the vinyl groups,xx Ti(iPrO)4 was added as a co-catalyst. However, this tactic was unavailing, and 17 was again recovered.
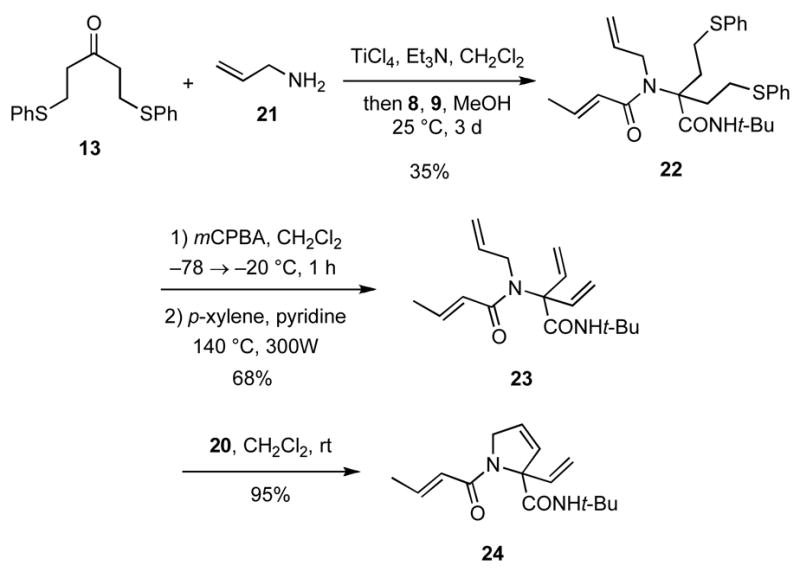
In order to obviate formation of unreactive chelates involving amides, a related sequence of reactions was conducted in which allylamine (21) was condensed with 13 in the presence of TiCl4 and Et3N, and the resultant imine was allowed to react with the carboxylic acid 8 and the isocyanide 9 to provide the allylic amine 22 in 35% yield. (Scheme 4). Selective S-oxidation of the two sulfide moieties as before and subsequent thermolysis gave 23 in 65% yield. Gratifyingly, the RCM of 23 with Grubbs 2nd generation catalyst 20 (5 mol%) provided the highly substituted pyrrolidine 24 in 95% yield. A number of diverse and novel compounds related to 24 may be accessed by varying the acid and the isocyanide compounds in the Ugi reaction.
Scheme 4.
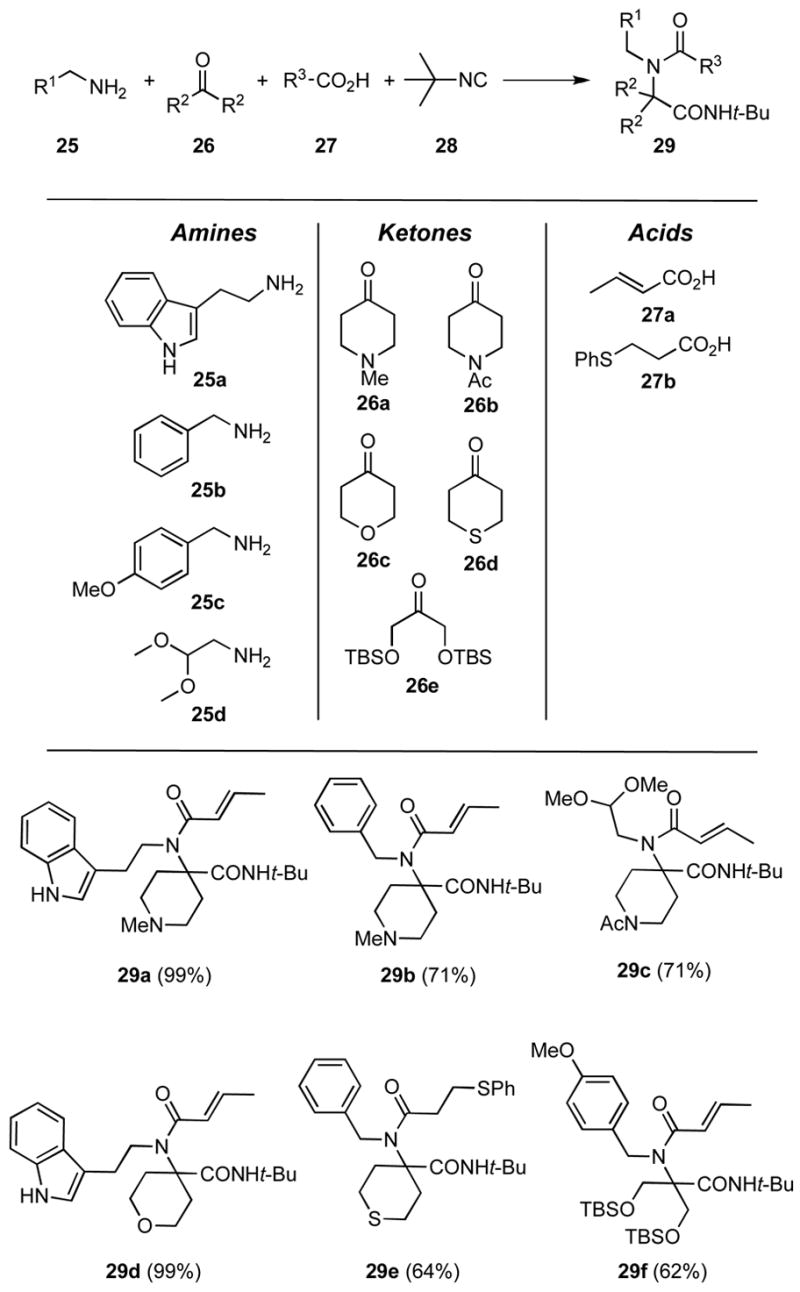
During the course of these studies, we discovered that a number of functionalized ketones containing heteroatoms could be employed that might serve as useful inputs in Ugi MCRs to give adducts in high yields according to the process generally depicted in Scheme 5. For example, the heterocyclic ketones 1-methyl-4-piperidone (26a), 1-acetyl-4-piperidone (26b), tetrahydro-4H-pyran-2-one (26c) and tetrahydro-4H-thiopyran-2-one (26d) were each found to participate in Ugi MCRs that proceeded readily in one-pot operations without preforming the ketimine.xxi Although the Ugi MCR involving 26a has been