Abstract
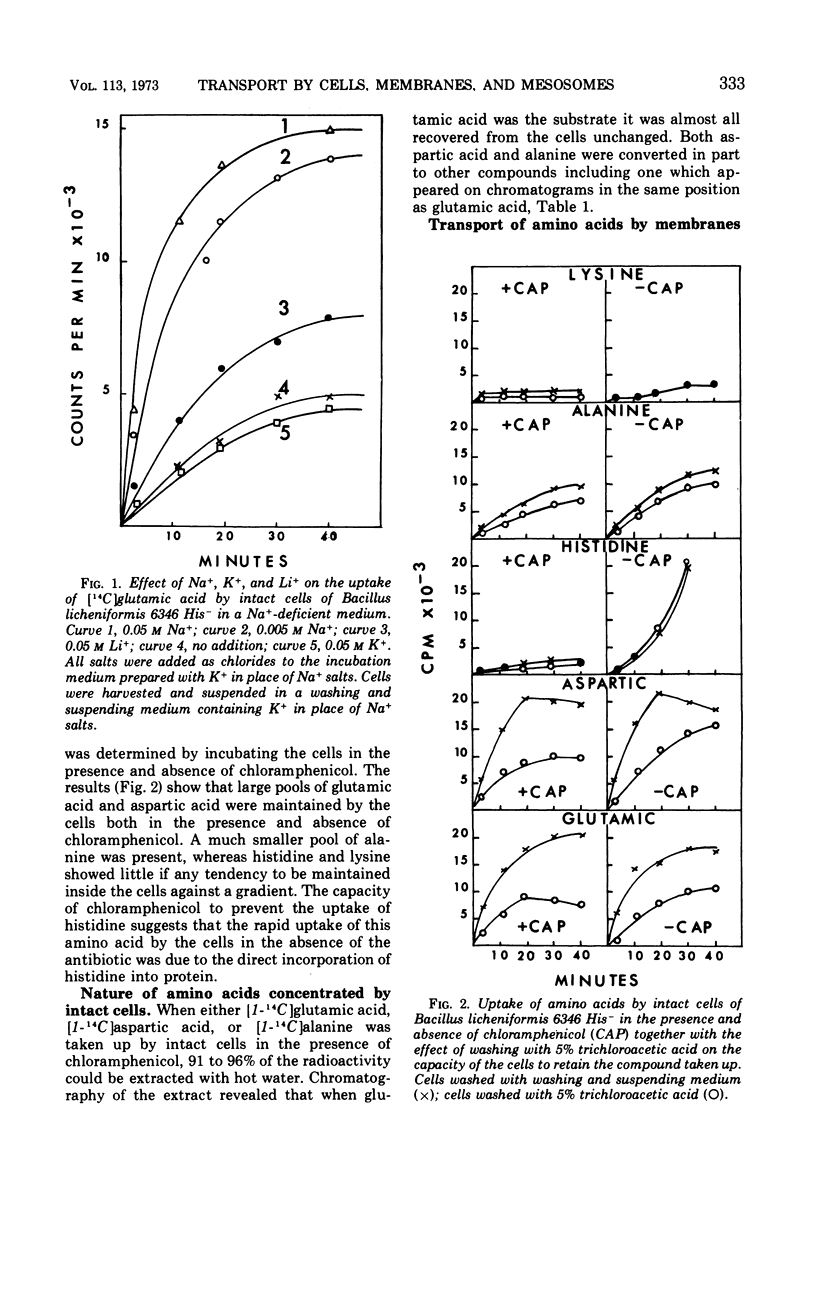
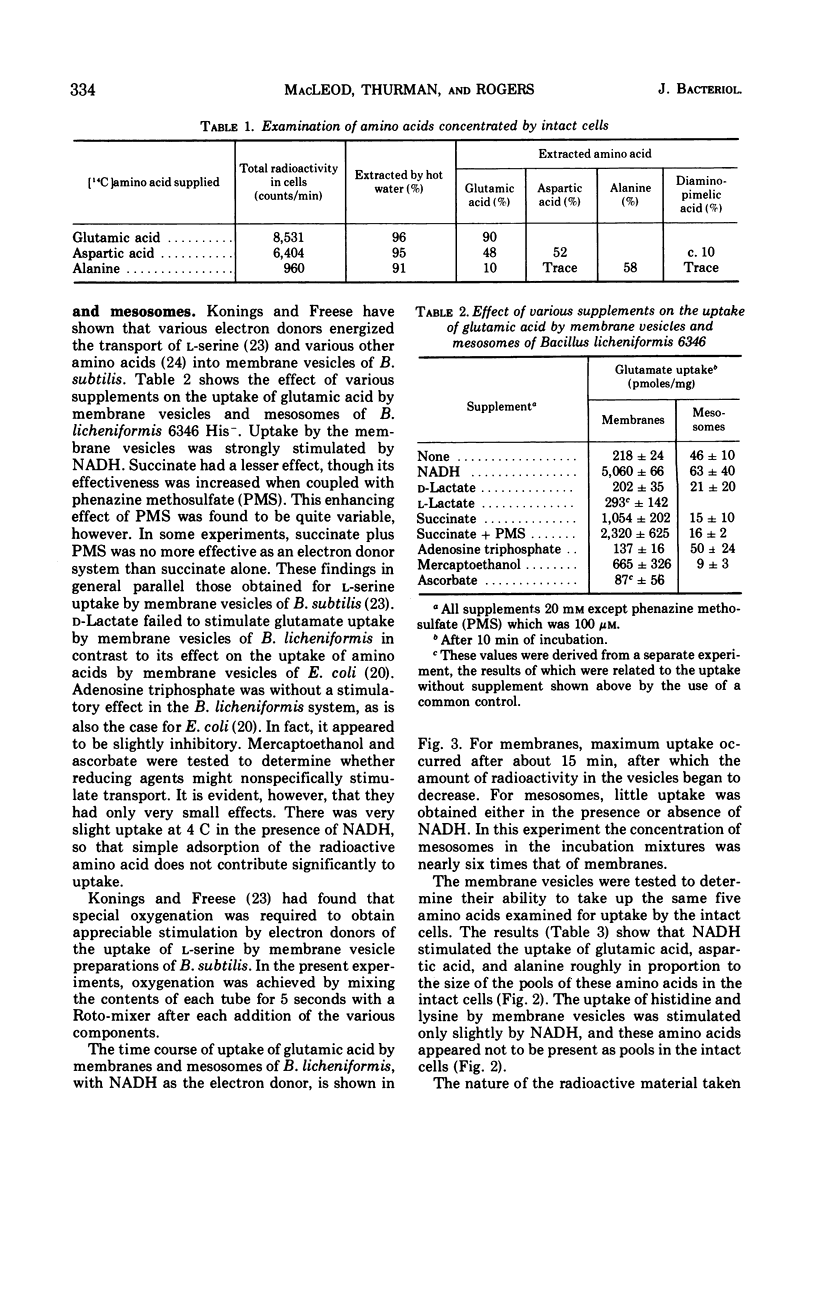
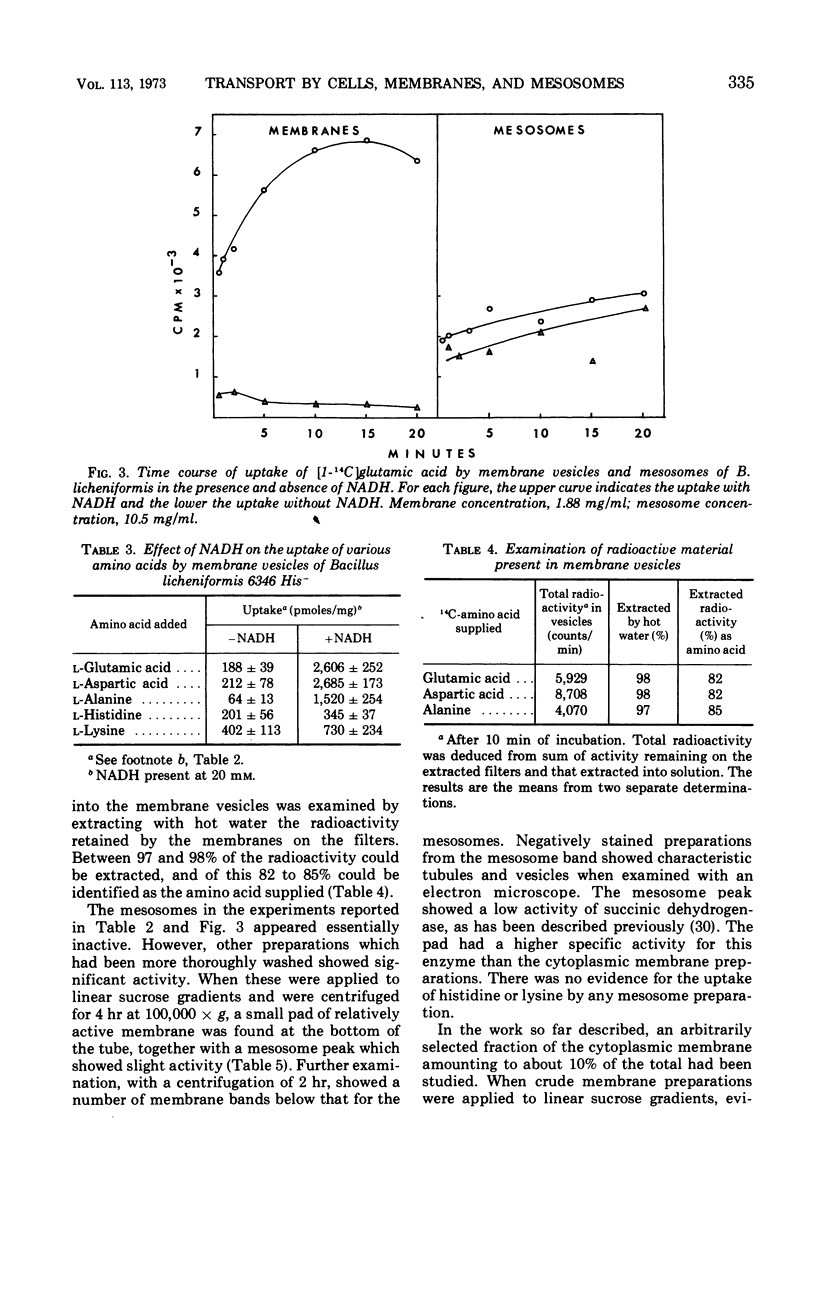
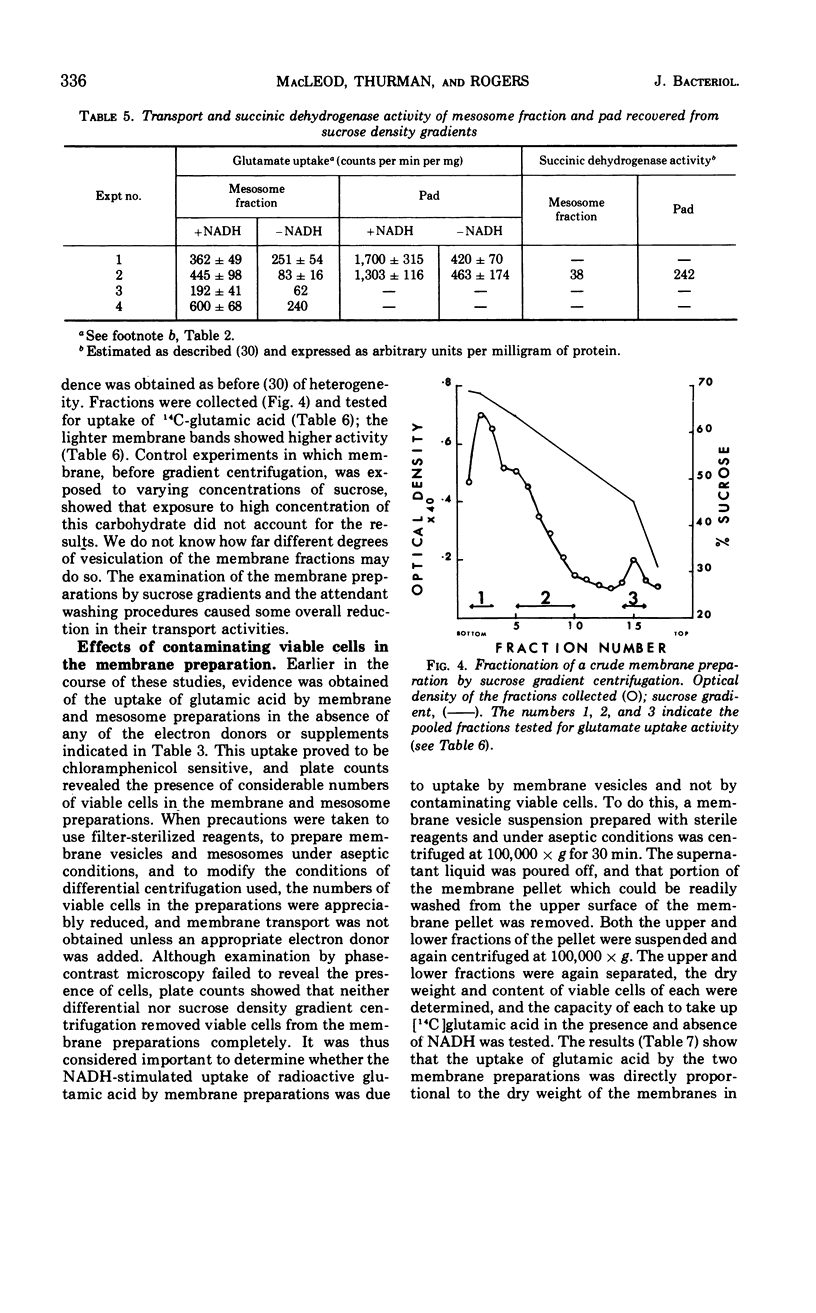
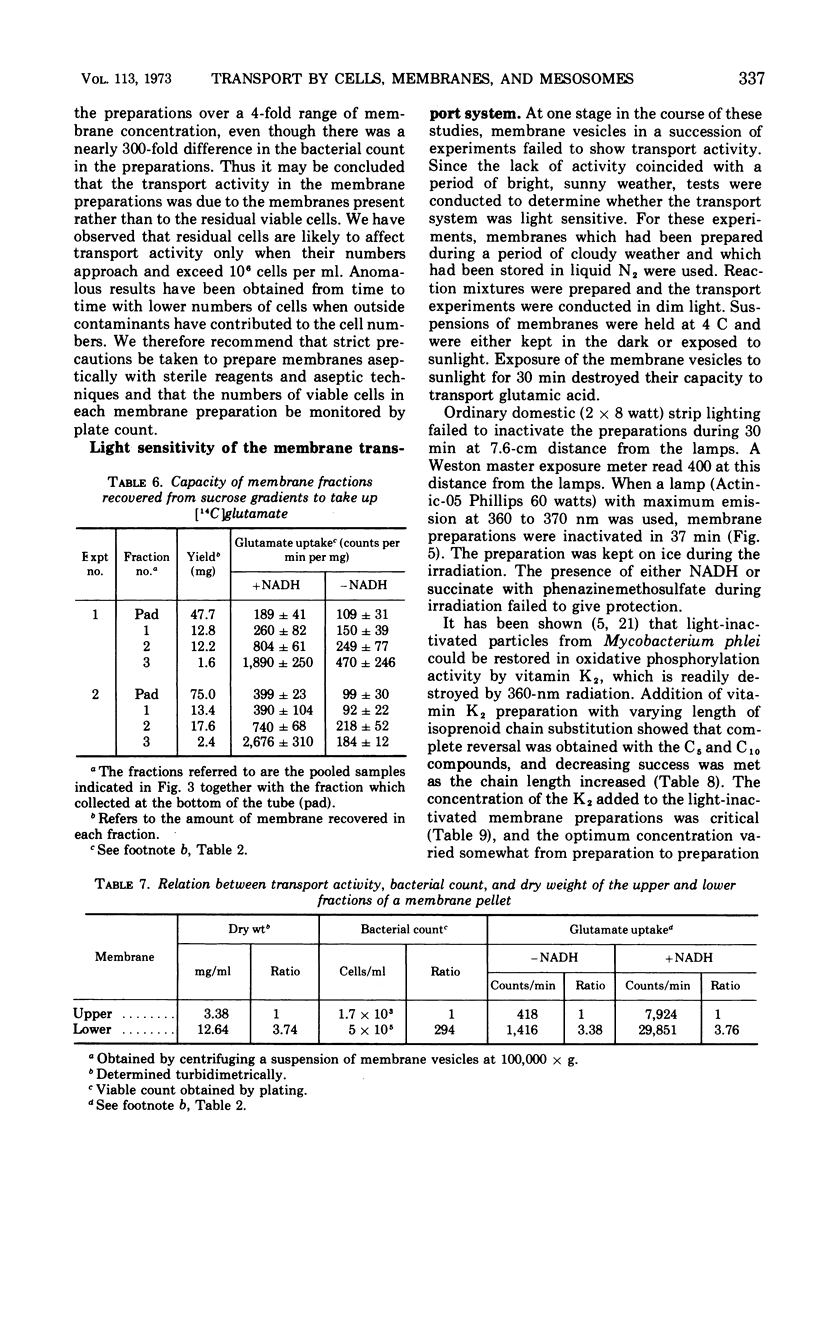
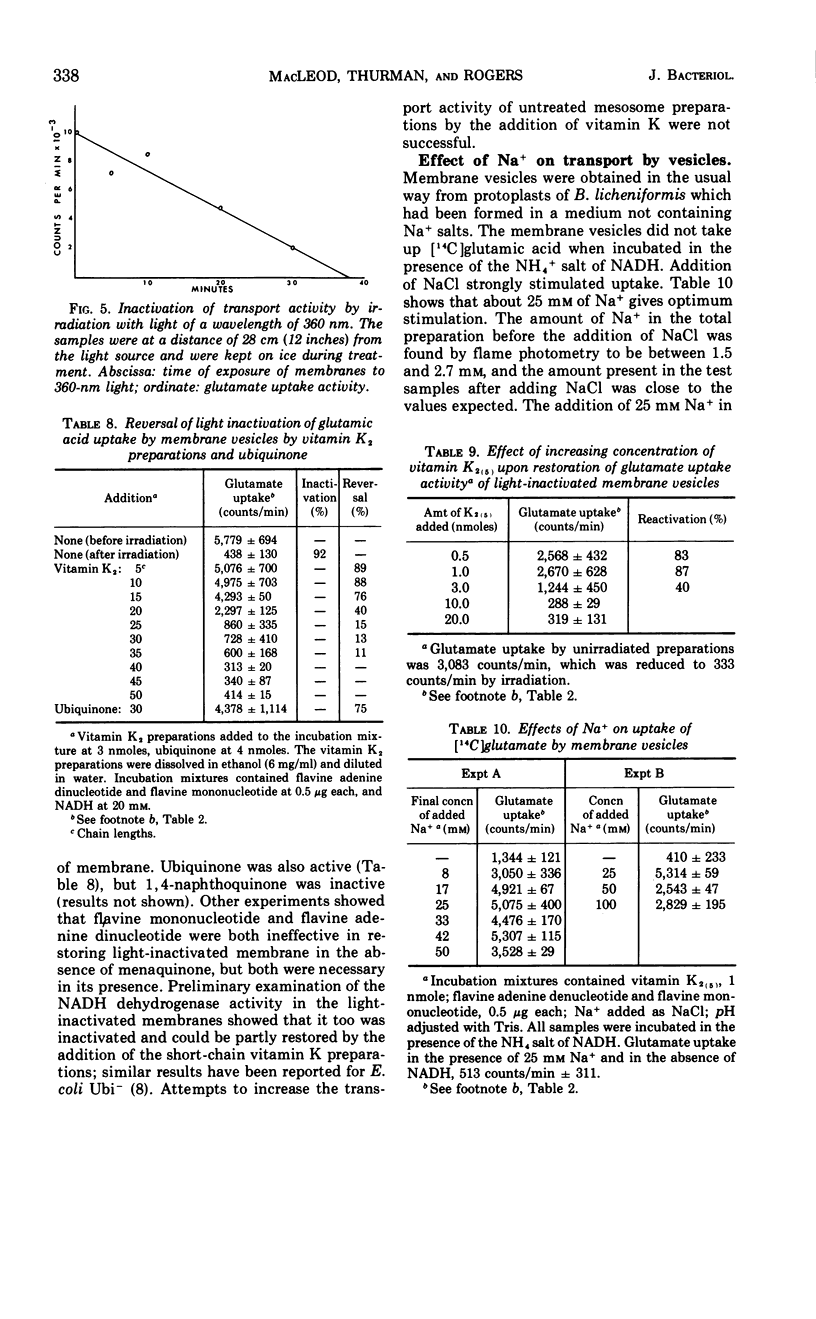
Sodium ion was shown to stimulate strongly the transport of l-glutamic acid into cells of Bacillus licheniformis 6346 His−. Lithium ion had a slight capacity to replace Na+ in this capacity, but K+ was without effect. Three of five amino acids tested. l-glutamic acid, l-aspartic acid, and l-alanine, were concentrated against a gradient in the cells. Intracellular pools of these amino acids were extractable with 5% trichloroacetic acid. Pools of l-histidine and l-lysine could not be detected. No