Abstract
β-catenin signaling is required for hair follicle development, but it is unknown whether its activation is sufficient to globally program embryonic epidermis to hair follicle fate. To address this we mutated endogenous epithelial β-catenin to a dominant active form in vivo. Hair follicle placodes were expanded and induced prematurely in activated β-catenin mutant embryos, but failed to invaginate or form multilayered structures. Eventually, the entire epidermis adopted hair follicle fate, broadly expressing hair shaft keratins in place of epidermal stratification proteins. Mutant embryonic skin was precociously innervated, and displayed prenatal pigmentation, a phenomenon never observed in wild-type controls. Thus β-catenin signaling programs the epidermis towards placode and hair shaft fate at the expense of epidermal differentiation, and activates signals directing pigmentation and innervation. In transcript profiling experiments we identified elevated expression of Sp5, a direct β-catenin target and transcriptional repressor. We show that Sp5 normally localizes to hair follicle placodes and can suppress epidermal differentiation gene expression. We identified the pigmentation regulators Foxn1, Adamsts20 and Kitl, and the neural guidance genes Sema4c, Sema3c, Unc5b and Unc5c, as potential mediators of the effects of β-catenin signaling on pigmentation and innervation. Our data provide evidence for a new paradigm in which, in addition to promoting hair follicle placode and hair shaft fate, β-catenin signaling actively suppresses epidermal differentiation and directs pigmentation and nerve fiber growth. Controlled downregulation of β-catenin signaling is required for normal placode patterning within embryonic ectoderm, hair follicle downgrowth, and adoption of the full range of follicular fates.
Keywords: epidermis, hair follicle, mouse embryo, β-catenin, Wnt
Introduction
The embryonic surface ectoderm stratifies to form the epidermis, providing an essential protective barrier. Subsets of surface ectodermal cells, rather than stratifying, develop into hair follicles and other ectodermal appendages (Mikkola and Millar, 2006). Hair follicle development is initiated by signaling from the dermis, which induces the formation of local thickenings, or placodes, in the overlying epithelium (Hardy, 1992). While it is currently unclear whether the initiating dermal signal is uniform or generated by labile local dermal condensates, the final location of placodes appears to be influenced by reaction-diffusion mechanisms involving competing epithelial signals (Jiang et al., 2004). These interactions result in the formation of a hexagonal array of primary placodes that appears at approximately embryonic day (E) 14.5 in mouse embryos, and gives rise to the large guard over-hairs of the coat. Secondary placodes are initiated in several waves between E15.5 and birth and develop into awl, auchene and zigzag hair follicles.
Signaling from placode epithelial cells causes condensation of mesenchymal cells that in turn stimulate proliferation and downgrowth of the placode (Hardy, 1992). Rapidly proliferating hair matrix cells surround the dermal condensate, thereafter referred to as a dermal papilla, and differentiate to form the hair shaft and inner root sheath (Fuchs, 2007; Millar, 2002). An outer root sheath surrounds the follicle and is contiguous with the basal epidermis. Incompletely characterized signals direct melanoblasts and nerve fibers to developing hair follicles (Jordan and Jackson, 2000; Peters et al., 2002).
Although the molecular nature of the primary dermal signal has not been established, several families of secreted signaling molecules participate in hair placode induction. These factors include the TNF family member ectodysplasin (EDA), which signals via its receptor EDAR and NF-κB to regulate primary placode formation; BMP family members, that negatively regulate placode formation and compete with EDA in establishing a regular patterned placodal array; and Sonic hedgehog (SHH), which controls proliferation and downgrowth of hair follicle epithelium (reviewed in Fuchs, 2007; Millar, 2002; Schmidt-Ullrich and Paus, 2005). Stable primary placodes fail to form in EDA pathway mutants, but transient pre-placode structures can be detected, suggesting the existence of earlier patterned signals (Schmidt-Ullrich et al., 2006).
Wnt/β-catenin signaling regulates many aspects of development and disease. Signaling is activated by binding of a secreted Wnt ligand to a Frizzled (FZ) receptor and LDL related protein (LRP) 5/6 co-receptor, leading to inactivation of a complex of proteins that phosphorylates cytoplasmic β-catenin and targets it for degradation. Cytoplasmic β-catenin accumulates, translocates to the nucleus, and complexes with LEF/TCF transcription factor family members to activate target gene transcription (Gordon and Nusse, 2006). Wnt/β-catenin signaling is required for hair follicle placode induction and pattered expression of Edar (Andl et al., 2002). Wnt reporter transgene expression is elevated in epithelial and dermal condensate cells at early stages of follicular development, and is then downregulated in the epithelium until the onset of hair shaft differentiation when it appears in hair shaft precursor cells (DasGupta and Fuchs, 1999; Maretto et al., 2003). β-catenin/LEF complexes can directly regulate expression of hair shaft keratin genes in vitro (Merrill et al., 2001), suggesting a key role for Wnt/β-catenin signaling in hair shaft differentiation.
Deletion of the intracellular Wnt/β-catenin pathway inhibitor Apc in skin epithelial cells results in formation an increased number of embryonic hair follicles, and hair follicle abnormalities, but does not affect development and stratification of interfollicular epidermis (Kuraguchi et al., 2006). Transgenic epidermal expression of stabilized β-catenin causes ectopic hair follicle induction in adult skin; however alterations in embryonic skin or hair follicle development were not observed (Gat et al., 1998) or not reported (Lo Celso et al., 2004; Silva-Vargas et al., 2005). Thus, Wnt/β-catenin signaling is required for hair follicle placode induction, but it is not clear whether activation of this pathway in mammalian embryonic surface ectoderm is sufficient to globally program ectodermal cells to hair follicle fate. It is also not known whether Wnt/β-catenin signaling activates the factors that attract nerve fibers and melanoblasts to hair follicles, or whether it suppresses epidermal stratification to allow placode development. To address these questions, we mutated the endogenous β-catenin gene to a constitutively active form in vivo using an efficient KRT14-Cre transgenic line.
Materials and Methods
Generation and genotyping of mouse lines
KRT14-Cre Ctnnb1(Ex3)fl/+ and Krt5-rtTA tetO-Dkk1 mice were generated as described previously (Chu et al., 2004; Liu et al., 2007). For tetracycline inducible β-catenin mutation, mice carrying tetO-Cre (Mucenski et al., 2003) and Krt5-rtTA (Diamond et al., 2000) transgenes and Ctnnb1(Ex3)fl (Harada et al., 1999) were placed on doxycycline chow (1 mg/kg, Bio-serv, Laurel, MD). To generate 4-hydroxytamoxifen inducible β-catenin mutants, 50 μl of 4-hydroxytamoxifen in 95% ethanol (10 mg/ml, Sigma-Aldrich, St. Louis, MO) or vehicle control was applied to dorsal back skin after hair clipping. Mice were genotyped by PCR analysis of tail biopsy DNA. All experiments were performed with approved animal protocols according to University of Pennsylvania and University of Michigan institutional guidelines.