recently reported to be efficient,viii the only example of which we are aware of the use of 26d is in a solid-phase Ugi process that was low yielding.xxii To our knowledge, there are no reports in the literature of employing 26b and 26c in Ugi MCRs; however, 26c and 26d have been used in some specialized isocyanide based MCRs.xxiii,xxiv Protected dihydroxyacetone derivatives such as 26e have not been used in Ugi MCRs. The amino inputs employed in these exploratory investigations were tryptamine (25a), benzylamine (25b), 4-methoxybenzylamine (25c), and aminoacetaldehyde dimethyl acetal (25d), whereas the carboxylic acid components were crotonic acid (27a) and β-phenylthiopropionic acid (27b). Because of its commercial availability, tert-butylisocyanide (28) was chosen as the universal isocyanide component. The utility of the method is exemplified by the preparation of the highly functionalized adducts 29a–29f.
Scheme 5.
In summary, we have found that a variety of ketones participate in either stepwise or one-pot Ugi MCRs to give good to excellent yields of adducts having a number of different functional groups that can be utilized in various post-condensation reactions. In one novel application of this strategy, we developed a route to highly substituted pyrrolidines via an Ugi reaction followed by a cyclization via RCM. The applications of these and related processes to the syntheses of biologically active natural and unnatural products are the subject of current investigations, the results of which will be reported in due course.
