Abstract
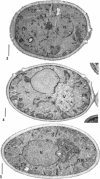
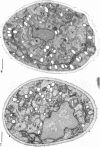
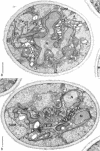
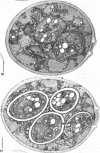
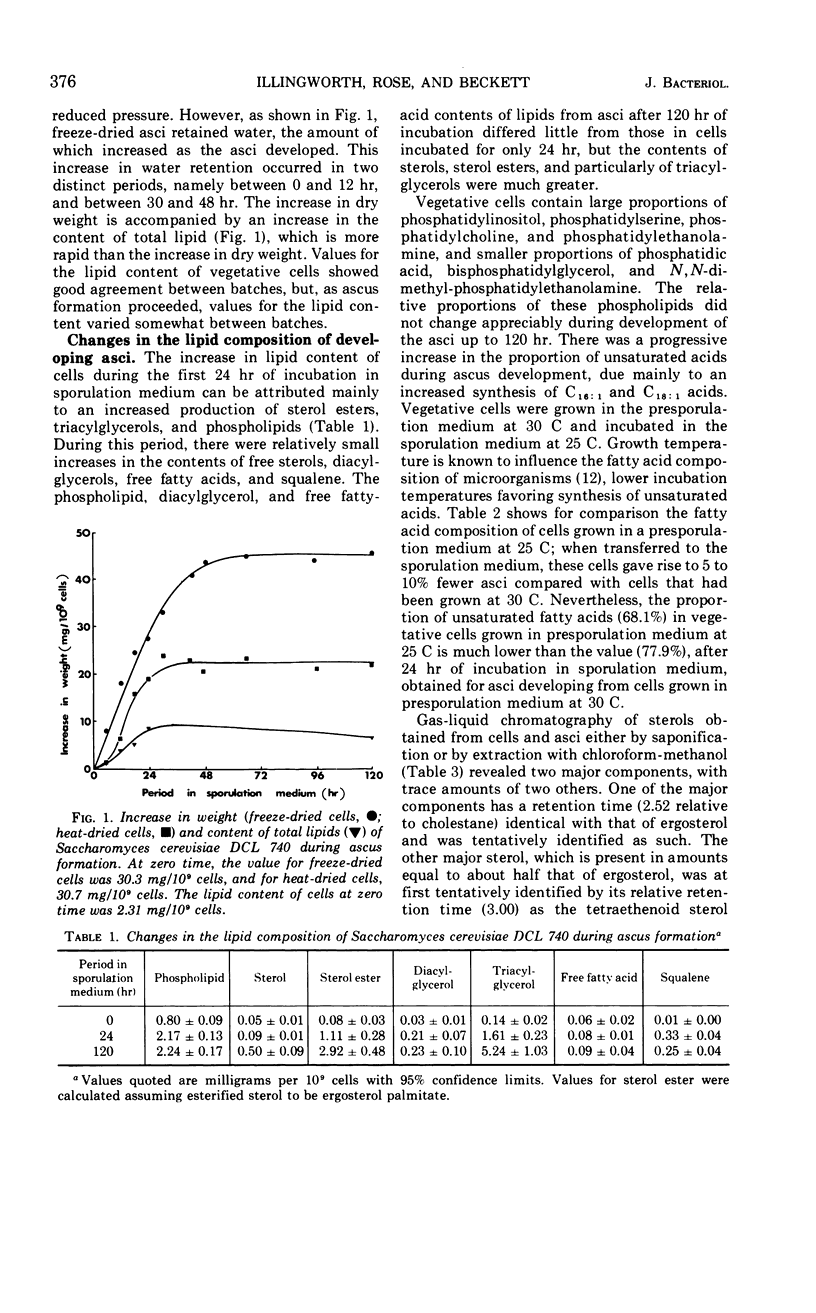
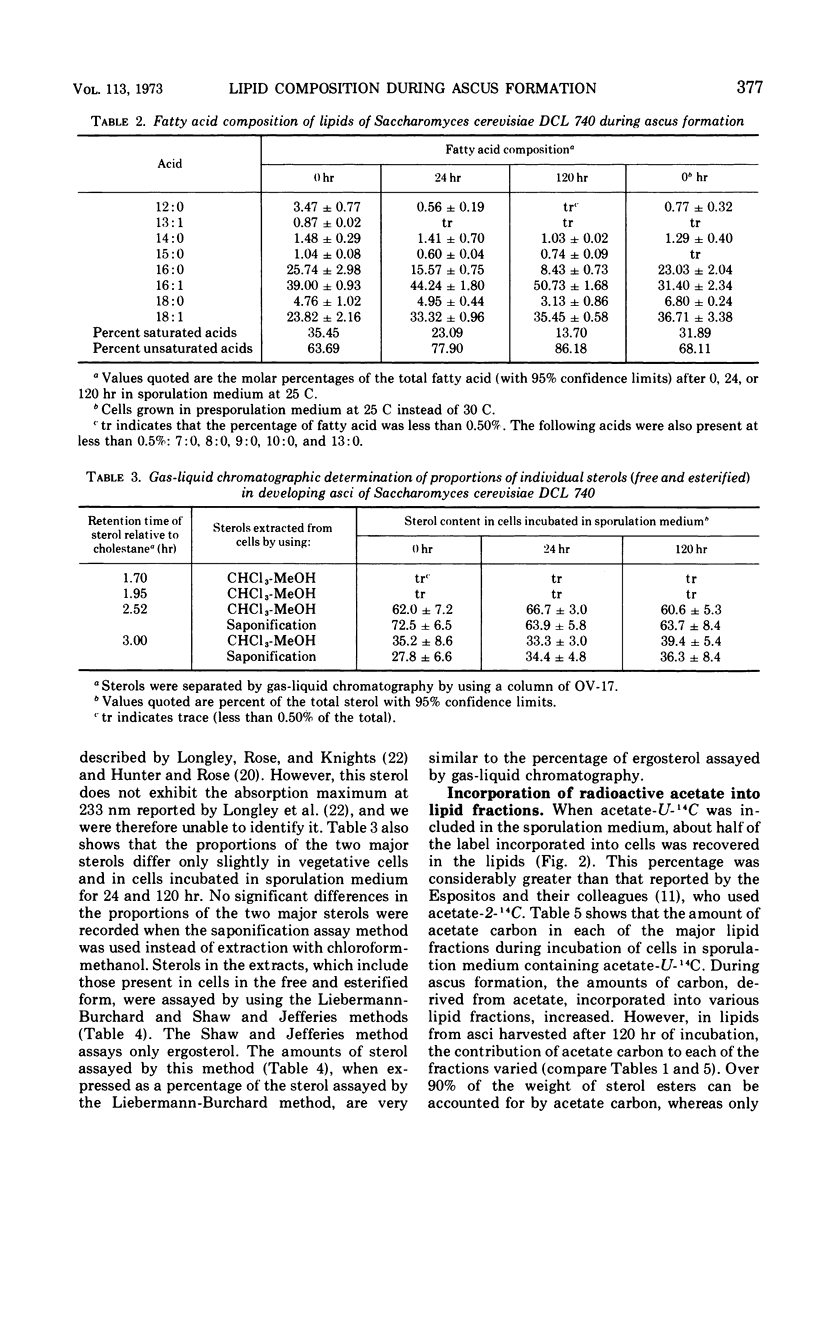
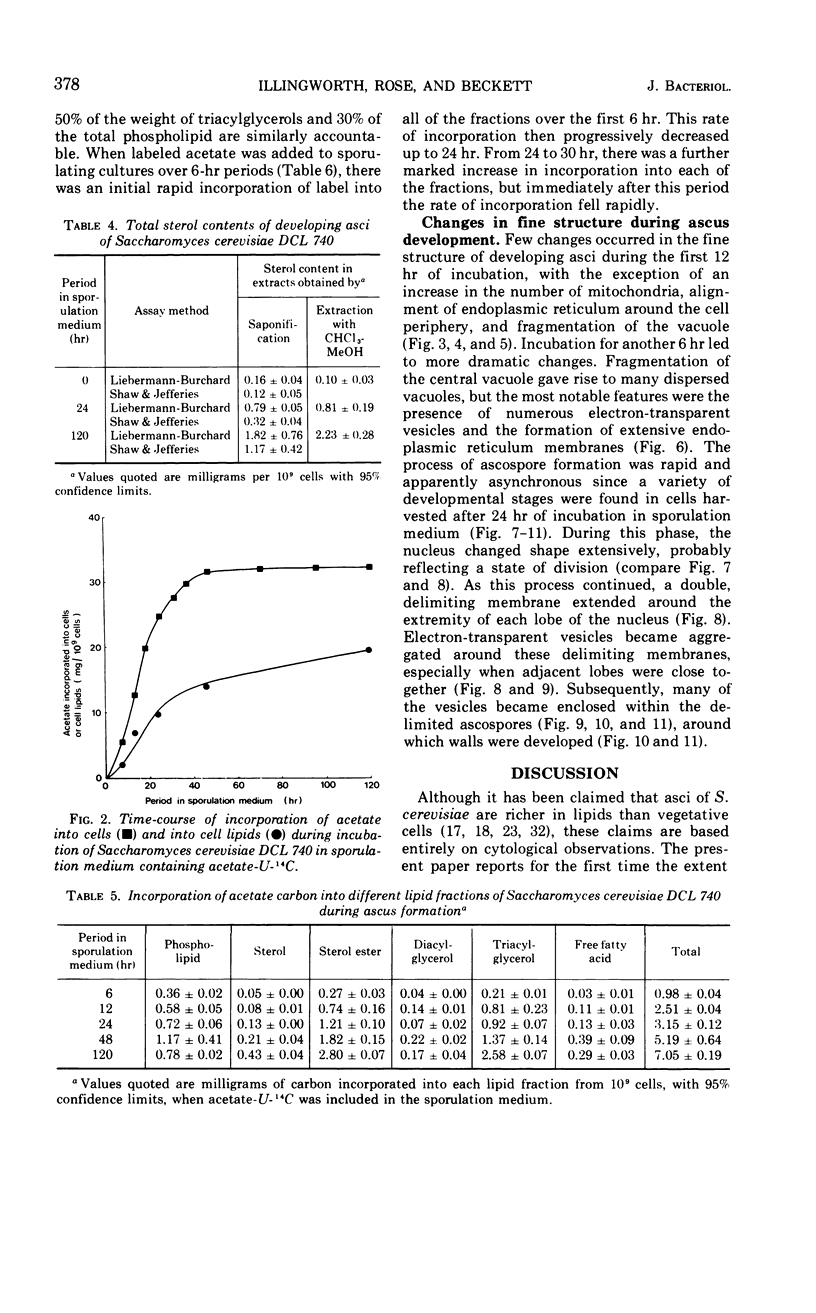
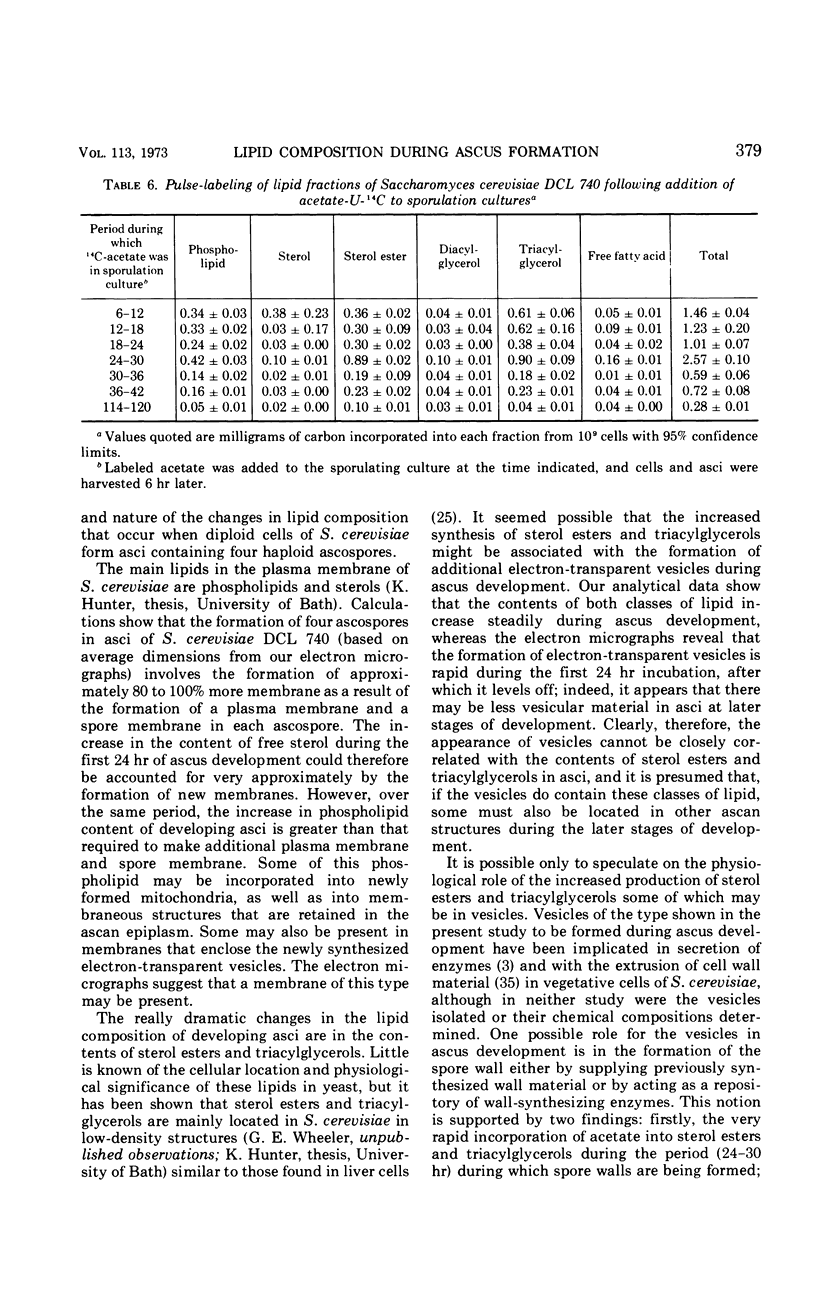
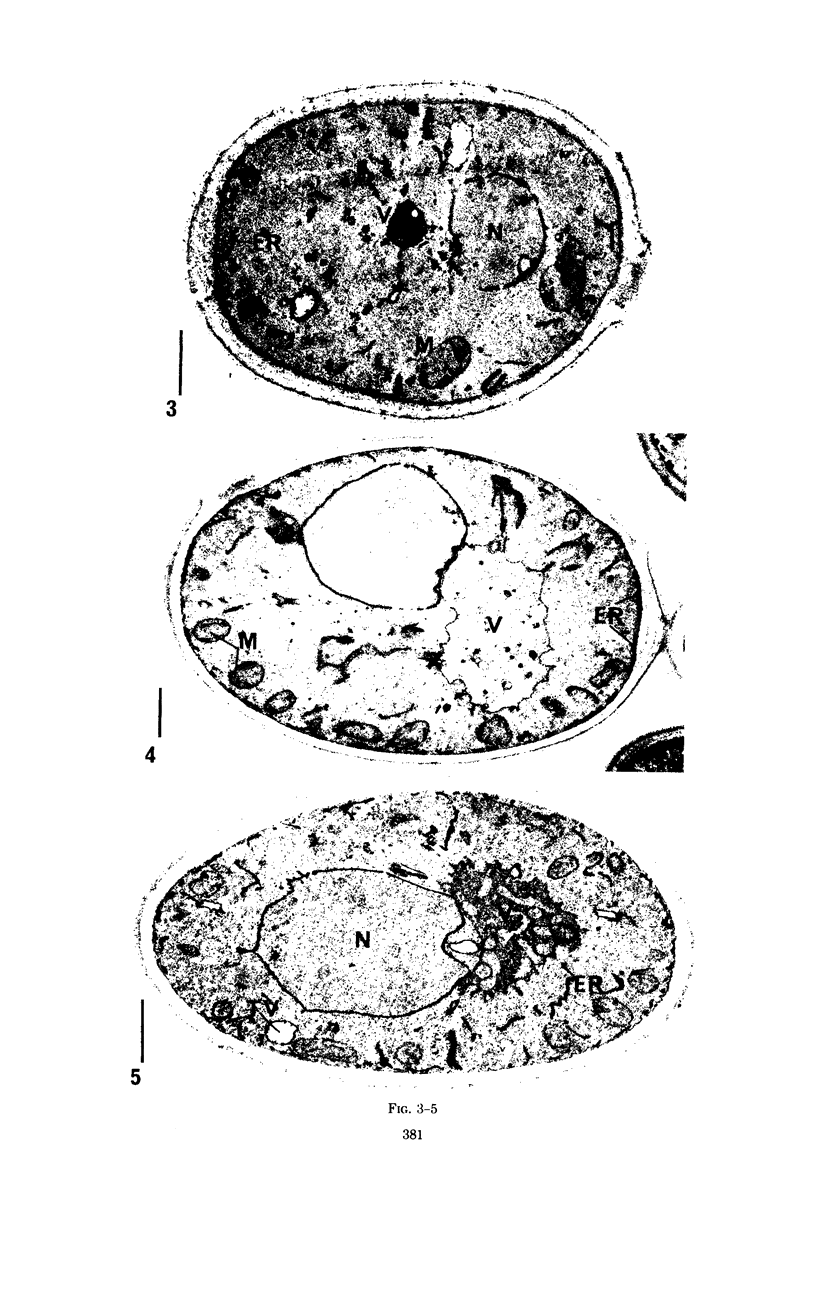
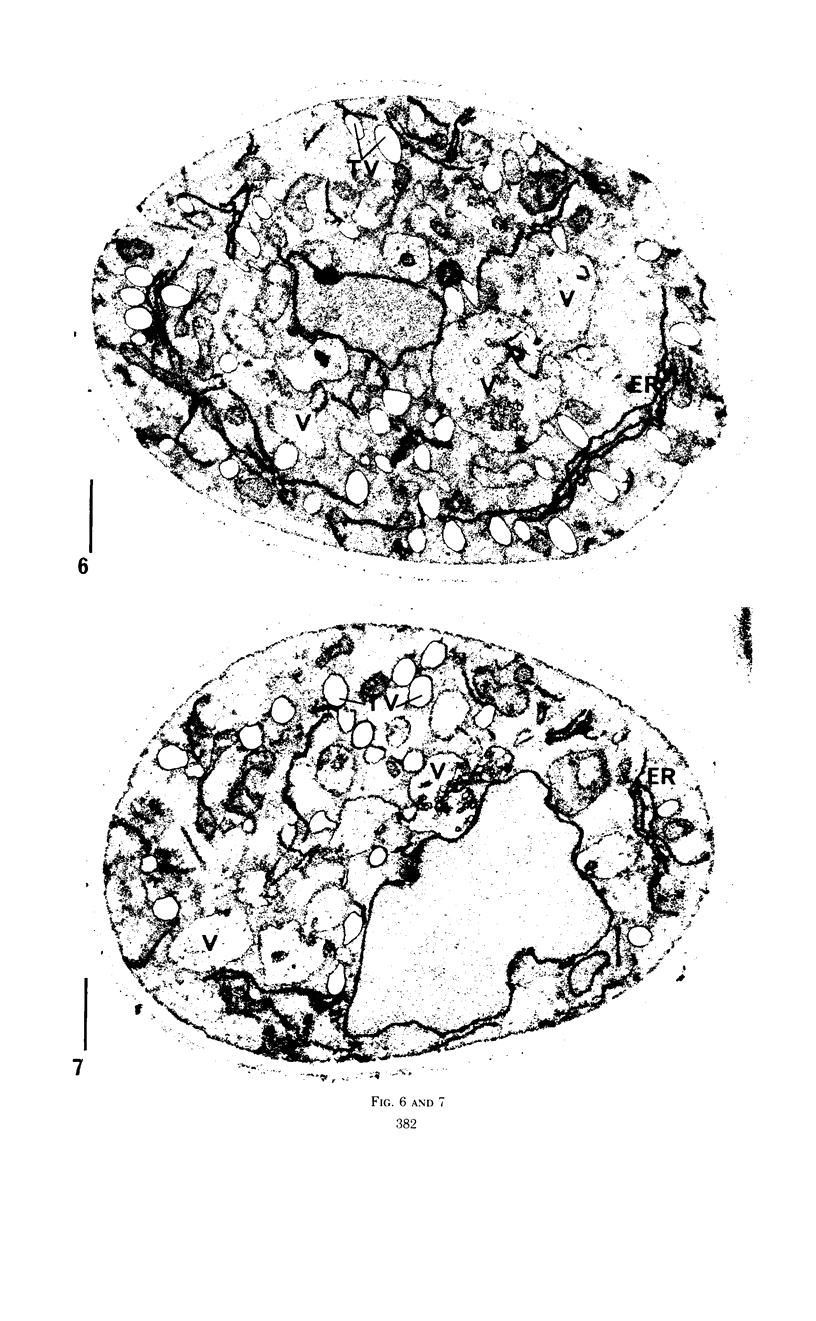
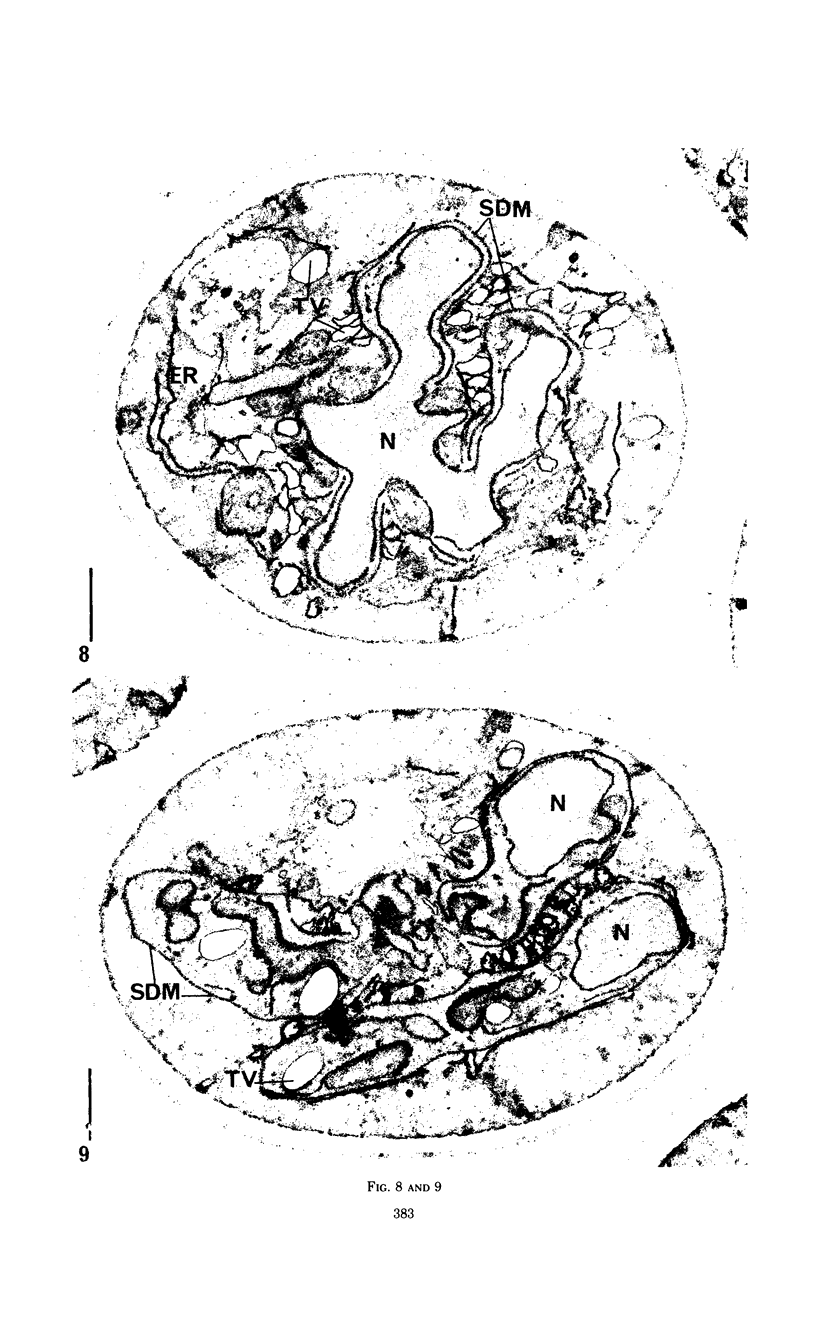
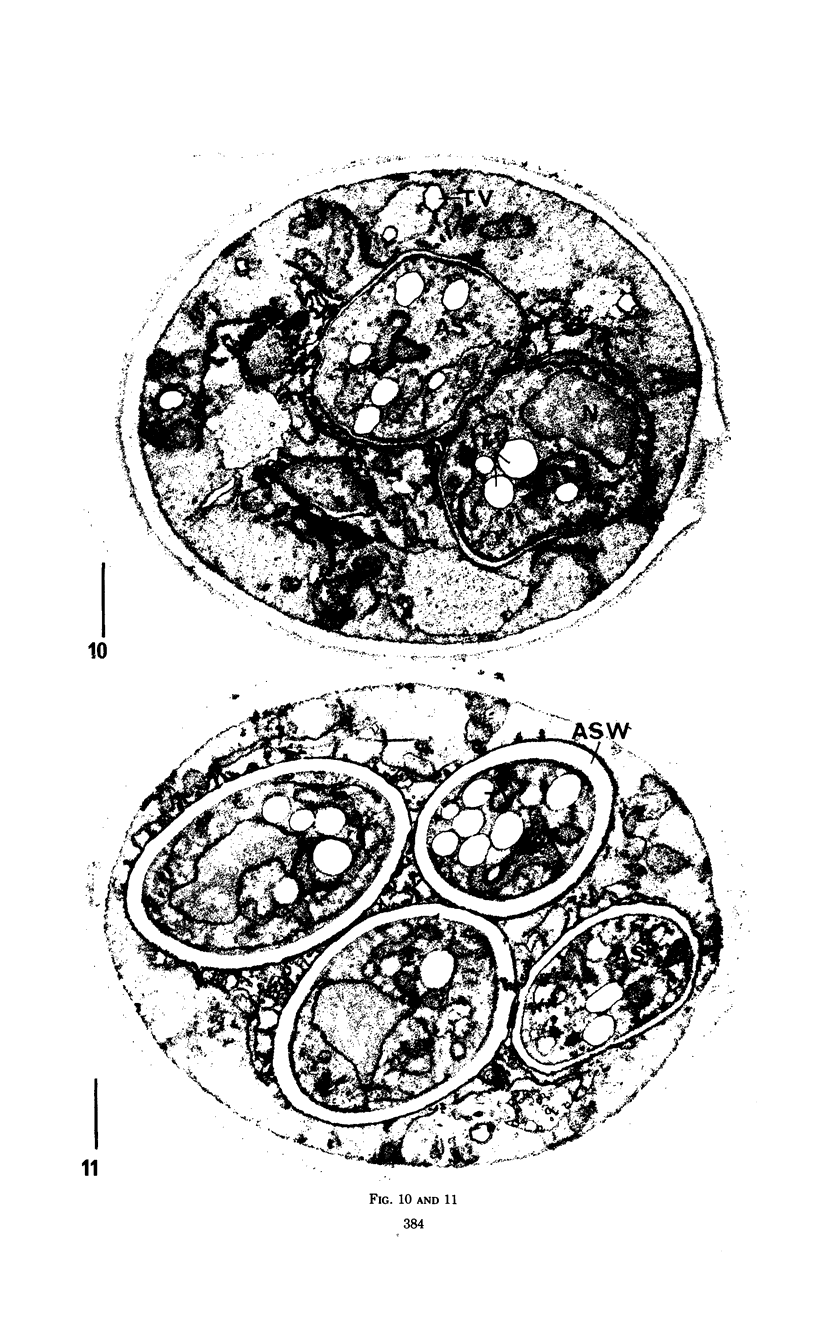
Eighty to ninety percent of vegetative cells of Saccharomyces cerevisiae DCL 740 incubated in KCl-acetate medium form asci, the majority of which are four-spored. Ascospores are visible in