Phenotypic analyses
Histological analysis, whole mount and section in situ hybridization and immunostaining, TUNEL, X-gal staining, Oil Red O staining and TEM were performed as described previously (Allen et al., 2003; Andl et al., 2004; Andl et al., 2006; Braun et al., 2003; Chu et al., 2004; Hutchin et al., 2005; Langbein et al., 2007; Liu et al., 2007). For epidermal barrier assays methanol-fixed embryos were stained in 1% Toluidine Blue O solution (Sigma-Aldrich, St. Louis, MO).
Semi-quantitative PCR and quantitative RT-PCR
Dissected dorsal skin was dispase treated (BD Bioscience, Sparks, MD) to separate epidermis and dermis. Wild type, floxed and deleted-floxed Ctnnb1 alleles were detected by PCR of extracted genomic DNA. RNA was extracted using RNeasy Mini Kit (Qiagen, Inc, Valencia, CA). Primer pairs for qRT-PCR were purchased from Superarray Bioscience. Reactions were performed in triplicate using SYBR green on an MJ Opticon II thermocycler (Bio-Rad, Hercules, CA). Relative expression levels were standardized using β-actin as an internal control. Data were analyzed using the Opticon III program.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using the Chromatin Immunoprecipitation kit (Upstate, Charlottesville, VA). Epidermal cells were dissociated, fixed in 1% formaldehyde for 15 minutes at RT, sonicated and incubated with anti-β-catenin antibody (14, BD Bioscience, San Jose, CA) or control IgG overnight at 4°C, followed by addition of Protein A agarose Beads. Purified DNA was subjected to semi-quantitative PCR with primers that amplify the Sp5 promoter, or Gapdh sequences.
Microarray analyses
Total RNA was subjected to one round of amplification/labeling to obtain biotinylated cRNA for hybridization to Affymetrix GeneChip Mouse Genome MOE430 2.0 oligonucleotide microarrays at the Microarray Core, University of Pennsylvania (Philadelphia, PA). Two entirely independent samples of each tested condition were used for data analyses. Scanned microarray images were imported into Gene Chip Operating Software (GCOS, Affymetrix, Santa Clara, CA) to generate signal values and present/absent calls for each probe set using the MAS 5.0 statistical expression algorithm. Data files were imported into GeneSpring 7.3.1 (Agilent, Santa Clara, CA), and replicate microarrays grouped and compared. Transcripts that were at least 2-fold increased in one population over the other and called present in both replicates were considered significant for further analysis.
Keratinocyte transfection
Full-length mouse Sp5 cDNA cloned in pCMV-3Tag (Stratagene, La Jolla, CA) or pCMV-3Tag control plasmid were transfected into HaCAT cells using the Cell Line Nucleofector Kit V (Amaxz GmbH, Gaithersburg, MD). mRNA was extracted after 3 weeks of culture in 500 μg/ml G-418 and subjected to quantitative RT-PCR for Sp5, KRT10, involucrin and DLX3.
Results
Generation of a dominant stabilizing epithelial β-catenin mutant
To determine whether forced activation of Wnt/β-catenin signaling alters the fate of embryonic surface ectoderm, we stimulated β-catenin signaling in vivo by mutating the endogenous β-catenin gene (Ctnnb1) to a dominant active form in epithelial cells. Ctnnb1(Ex3)fl/fl mice, in which exon 3 of Ctnnb1, encoding all phosphorylation target serine/threonine residues, is flanked by loxP sequences that are targets for Cre-mediated recombination, were mated to KRT14-Cre line 43 transgenic mice expressing Cre recombinase under the control of a keratin 14 promoter (Liu et al., 2007). KRT14-Cre mediated recombination of a ROSA26R Cre reporter gene (Soriano, 1999) was observed in surface epithelium by E11.5 (Supplementary Figure S1).
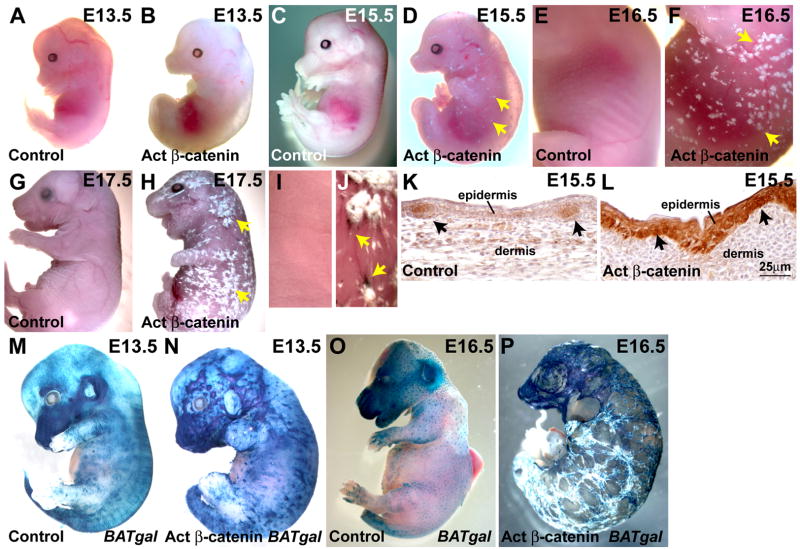
KRT14-Cre Ctnnb1(Ex3)fl/+ embryos died perinatally and displayed limb and craniofacial defects. Their skin appeared grossly abnormal by E14.5, with multiple raised papules, and by birth was almost completely covered in large keratinized plaques (Figure 1A–J). Precocious pigmentation was observed in embryonic ectoderm associated with keratinized plaques (Figure 1J). Control mice displayed hair shaft pigmentation several days after birth, but control embryo skin was never pigmented (Figure 1I).
Figure 1. Abnormal skin development and Wnt/β-catenin pathway activation in stabilized β-catenin mutant embryos.
(A – H) Control littermate and KRT14-Cre Ctnnb1(Ex3)fl/+ (Act β-catenin) embryos at the stages indicated. Arrows indicate keratinized plaques in mutant embryos. (I, J) Higher magnification views of dorsal skin from (G, H), respectively. Pigment (arrows) accumulates in mutant skin. (K, L) Immunohistochemistry for β-catenin at E15.5 showing elevated β-catenin levels in control placodes (K) and cytoplasmic and nuclear β-catenin throughout mutant ectoderm (L) (arrows). (M – P) X-gal stained control BATgal (M, O) and KRT14-Cre Ctnnb1(Ex3)fl/+ BATgal (Act β-catenin BATgal) (N, P) littermates at E13.5 (M, N) and E16.5 (O, P) showing increased BATgal expression and its irregular pattern in the mutant. Size bar in (L) applies to (K, L).