evidence of active transport of lysine into cells could be detected, and histidine was taken up in the absence of chloramphenicol but not in its presence. The uptake of glutamic acid by membrane vesicle preparations was strongly stimulated by reduced nicotinamide adenine dinucleotide (NADH) and to a lesser extent by succinate. The presence of phenazine methosulfate increased uptake in the presence of succinate. Either l- or d-lactate and adenosine triphosphate were without effect. None of these compounds stimulated the uptake of glutamic acid by mesosomes, although some mesosome preparations contained separable membrane which was very active. NADH strongly stimulated the uptake of aspartic acid and alanine by membrane vesicles but had only a slight effect on the uptake of histidine and lysine. No evidence of active transport of any of the amino acids into mesosomes could be detected either in the presence or absence of NADH. NADH stimulation of the uptake of glutamic acid by membrane vesicles was destroyed by exposure to light of 360 nm; this inactivation was reversible by vitamin K2(5) or K2(10). Sodium ion stimulated transport of glutamic acid by membrane vesicles.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODIE A. F., BALLANTINE J. Oxidative phosphorylation in fractionated bacterial systems. II. The role of vitamin K. J Biol Chem. 1960 Jan;235:226–231. [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. The structure and development of mesosomes studied in Bacillus licheniformis strain 6346. J Ultrastruct Res. 1972 Jan;38(1):113–133. doi: 10.1016/s0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. Preparation and some properties of active protoplasts of Bacillus megaterium. J Gen Microbiol. 1968 Oct;53(3):425–430. doi: 10.1099/00221287-53-3-425. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Freer J. H. The isolation and characterisation of mesosome material from Micrococcus lysodeikticus. J Gen Microbiol. 1969 Nov;58(3):vii–vii. [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandes B., Frehel C., Chaix P. Fractionment et purification des systèmes membranaires cytoplasmiques et mésosomiquees de lbacillus subtilis. Etude de quelques-unes de leurs propríetés oxydo-réductricwa