asci after about 24 hr, and spore formation is complete after about 48 hr. The dry weight of the cells increases by about 75% during 48 hr of incubation, while the lipid content of the cells increases by a factor of four. The increase in lipid content is attributed mainly to an increased synthesis of sterol esters and triacylglycerols and to a lesser extent of phospholipids. The phospholipid and sterol compositions do not change appreciably, but there is a marked increase in the proportion of unsaturated fatty acid residues in ascan lipids. Uniformly labeled 14C-acetate is incorporated mainly into sterol esters and triacylglycerols and phospholipids. Pulse-labeling by adding acetate-U-14C to sporulating cultures and harvesting after a further 6 hr of incubation reveal two main periods of acetate incorporation, namely between 0 and 18 hr, and between 24 and 30 hr. Electron micrographs of thin sections through developing asci show that the principal changes in fine structure occur between 18 and 24 hr and include the appearance of numerous electron-transparent vesicles which become aligned around the meiotic nucleus, and the laying down of extensive endoplasmic reticulum membranes. Changes in fine structure are discussed in relation to the alterations in lipid content and composition of asci.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Beckett A., Barton R., Wilson I. M. Fine structure of the wall and appendage formation in ascospores of Podospora anserina. J Gen Microbiol. 1968 Aug;53(1):89–94. doi: 10.1099/00221287-53-1-89. [DOI] [PubMed] [Google Scholar]
- Beteta P., Gascon S. Localization of invertase in yeast vacuoles. FEBS Lett. 1971 Mar 22;13(5):297–300. doi: 10.1016/0014-5793(71)80245-4. [DOI] [PubMed] [Google Scholar]
- Black S. H., Gorman C. The cytology of Hansenula. 3. Nuclear segregation and envelopment during ascosporogenesis in Hansenula wingei. Arch Mikrobiol. 1971;79(3):231–248. [PubMed] [Google Scholar]
- Briley M. S., Illingworth R. F., Rose A. H., Fisher D. J. Evidence for a surface protein layer on the Saccharomyces cerevisiae ascospore. J Bacteriol. 