Embryonic mutant skin showed elevated expression of β-catenin protein in the epithelium from E11.5 onwards, with many ectodermal cells exhibiting both cytoplasmic and nuclear localization of β-catenin (Figure 1K, L). To determine the pattern of Wnt/β-catenin pathway activation in mutant skin we generated KRT14-Cre Ctnnb1(Ex3)fl/+ mice carrying two independent Wnt reporter genes, BATgal (Maretto et al., 2003) or TOPGAL (DasGupta and Fuchs, 1999). Expression of either reporter was significantly elevated and more widespread in mutant compared with littermate control skin, and the normal regular pattern of hair follicle placode formation was disturbed in the mutant (Figure 1M – P).
Premature hair follicle placode development in stabilized β-catenin mutant embryos
Thickened epithelial structures expressing the placode marker Shh (St-Jacques et al., 1998) appeared in stabilized β-catenin mutant embryonic dorsal skin by E12.5, whereas hair follicle placodes were not apparent in control littermate embryos until E14.5 (Figure 2A – F).
Figure 2. Premature and expanded placode development in β-catenin mutant embryos.
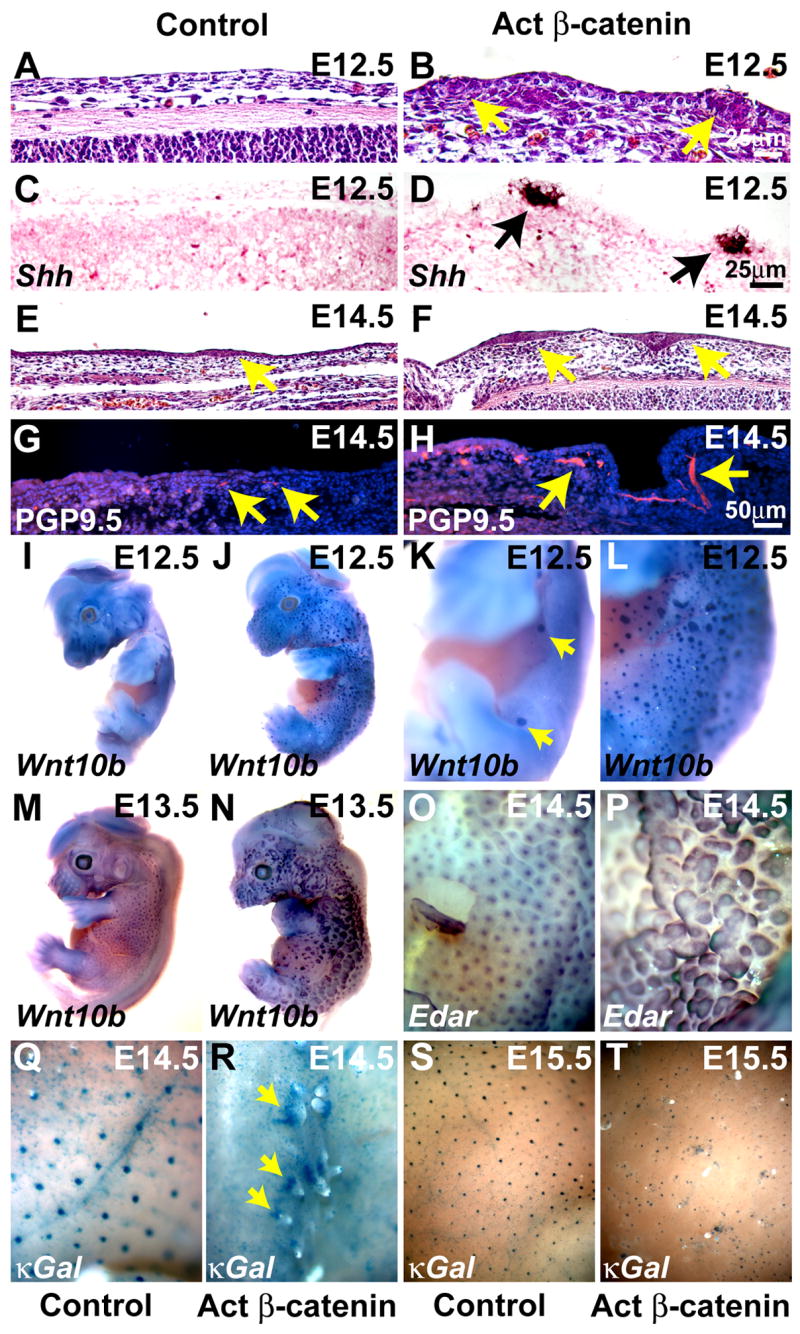
(A – D) Premature placode development at E12.5 indicated by H & E staining (A, B, arrows) and Shh mRNA expression (C, D) (purple brown) in KRT14-Cre Ctnnb1(Ex3)fl/+ (Act β-catenin) embryos compared with controls. (E – H) Placodes are enlarged in the mutant at E14.5 (E, F) and immunofluorescence for PGP9.5 reveals precocious development of enlarged nerve fibers (G, H, arrows). (I – P) Whole mount in situ hybridization for Wnt10b (I – N) or Edar (O, P) reveals accelerated formation of enlarged placodes in the mutants. (Q – T) Whole mount X-gal staining of control κGal (Q, S) and KRT14-Cre Ctnnb1(Ex3)fl/+ κGal (R, T) embryos reveals an irregular pattern of NFκB activity in the mutants. Size bars in (B), (D), (H) apply to (A, B); (C, D); (E – H), respectively.
Interestingly, immunofluorescence for the pan-neural marker PGP9.5 revealed that E14.5 mutant skin was more extensively innervated than control littermate skin, with larger, more developed nerve fibers (Figure 2G, H). These data indicate that secreted factors activated by cell autonomous mutant β-catenin accelerate attraction of nerve fibers to the skin.
