Abstract
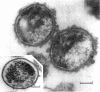
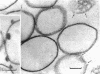
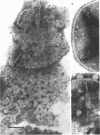
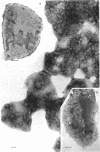
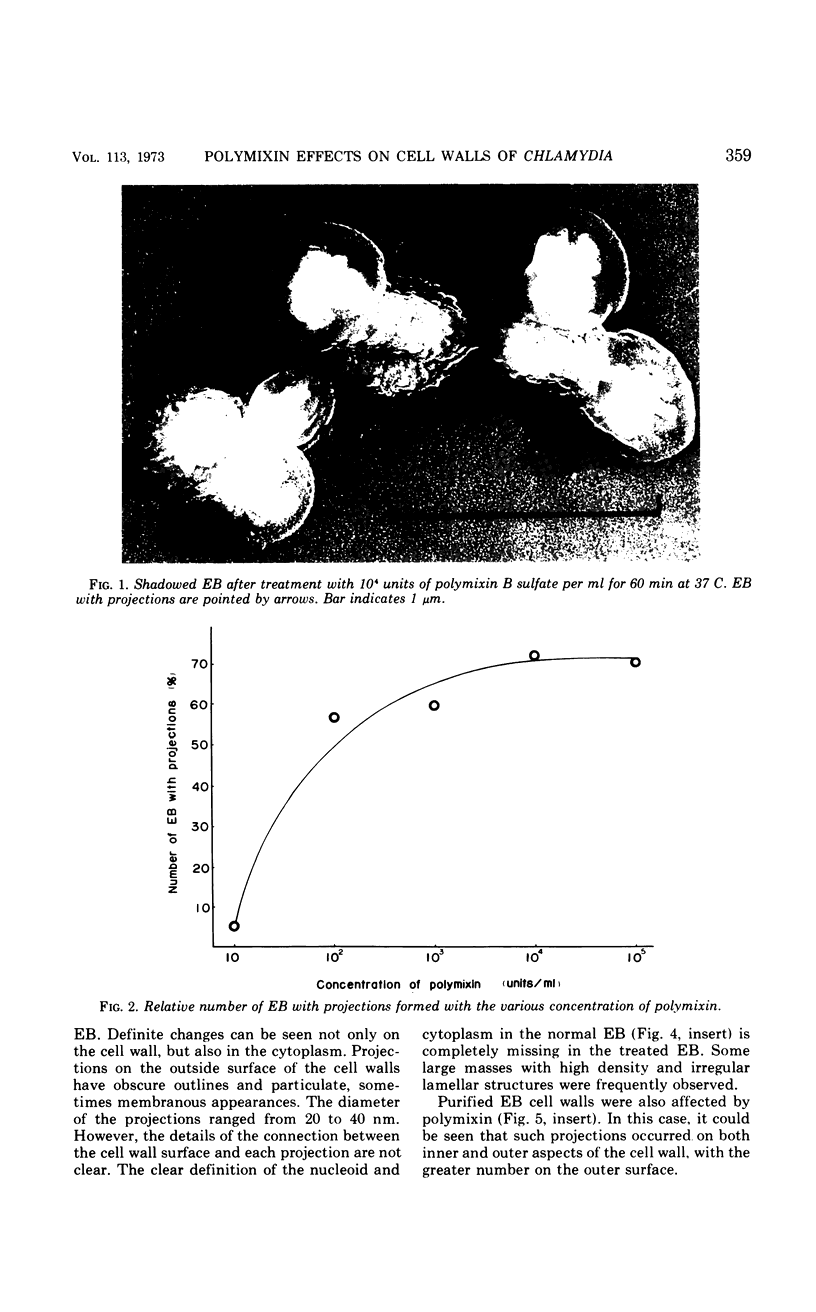
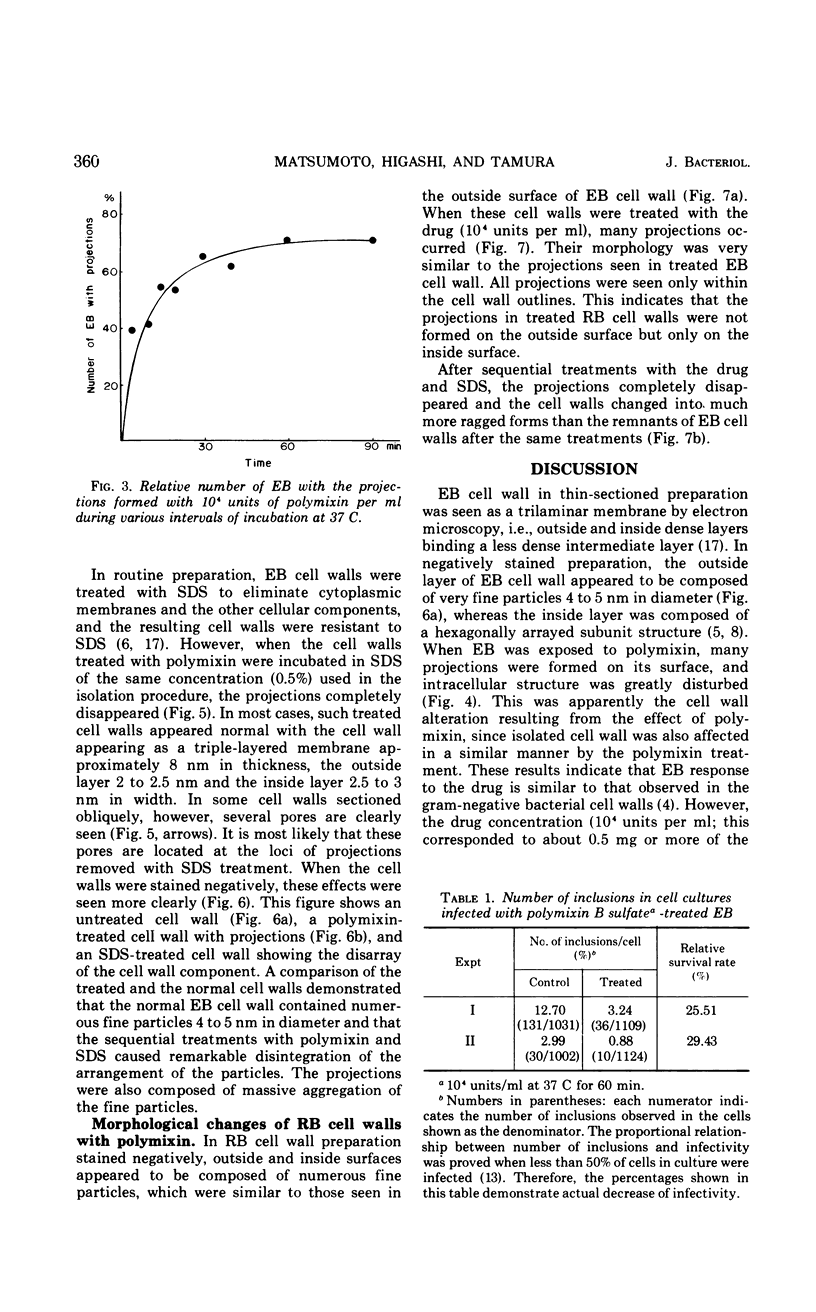
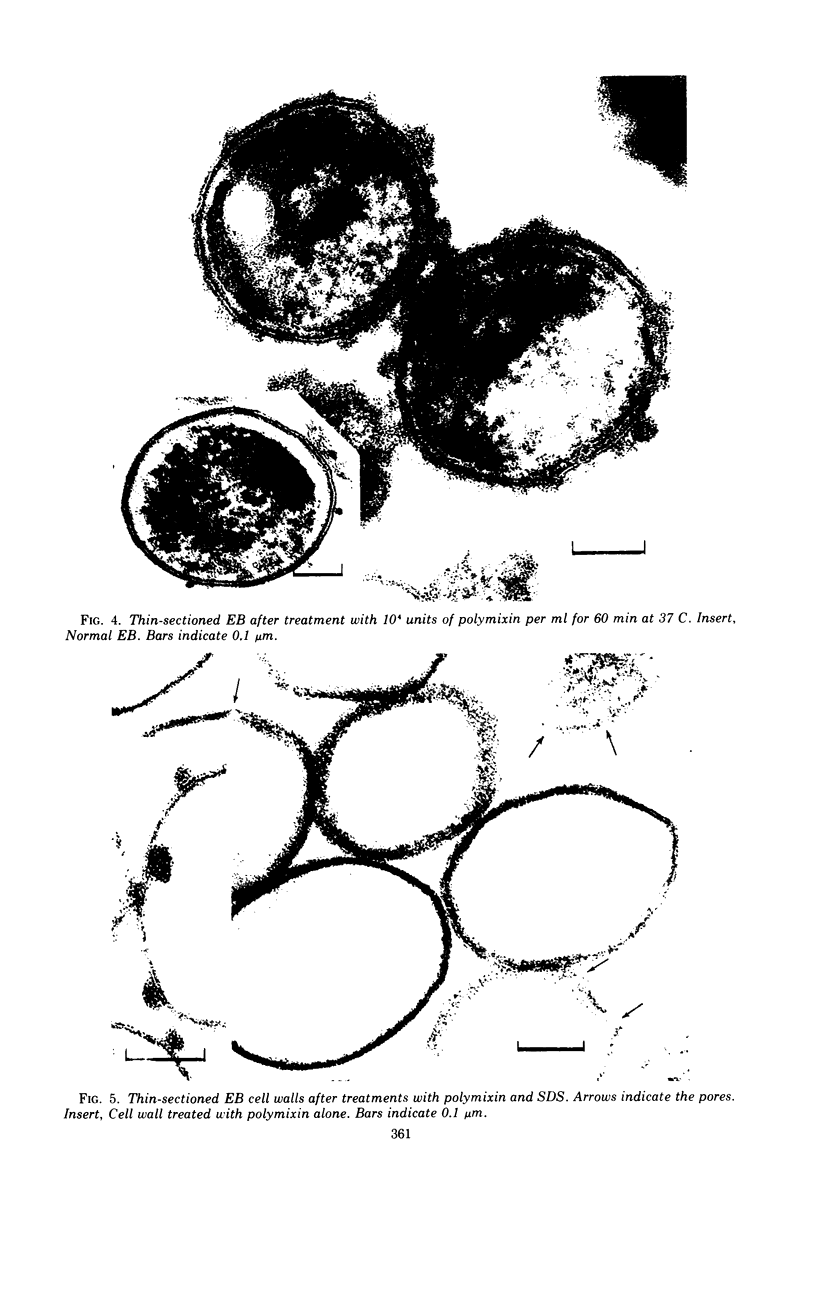
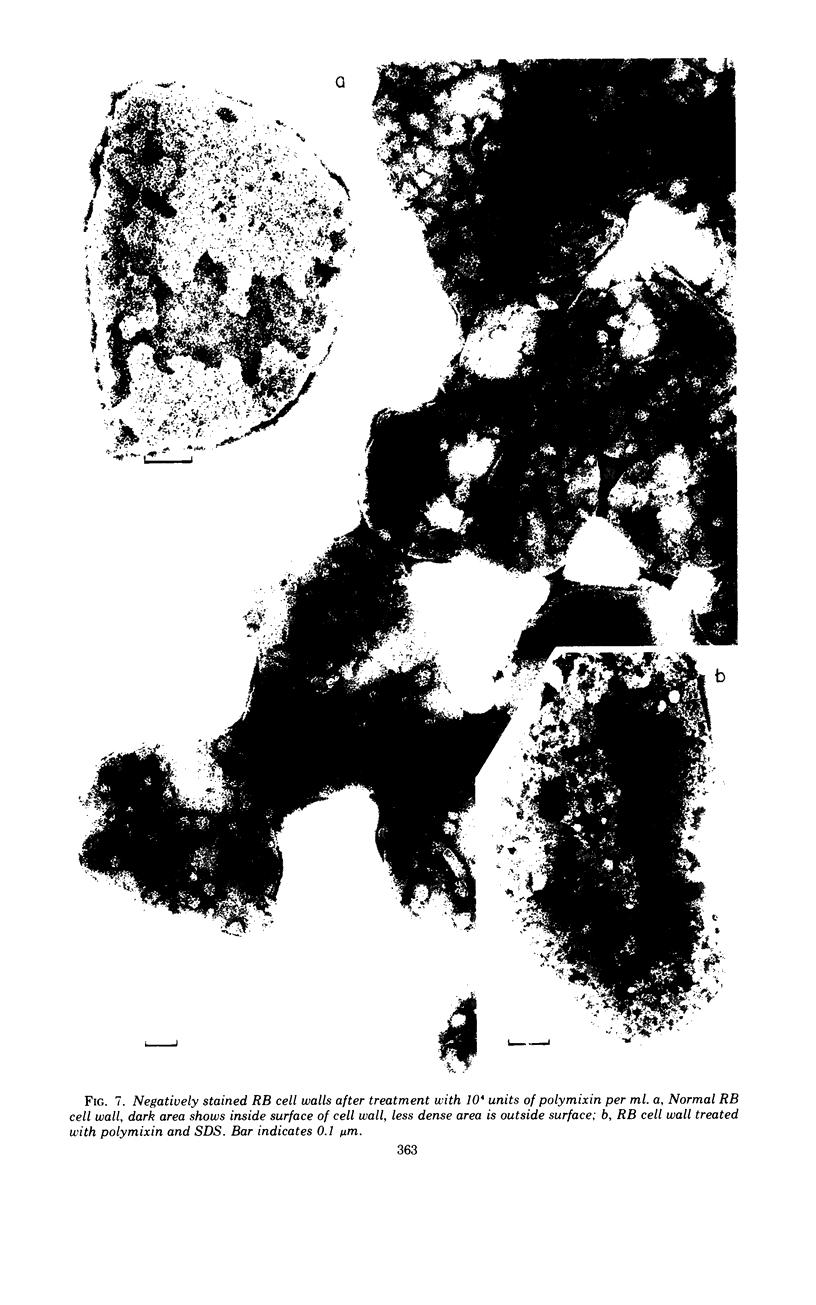
The effects of polymixin B sulfate on cell walls of mature elementary body (EB) and of immature developmental reticulate body (RB) of Chlamydia psittaci were investigated. When purified EB were treated with polymixin (104 units per ml or more) at 37 C for 60 min, about 70% of EB was found to be covered with a number of projections. Further incubation did not increase the percentage affected. The