Supplementary Material
Supplementary Material
Supplementary material (spectral and characterization data for all new compounds) associated with this article can be found in the online version at doi: #.
Acknowledgments
We are grateful to the National Institutes of Health (GM25439 and GM31077), the Robert A. Welch Foundation, Pfizer, Inc., Merck Research Laboratories, Hoffmann-La Roche, and Boehringer-Ingelheim for their generous support. We also thank Dr. Richard Pederson (Materia, Inc.) for catalyst support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References and Notes
- i.(a) Zhu J, Bienayme H, editors. Multicomponent Reactions. Wiley-VCH; Weinheim: 2005. [Google Scholar]; (b) Zhu J. Eur J Org Chem. 2003:1133–1144. [Google Scholar]; (c) Dömling A. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- ii.(a) Martin SF, Benage B, Geraci LS, Hunter JE, Mortimore M. J Am Chem Soc. 1991;113:6161–6171. [Google Scholar]; (b) Martin SF, Clark CC, Corbett JW. J Org Chem. 1995;60:3236–3242. [Google Scholar]; (c) Ito M, Clark CC, Mortimore M, Goh JB, Martin SF. J Am Chem Soc. 2001;123:8003–8010. doi: 10.1021/ja010935v. [DOI] [PubMed] [Google Scholar]
- iii.Sunderhaus JD, Dockendorff C, Martin SF. Org Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. and references therein. [DOI] [PubMed] [Google Scholar]
- iv.Dömling A, Ugi I. Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- v.For convertible isocyanides, see: Keating TA, Armstrong RW. J Am Chem Soc. 1995;117:7842–7843.Keating TA, Armstrong RW. J Am Chem Soc. 1996;118:2574–2583.Lindhorst T, Bock H, Ugi I. Tetrahedron. 1999;55:7411–7420.Linderman RJ, Binet S, Petrich SR. J Org Chem. 1999;64:336–337.Rikimaru K, Yanagisawa A, Kan T, Fukuyama T. Synlett. 2004;1:41–44.Pirrung MC, Ghorai S. J Am Chem Soc. 2006;128:11772–11773. doi: 10.1021/ja0644374.Gilley CB, Buller MJ, Kobayashi Y. Org Lett. 2007;9:3631–3634. doi: 10.1021/ol701446y.Tanaka Y, Hasui T, Suginome M. Org Lett. 2007;9:4407–4410. doi: 10.1021/ol701570c.
- vi.For selected examples, see: Mironov MA, Tokareva MI, Ivantsova MN, Mokrushin VS. Russ J Org Chem. 2004;40:847–853.Kalinski C, Umkehrer M, Gonnard S, Jäger N, Ross G, Hiller W. Tetrahedron Lett. 2006;47:2041–2044.
- vii.(a) Beck B, Hess S, Dömling A. Bioorg Med Chem Lett. 2000;10:1701–1705. doi: 10.1016/s0960-894x(00)00305-x. [DOI] [PubMed] [Google Scholar]; (b) Dömling A, Achatz S, Beck B. Bioorg Med Chem Lett. 2007;17:5483–5486. doi: 10.1016/j.bmcl.2007.04.066. [DOI] [PubMed] [Google Scholar]
- viii.Habashita H, Kokubo M, Hamano S, Hamanaka N, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. J Med Chem. 2006;49:4140–4152. doi: 10.1021/jm060051s. [DOI] [PubMed] [Google Scholar]
- ix.(a) Yamada T, Yanagi T, Omote Y, Miyazawa T, Kuwata S, Sugiura M, Matsumoto K. J Chem Soc, Chem Comm. 1990:1640–1641. [Google Scholar]; (b) Costa SPG, Maia HLS, Pereira-Lima SMMA. Org Biomol Chem. 2003;1:1475–1479. doi: 10.1039/b212473b. [DOI] [PubMed] [Google Scholar]; (c) Pinto FCSC, Pereira-Lima SMMA, Ventura C, Albuquerque L, Concalves-Maia R, Maia HLS. Tetrahedron. 2006;62:8184–8198. [Google Scholar]
- x.For a review of applications of RCM to the preparation of heterocycles, see: Deiters A, Martin SF. Chem Rev. 2004;104:2199–2238. doi: 10.1021/cr0200872. and references cited therein.