To visualize the global pattern of placode development we carried out whole mount in situ hybridization for the placode marker Wnt10b (St-Jacques et al., 1998). At E12.5 elevated Wnt10b expression localized specifically to mammary and vibrissa placodes in control embryos (Figure 2I, K). Stabilized β-catenin mutant embryos displayed numerous placodes covering the trunk, head and limbs (Figure 2J, L). At E13.5, mutant placodes were enlarged (Figure 2M, N). Counting of placodes in a defined area of head skin at E13.5 revealed a mean of 7±3 placodes in control embryos (n=3) and 33±9 placodes in mutant embryos (n=3) (p=0.03). Wnt10b-positive placodes were observed in footpads of E12.0 mutant but not control embryos (Supplementary Figure S2). Thus activated β-catenin can promote placode development even in normally hairless skin regions. Accelerated development of sweat glands was not observed in mutant footpads at later embryonic stages, suggesting that footpad placodes did not represent precocious sweat gland anlagen. Mammary glands developed in mutant female embryos, but exhibited histological abnormalities that will be described elsewhere.
Expression of Edar, assayed at E14.5, was elevated and more widespread in mutant than in littermate control skin (Figure 2O, P). To determine whether EDAR signaling is activated in mutant placodes, we generated KRT14-Cre Ctnnb1(Ex3)fl/+ mice carrying the NF-κB reporter gene (Igκ)3xcona-lacZ (κGal) (Schmidt-Ullrich et al., 1996). NF-κB activity was detected in KRT14-Cre Ctnnb1(Ex3)fl/+ κGal skin at E14.5 and E15.5, but failed to show its normal regular pattern (Figure 2Q – T). NF-κB activity was not detected in mutant skin at earlier stages despite placodal expression of Wnt10b and Shh. Precociously initiated placodes may thus be more similar to secondary awl than primary hair follicle placodes, as development of the former but not the latter is independent of NF-κB signaling.
Impaired hair follicle development and broad adoption of hair follicle fate in activated β-catenin mutant surface ectoderm
Despite formation of enlarged placodes in stabilized β-catenin mutant embryos at E14.5, hair follicles failed to extend into the dermis or form multilayered structures (Figure 3A – J). In some regions of the mutant embryos, large evaginations were noted that accumulated keratinized material and corresponded to the keratinized papules seen in whole mounts (Figure 3B, D, J). Stratification of the epidermis appeared impaired, and fibroblasts in the upper dermis were more densely packed than those in littermate controls (Figure 3G, H).
Figure 3. Aberrant hair follicle development and global expression of placode and hair shaft markers in activated β-catenin mutant embryos.
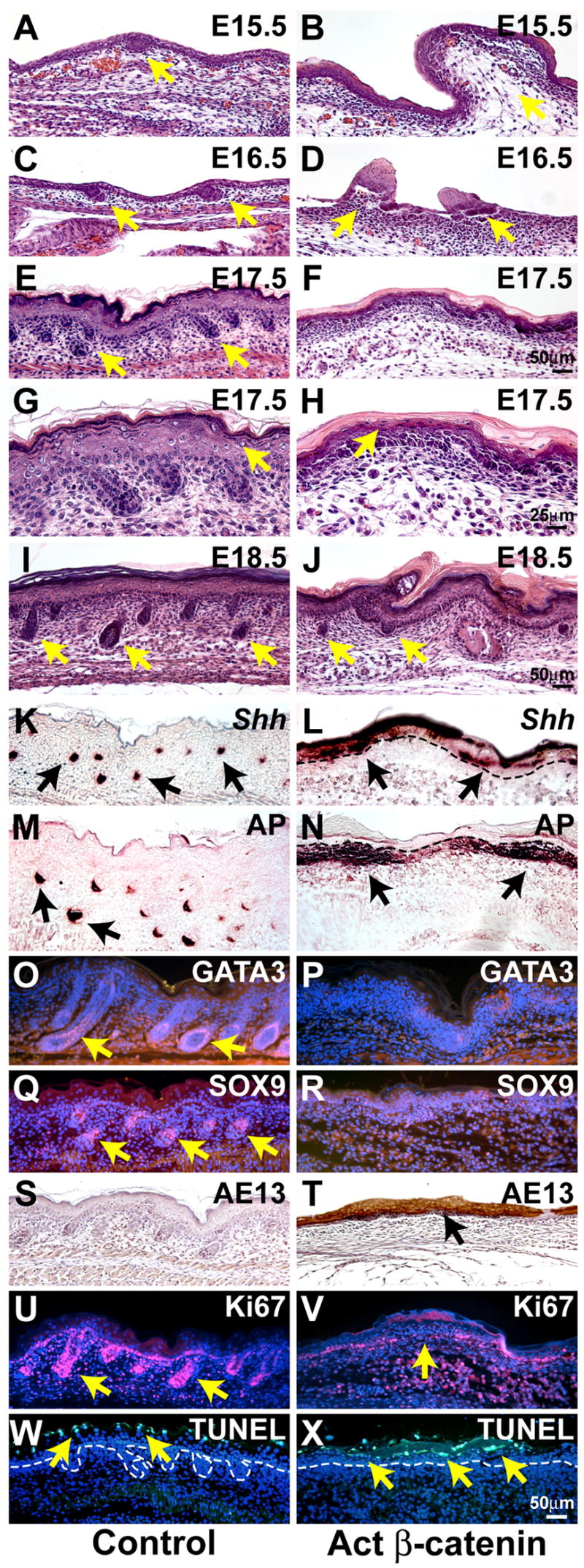
(A – J) Histological analysis of dorsal skin from control littermate and KRT14-Cre Ctnnb1(Ex3)fl/+ mutant embryos reveals failure of hair follicle downgrowth in mutants. Mutant skin displays evaginations (B, D, J) and fails to stratify normally (H). (G, H) are higher magnification views of (E, F) respectively. The placode marker Shh (in situ hybridization, brown) (K,L) and dermal condensate marker alkaline phosphatase (AP) (enzymatic assay, purple brown) (M,N) are expressed broadly in the mutant. Expression of the inner root sheath marker GATA3 (O,P) and the outer root sheath marker SOX9 (Q,R) (immunofluorescence, red) is reduced or absent, but hair shaft keratins (AE13 antibody, immunohistochemistry, brown) are ectopically expressed in mutant ectoderm (S, T). Proliferation is decreased (U,V) (Ki67 immunofluorescence, red) and the pattern of apoptosis/terminal differentiation is altered (W, X) (TUNEL staining, green) in mutant ectoderm. Dashed lines mark dermal-epidermal boundaries. Samples were at E17.5 (K –N; Q – X) or newborn (O, P). Size bars in (F), (H), (J), (X) apply to (A – F); (G, H); (I, J); (K – X), respectively.