infectivity after treatment as assayed by the inclusion counting technique was reduced by 70% of the original titer. These results suggest that EB with the projections are no longer infective. The projections had obscure outlines and were 20 to 40 nm in diameter when seen in thin sections. In the negatively stained preparations, the projections were composed of aggregations of fine particles 4 to 5 nm in diameter. Treatment with sodium dodecyl sulfate at the same concentration used for cell wall isolation removed the projections completely, and the cell walls were converted to rather ragged forms apparently composed of outside and inside layers. When RB cell walls prepared from infected cells at 18 hr after infection were treated with polymixin at the same concentration, the projections having the same morphology with those seen on treated EB cell walls were observed only on the inside surface of cell wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- Higashi N. The mode of reproduction of psittacosis-lymphogranuloma-trachoma (PLT) group viruses. Int Rev Exp Pathol. 1964;3:35–64. [PubMed] [Google Scholar]
- KAYE J. J., CHAPMAN G. B. CYTOLOGICAL ASPECTS OF ANTIMICROBIAL ANTIBIOSIS. III. CYTOLOGICALLY DISTINGUISHABLE STAGES IN ANTIBIOTIC ACTION OF COLISTIN SULFATE ON ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:536–543. doi: 10.1128/jb.86.3.536-543.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P. Structure of purified cell walls of dense forms of meningopneumonitis organisms. J Bacteriol. 1966 Jan;91(1):409–413. doi: 10.1128/jb.91.1.409-413.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- TAMURA A., IWANAGA M., HIGASHI N. [Biochemical studies of meningopneumonitis virus multiplication in L cell suspension culture]. Virus. 1961 Dec;11:386–393. doi: 10.2222/jsv.11.386. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci). J Bacteriol. 1968 Oct;96(4):875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Preparation and chemical composition of the cell membranes of developmental reticulate forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1184–1188. doi: 10.1128/jb.94.4.1184-1188.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]