1970 Oct;104(1):588–589. doi: 10.1128/jb.104.1.588-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G. C. The ultrastructure of ascospore delimitation in Saccobolus kerverni. J Cell Biol. 1967 Apr;33(1):218–224. doi: 10.1083/jcb.33.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farrell J., Rose A. Temperature effects on microorganisms. Annu Rev Microbiol. 1967;21:101–120. doi: 10.1146/annurev.mi.21.100167.000533. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO T., CONTI S. F., NAYLOR H. B. Fine structure of microorganisms. III. Electron microscopy of resting and germinating ascospores of Saccharomyces cerevisiae. J Bacteriol. 1958 Oct;76(4):406–416. doi: 10.1128/jb.76.4.406-416.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., CONTI S. F., NAYLOR H. B. Studies of the fine structure of microorganisms. IV. Observations on budding Saccharomyces cerevisiae by light and electron microscopy. J Bacteriol. 1959 Mar;77(3):344–354. doi: 10.1128/jb.77.3.344-354.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Longley R. P., Rose A. H., Knights B. A. Composition of the protoplast membrane from Saccharomyces cerevisiae. Biochem J. 1968 Jul;108(3):401–412. doi: 10.1042/bj1080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn R. R., Magee P. T. Development of the spore wall during ascospore formation in Saccharomyces cerevisiae. J Cell Biol. 1970 Mar;44(3):688–692. doi: 10.1083/jcb.44.