To determine the molecular basis for these phenotypes we examined expression of hair follicle markers at E17.5. Shh is a specific marker for hair follicles within the skin (Chiang et al., 1999; St-Jacques et al., 1998), and at this stage its expression was confined to control hair follicle bulbs (Figure 3K). In mutant embryos, expression of Shh was observed throughout the surface ectoderm, suggesting widespread acquisition of hair follicle fate (Figure 3L). Alkaline phosphatase activity marks the hair follicle dermal condensate and dermal papilla (Figure 3M). Strikingly, in mutant embryos alkaline phosphatase activity was detected broadly throughout most of the upper dermis (Figure 3N). Together with the increased density of upper dermal fibroblasts, this abnormal staining indicated that upper dermal cells had broadly acquired at least some characteristics of hair follicle dermal condensate. These data suggest that secreted factor(s) expressed in response to cell autonomous stabilized epithelial β-catenin induced dermal condensation and alkaline phosphatase expression.
To determine whether markers for hair follicle differentiation were induced in mutant surface ectoderm, we analyzed expression of GATA3, which is specifically expressed in inner root sheath precursors within the hair follicle and is required for their differentiation (Kaufman et al., 2003); Sox9, a marker for the hair follicle outer root sheath (Vidal et al., 2005); and hair shaft keratins that are specifically recognized by the AE13 antibody (Lynch et al., 1986). Expression of GATA3 and Sox9 was detected in the inner and outer root sheaths of control hair follicles, respectively, between E17.5 and birth, but expression of either marker was barely detectable in mutant ectoderm (Figure 3O – R). Immunostaining for AE13 was confined to hair shaft precursor cells in controls, but, remarkably, in the mutant was broadly and strongly present throughout the surface ectoderm (Figure 3S, T). Thus, among the various hair follicle epithelial lineages, mutant ectodermal cells appeared to differentiate predominantly towards hair shaft rather than inner or outer root sheath.
Consistent with hair shaft-like differentiation of mutant ectodermal cells, at E17.5 mutant dorsal skin displayed a mean of 10±3 Ki67 positive cells per field at 20x compared with 29±5 in the equivalent region of control skin (n=3 embryos and 2 fields counted per embryo for each genotype) (p=0.0024) (Figure 3U, V). Decreased proliferation in mutant ectoderm was largely due to absence of defined hair follicle matrix cell populations. TUNEL-positive cells were detected broadly in mutant surface ectoderm, but in controls were mostly detected in terminally differentiating upper layers of the epidermis (Figure 3W, X).
Induction of the stabilizing β-catenin mutation in embryonic surface epithelium after placode initiation causes defects in hair follicle development
Abnormal hair follicle development could be due to global alterations in the surface ectoderm prior to the stage when placodes are normally initiated, rather than to specific effects within developing follicles. To test this, we generated Krt5-rtTA tetO-Cre Ctnnb1(Ex3)fl/+ embryos in which mutation of β-catenin to a stabilized form can be induced in epithelial cells by dosage of the pregnant mother with oral doxycycline, and Krt5-rtTA tetO-Cre Ctnnb1+/+ littermate controls. Untreated Krt5-rtTA tetO-Cre Ctnnb1(Ex3)fl/+ embryos and adult mice lacked overt phenotypes. Induction of β-catenin mutation from E13.5, the stage at which primary placode initiation is just beginning, resulted in formation of enlarged, abnormal placodes, and failure of hair follicle downgrowth (Figure 4A, B). Similarly, induction from E15.5, a stage after the formation of primary hair follicle placodes, blocked hair germ extension and resulted in defects in epidermal stratification, assayed at birth (Figure 4C – F). Pigment accumulation was noted in mutant but not control littermate skin at birth (Figure 4G, H). Thus the activated β-catenin mutation causes defective hair follicle development and abnormal pigmentation when induced after the onset of hair follicle placode formation.
Figure 4. Mutation of β-catenin after placode initiation blocks hair follicle downgrowth and causes abnormal epidermal stratification and pigmentation.
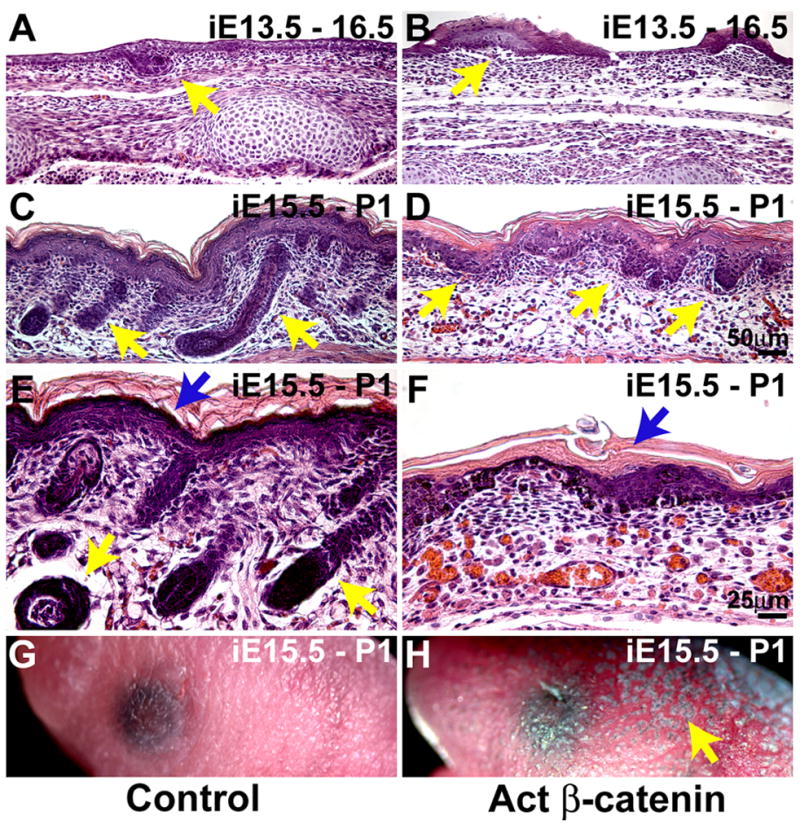
(A – F) H & E stained sections of E16.5 (A, B) or P0.5 (C – F) dorsal skin from littermate control and Krt5-rtTA tetO-Cre Ctnnb1(Ex3)fl/+ embryos doxycycline treated from E13.5 (A, B) or E15.5 (C – F). (E, F) are higher magnification views of (C, D). Placodes are abnormal and expanded at E16.5 (A, B), but hair follicle downgrowth fails (C, D). Epidermal stratification is abnormal in the mutants (E, F). (G, H) Precocious pigmentation (arrow) of newborn mutant induced from E15.5 (G, H). Size bars in (D), (F) apply to (A – D) and (E, F), respectively.