- xi.Laronze JY, Sapi J, Levy J. Synthesis. 1988:619–621. [Google Scholar]
- xii.For reviews on enantioselective olefin metathesis, see: Hoveyda AH. Chapter 2.3 In: Grubbs RH, editor. Handbook of Metathesis. Vol. 2. Wiley-VCH; Weinheim, Germany: 2003. Schrock RR, Hoveyda AH. Angew Chem Int Ed. 2003;42:4592–4633. doi: 10.1002/anie.200300576.Hoveyda AH, Schrock RR. Chem Eur J. 2001;7:945–950. doi: 10.1002/1521-3765(20010302)7:5<945::aid-chem945>3.0.co;2-3.
- xiii.Krelaus P, Westermann B. Tetrahedron Lett. 2004;45:5987–5990. [Google Scholar]
- xiv.Banfi L, Basso A, Guanti G, Riva R. Tetrahedron Lett. 2003;44:7655–7658. [Google Scholar]
- xv.Hebach C, Kazmaier U. Chem Commun. 2003:596–597. [PubMed] [Google Scholar]
- xvi.For a related example of a van Leusen MCR/RCM sequence, see: Gracias V, Gasiecki AF, Djuric SW. Org Lett. 2005;7:3183–3186. doi: 10.1021/ol050852+.
- xvii.Blicke F, McCarty F. J Org Chem. 1959;24:1376–1379. [Google Scholar]
- xviii.Angiolini L, Carlini C, Tramontini M, Ghedini N. Polymer. 1989;30:564–569. [Google Scholar]
- xix.Moss N, Gauthier J, Ferland JM. Synlett. 1994:142–144. [Google Scholar]
- xx.Grubbs RH, Miller SJ, Fu GC. Acc Chem Res. 1995;28:446–452.Arisawa M, Nishida A, Nakagawa M. J Organomet Chem. 2006;691:5109–5121.Fürstner A, Langemann K. J Am Chem Soc. 1997;119:9130–9136. See also: Ghosh AK, Cappiello J, Shin D. Tetrahedron Lett. 1998;39:4651–4654. doi: 10.1016/S0040-4039(98)00887-9.Yang Q, Xiao WJ, Yu Z. Org Lett. 2005;7:871–874. doi: 10.1021/ol047356q.
- xxi.General procedure for preparing 29a–f: The isocyanide 28 (1.1 mmol) was added to a solution of amine 25a–e (1.1 mmol), ketone 26a–d (1 mmol), acid 27a,b (1.1 mmol) in MeOH (1 mL) at room temperature, and the solution was stirred for 18 h. The solvent was removed under reduced pressure, and the residue was dissolved in EtOAc (20 mL). The organic solution was washed with sat. aq. NaHCO3 (2 × 20 mL) and brine (20 mL) and dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product was purified either via flash column chromatography on silica gel (eluting with 1–2% MeOH in CH2Cl2) or by crystallization from EtOH.
- xxii.Szardenings AK, Burkoth TS, Lu HH, Tien DW, Campbell DA. Tetrahedron. 1997;53:6537–6593. [Google Scholar]
- xxiii.Bossio R, Marcaccini R, Pepino R. Liebigs Ann Chem. 1993;11:1229–1231. [Google Scholar]
- xxiv.Marcaccini S, Pepino R, Polo C, Pozo MC. Synthesis. 2001;1:85–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary material (spectral and characterization data for all new compounds) associated with this article can be found in the online version at doi: #.