associées à la chaine respiratoire. Biochim Biophys Acta. 1970 Dec 8;223(2):292–308. doi: 10.1016/0005-2728(70)90186-6. [DOI] [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. X. Different roles for the natural quinones of Escherichia coli W in oxidative metabolism. J Biol Chem. 1963 Jul;238:2564–2570. [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Konings W. N., Freese E. L-serine transport in membrane vesicles of Bacillus subtilis energized by NADH or reduced phenazine methosulfate. FEBS Lett. 1971 Apr 12;14(1):65–68. doi: 10.1016/0014-5793(71)80276-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Stocker B. A. How genes determine the structure of the Salmonella lipopolysaccharide. J Gen Microbiol. 1969 Aug;57(3):vi–vi. [PubMed] [Google Scholar]
- Patch C. T., Landman O. E. Comparison of the biochemistry and rates of synthesis of mesosomal and peripheral membranes in Bacillus subtilis. J Bacteriol. 1971 Jul;107(1):345–357. doi: 10.1128/jb.107.1.345-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J. The specific requirement for sodium chloride for the active uptake of L-glutamate by Halobacterium salinarium. Biochem J. 1966 May;99(2):257–260. doi: 10.1042/bj0990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILBRANDT W., ROSENBERG T. The concept of carrier transport and its corollaries in pharmacology. Pharmacol Rev. 1961 Jun;13:109–183. [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971 Feb 25;246(4):1032–1040. [PubMed] [Google Scholar]