Stabilized β-catenin mutants lack epidermal differentiation and barrier function
To analyze defects in epidermal stratification at the molecular level, we carried out immunofluorescence for specific markers for basal, spinous and granular epidermal layers at E17.5. Stabilized β-catenin mutants exhibited reduced levels of the basal layer marker KRT14 compared with littermate controls (Figure 5A, B). Immunofluorescence for the spinous layer markers KRT1 and KRT10 and the granular layer markers filaggrin and loricrin, was prominent in control littermate embryos, but virtually undetectable in mutant surface ectoderm (Figure 5C – H). Expression of the desmosomal components DSC1 and DSG1, which normally localize to suprabasal epidermal layers, was markedly reduced in mutant surface ectoderm (Figure 5I – L). These data are consistent with a complete failure of epidermal differentiation and global conversion of ectodermal cells to a hair follicle fate.
Figure 5. Epidermal differentiation fails in activated β-catenin mutants.
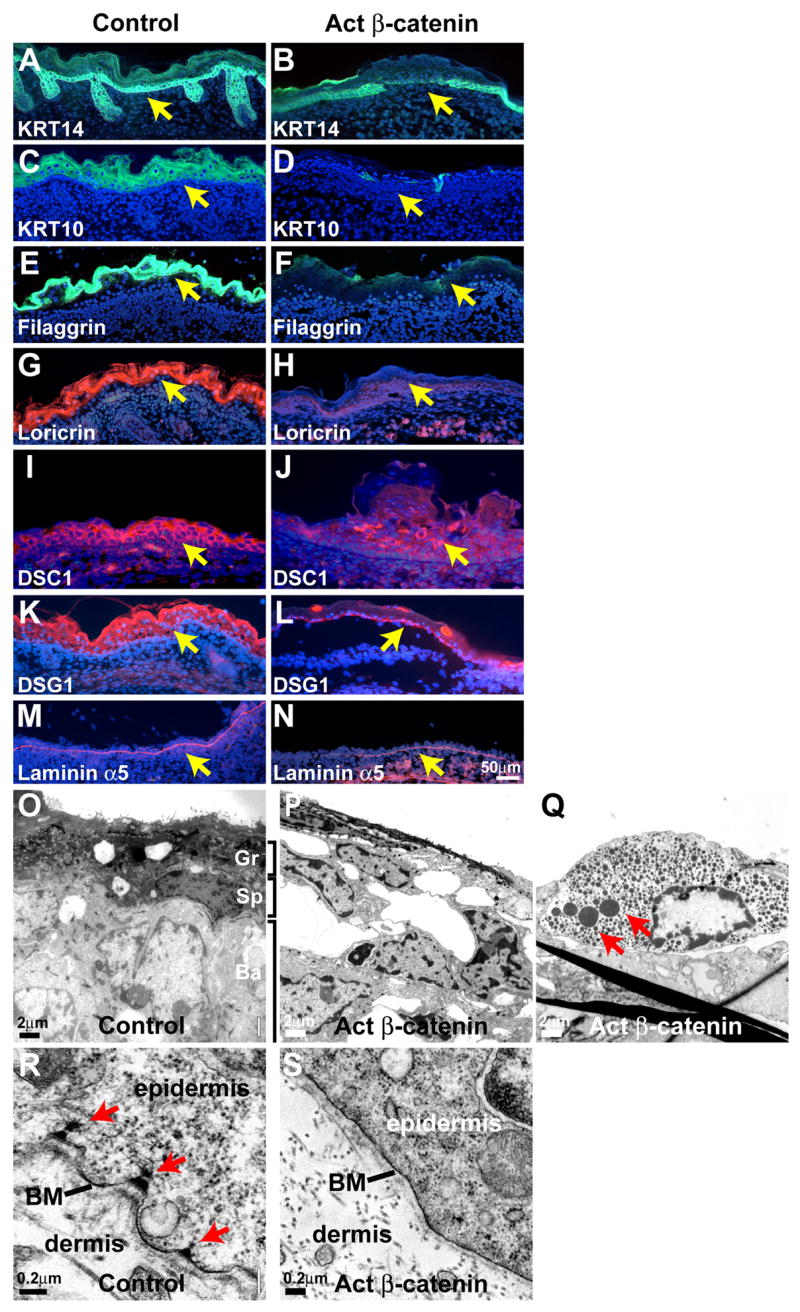
(A–N) Expression of KRT14 (A, B), DSC1 (I, J) and DSG1 (K, L) is decreased and expression of KRT10, filaggrin and loricrin (C – H) is absent in stabilized β-catenin mutants, but laminin α5 expression (M, N) indicates that the basement membrane remains intact. Size bar in (N) applies to (A – N). (O – S) TEM of E16.5 control (O, R) and mutant (P, Q, S) dorsal epidermis (O – Q) or epidermal-dermal junction region (R, S) reveals presence of basal (Ba), spinous (Sp) and granular (Gr) layers in control epidermis (O) and their absence in the mutant (P). Pigment granules are present in mutant ectoderm (red arrows in Q). The basement membrane (BM) is intact in the mutant, but hemidesmosomes (red arrows in (R)) are reduced in number (S).
The striking defects in epidermal stratification in stabilized β-catenin mutants suggested that barrier formation was abnormal. To test this, we subjected mutant and control littermate embryos to dye exclusion assays between E16.5 and E18.5. Control embryos exhibited barrier formation on their dorsal surface at E17.5 and were completely able to exclude dye by E18.5. By contrast, mutant embryos totally failed to exclude dye at all stages examined (Supplementary Figure S3).
To determine the impact of these changes on skin ultrastructure we subjected control and stabilized mutant skin to transmission electron micrography at E16.5. Control epidermis displayed the expected stratifying structure, with readily identifiable basal cells; spinous cells characterized by dense networks of keratin filaments; and granular cells containing keratohyalin granules. In contrast, mutant surface ectoderm lacked characteristics of suprabasal, spinous and granular cells (Figure 5O, P). Pigment granules were noted in some regions of the mutant ectoderm (Figure 5Q). Consistent with unaffected expression of laminin α5 in mutant skin (Figure 5M, N), the basement membrane was present in the mutant, and lamina lucida and lamina densa structures were similar to controls. However, hemidesmosomes were reduced in number compared with control skin (Figure 5R, S). Notably, large spaces were apparent between mutant epithelial cells (Figure 5P), suggesting defective cell-cell adhesion. Consistent with cell adhesion defects, E-cadherin was downregulated in mutant basal cells; by contrast, P-cadherin localized intensely to control hair germs, but in mutants was strongly expressed throughout the surface epithelium (Supplementary Figure S4).