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P. R., BAUMANN C. A. Skin sterols. I. Colorimetric determination of cholesterol and other sterols in skin. J Biol Chem. 1952 Apr;195(2):615–621. [PubMed] [Google Scholar]
- Moens P. B. Fine structure of ascospore development in the yeast Saccharomyces cerevisiae. Can J Microbiol. 1971 Apr;17(4):507–510. doi: 10.1139/m71-084. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudoerffer T. S., Lea C. H. Antioxidants for the (thin-layer) chromatography of lipids. J Chromatogr. 1966 Jan;21(1):138–140. doi: 10.1016/s0021-9673(01)91276-2. [DOI] [PubMed] [Google Scholar]
- Plurad S. B. Fine structure of ascosporogenesis in Nematospora coryli Peglion, a pathogenic yeast. J Bacteriol. 1972 Feb;109(2):927–929. doi: 10.1128/jb.109.2.927-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Carbohydrate accumulation during the sporulation of yeast. J Bacteriol. 1970 Jan;101(1):53–57. doi: 10.1128/jb.101.1.53-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The formation of buds in yeast. J Gen Microbiol. 1969 Mar;55(3):393–398. doi: 10.1099/00221287-55-3-393. [DOI] [PubMed] [Google Scholar]
- VAN HANDEL E., ZILVERSMIT D. B. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957 Jul;50(1):152–157. [PubMed] [Google Scholar]