Global changes in gene transcription patterns in activated β-catenin mutant skin
To begin to dissect the molecular mechanisms underlying the dramatic effects of stabilized β-catenin on embryonic surface ectodermal development, innervation and pigmentation, we used microarray analysis to obtain global transcript profiles of mutant and stabilized β-catenin mutant dorsal dermis and epidermis at E15.5 and unseparated dorsal skin at E14.5 (GEO accession number GSE10733). We analyzed >18,000 transcripts that were flagged as present in at least 2 of 4 samples, representing >14,000 genes. Genespring software was used to normalize the raw data and identify genes with a Student’s t test p value of less than 0.05 and a change in relative expression levels of at least 2-fold in KRT14-Cre Ctnnb1(Ex3)fl/+ compared with control samples.
Analysis at E15.5 revealed major changes in the transcriptional profile of mutant compared with control epithelium, with 3124 probe sets identified as >2-fold upregulated and 3173 probe sets >2-fold downregulated in mutant epithelium at E15.5 (Supplementary Table S1, GEO accession number GSE10728). These numbers overestimate the actual number of differentially expressed transcripts as some transcripts were detected by multiple probe sets. Selected genes differentially expressed in mutant and control epithelium at E15.5 are listed in Table 1. A smaller number of probe sets displayed differential expression in mutant compared with control dermis at E15.5 (97 >2-fold upregulated and 55 >2-fold downregulated) (Supplementary Table S2, GEO accession number GSE10727). The altered gene transcript profile in mutant dermis presumably occurs in response to secreted factors expressed downstream of mutant epithelial β-catenin. A subset of the affected epidermal and dermal genes, including the placode markers Shh and Ptch1, already demonstrated altered expression at E14.5 (121 >2-fold upregulated and 49 >2-fold downregulated signals) (Supplementary Table S3, GEO accession number GSE10726). Upregulation of additional Shh pathway members Ptch2, Smo and Gli3 was detected in mutant ectoderm at E15.5. Note that expression of some epithelial-associated genes appears in the E15.5 dermal transcript data set. In situ hybridization and immunostaining experiments indicate that these aberrations are due to difficulties in completely separating mutant epithelial and dermal tissue before RNA extraction, rather than to mis-expression (Figure 3S, T and data not shown). Wnt/β-catenin signaling pathway genes known to be Wnt-regulated showed increased expression in mutant ectoderm, providing validation of the microarray data (Table 1).
Table 1.
| Table 1A: Selected genes upregulated in activated β-catenin mutant epidermis at E15.5 | |
| Wnt/β-catenin pathway | Lef1, Tcf4, Nkd1, Nkd2, Dkk4, Dact1, Wif1, Axin2, Krm1, Wisp1, Wnt5a, Wnt11, Wnt10a |
| Non-canonical Wnt pathway | Daam2, Prickle2, Vangl2, Ror2, Wnt5a, Wnt11 |
| SHH pathway | Shh, Ptch1, Ptch2, Smo, Gli3 |
| BMP pathway | Bmp2, Bmp4, Msx1, Msx2 |
| Notch pathway | Hey1, Hes6, Deltex-1, Dll1 |
| Axon guidance | Sema4c, Sema3c, Unc5b, Unc5c |
| Neuronal | Robo1, Netrin G1 |
| Melanoblast regulatory | Adamts20, Kitl |
| Melanoblast / melanocyte expressed | Mlana, Mitf, Mc1r |
| Pigment deposition | Foxn1 |
| Hair shaft precursor-associated | S100a3, Mt4, Bmp2, Bmp4, Msx1, Msx2, Hoxc13, Foxq1, Foxn1 |
| Buttonhead family transcription factors | Sp5, Sp8 |
| Hair shaft keratins | Krt31, Krt32, Krt33a, Krt34, Krt35, Krt82, Krt85, mHa3 |
| Hair shaft keratin associated proteins | Krtap16 family |
| Keratins specific for IRS cuticle | Krt72, Krt73, Krt26 |
| Keratins expressed in IRS cuticle and other IRS layers | Krt25, Krt27, Krt71 |
| Table 1B: Selected genes downregulated in activated β-catenin mutant epidermis at E15.5 | |
| Gata3 regulated genes | Gata3, Ptgs1, Sgpp1 |
| Signaling molecules | Wnt7a, Tgfbr3 |
| Homeobox containing transcription factors | Hoxa3, Hoxa5 and Hoxa7, Irx1, Irx2 |
| Stem cell-associated | Lrig1, Krt15 |
| Epidermal stratification | Krt14, Krt1, Krt10, Lor, Casp14, Clca1, Clca2, Hrnr |
| Desmosomal components | Dsc1, Dsc3, Dsg1 |
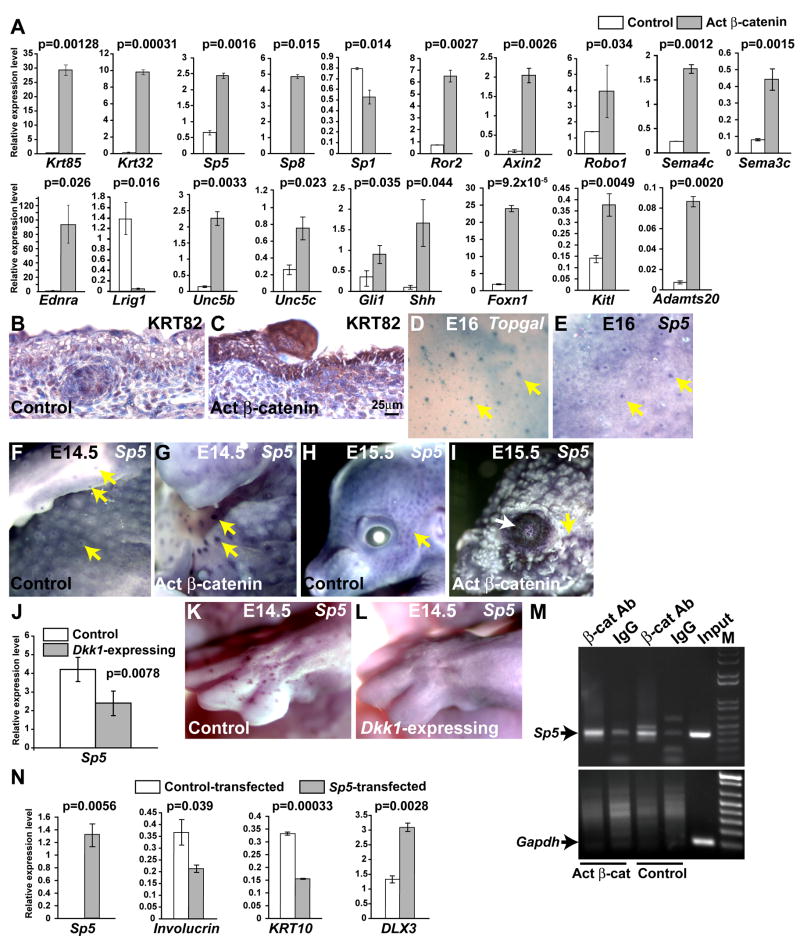
Consistent with the results of immunostaining experiments with AE13, transcript levels for multiple hair shaft specific keratins and keratin-associated proteins were dramatically increased in the mutant. These data were confirmed for selected hair keratins by quantitative RT-PCR (Krt85 and Krt32) (Figure 6A) and immunohistochemistry (KRT82) (Figure 6B, C). Also markedly upregulated was a battery of transcripts expressed in hair shaft precursor cells and/or required for hair shaft differentiation (Table 1). Adamts20 and Kitl that regulate melanoblast migration (Rao et al., 2003) and Foxn1, that controls pigment deposition (Weiner et al., 2007) were over-expressed, and could contribute to the premature pigmentation observed in mutant skin. In line with our finding of increased nerve fiber density in mutant skin, transcripts for several axon guidance molecules including Sema4c, Sema3c and the netrin receptor genes Unc5b and Unc5c were markedly upregulated in the mutant.
Figure 6. Verification of array data and identification of Sp5 as a direct β-catenin target capable of suppressing epidermal differentiation gene expression.
(A) qRT-PCR assays for the indicated transcripts in epidermal extracts from control and activated β-catenin mutant E15.5 embryos. Average relative expression levels, measured in triplicate assays of two independent pairs of experimental and control samples, were normalized to levels of actin transcripts. (B, C) Immunohistochemistry for hair keratin KRT82 (brown) demonstrates its upregulation in activated mutant epithelium at E16.5. (D, E) Similar patterns of Topgal (whole mount X-gal staining, blue) and Sp5 (whole mount in situ hybridization, purple) expression in wild type primary hair follicle placodes at E16. The less sensitive in situ hybridization assay does not detect Sp5 expression in all secondary hair follicles at this stage. (D, E) were photographed at the same magnification. (F – I) Expanded Sp5 expression in KRT14-Cre Ctnnb1(Ex3)fl/+ mutant embryos at E14.5 (F, G) and E15.5 (H, I) (yellow arrows) and its ectopic expression in mutant cornea (white arrow). (J) qRT-PCR shows decreased expression of Sp5 in epidermal extracts of K5-rtTA tetO-Dkk1 E14.5 embryos doxycycline treated from E0.5 compared with control littermates. (K, L) Absence of placodal Sp5 expression in Dkk1-expressing (L) compared with littermate control (K) E15.5 embryos. (M) ChIP assay for β-catenin binding to a region of the Sp5 promoter containing multiple conserved LEF/TCF binding sites. Chromatin prepared from E15.5 control and KRT14-Cre Ctnnb1(Ex3)fl/+ epidermis was incubated with anti-β-catenin antibody (β-cat Ab) or control IgG. Semi-quantitative PCR detects β-catenin binding to the Sp5 promoter in control extracts and increased binding in KRT14-Cre Ctnnb1(Ex3)fl/+ extracts. β-catenin does not bind to Gapdh sequences (bottom panel). (N) qRT-PCR detects expression of transfected mouse Sp5, decreased levels of endogenous KRT10 and involucrin, and increased expression of endogenous DLX3 in Sp5-transfected compared with empty vector control-transfected HaCAT cells. Average relative expression levels measured in triplicate assays of three independent transfection experiments are shown, normalized to levels of actin transcripts.
The stem cell-associated genes Lrig1 (Jensen and Watt, 2006) and Krt15 (Morris et al., 2004) were downregulated in mutant ectoderm, consistent with lack of expression of Sox9, that is required for establishing the hair follicle stem cell compartment (Vidal et al., 2005), and with our observation that in this developmental context, stabilized β-catenin promotes ectodermal differentiation towards hair shaft, rather that maintaining cells in an undifferentiated state.
An unexpected finding was that the transcription factor gene Sp5 and its relative Sp8 were substantially upregulated in mutant epidermis. Wnt regulates Sp8 expression in developing limb (Kawakami et al., 2004). Sp5 is Wnt-regulated in diverse developmental and disease processes and its proximal promoter region contains five evolutionarily conserved TCF/LEF binding sites that mediate direct activation by canonical Wnt signaling (Fujimura et al., 2007; Takahashi et al., 2005; Weidinger et al., 2005). Differential expression of Sp5, Sp8 and selected additional genes from the microarray analyses was confirmed by quantitative RT-PCR (Figure 6A). Sp5 expression localized to hair follicle placodes in wild type embryos in a similar pattern to Topgal Wnt reporter activity (Figure 6D, E), and was increased in gain of function β-catenin mutant ectoderm (Figure 6F – I). Conversely, in ectoderm from embryos expressing the Dkk1 Wnt inhibitor under the control of a Krt5 promoter (Chu et al., 2004), Sp5 expression, analyzed by quantitative RT-PCR and in situ hybridization, was downregulated (Figure 6J – L). We confirmed by ChIP analysis that Sp5 is a direct target of β-catenin in surface ectodermal cells in vivo (Figure 6M).
Sp5 represses KRT10 and involucrin expression in keratinocytes
Sp5 can function as a transcriptional repressor (Fujimura et al., 2007), suggesting it as a candidate factor mediating downregulation of epidermal differentiation genes in mutant β-catenin surface ectoderm. To test this, we transfected an expression vector for mouse Sp5 into the HaCAT human keratinocyte cell line under differentiating conditions. Sp5 suppressed expression of mRNAs encoding the squamous differentiation markers KRT10 and involucrin relative to their levels in empty vector-transfected cells (Figure 6N). Thus, Sp5 may mediate some of the effects of β-catenin in downregulating a subset of epidermal differentiation genes. Interestingly, expression of DLX3, which is predominantly associated with hair shaft differentiation (Morasso et al., 1995), was increased by transfected Sp5.
Stabilized β-catenin induced in adult ectoderm causes formation of functional ectopic hair follicles
To test the effects of inducing mutation of β-catenin in adult epidermis we analyzed hair follicle development in Krt5-rtTA tetO-Cre Ctnnb1(Ex3)fl/+ experimental and Krt5-rtTA tetO-Cre Ctnnb1+/+ littermate control mice placed on oral doxycycline from postnatal day (P) 35 – 40 or P50 – 70, and in adult KRT5-CreERT2 Ctnnb1(Ex3)fl/+ mice treated from P56 – 63 with tamoxifen or vehicle control applied topically to dorsal skin after hair clipping. For both methods of Cre induction, histological analysis of induced mutant skin revealed initiation of hair follicle development from existing hair follicle outer root sheaths and epidermis, resulting in development of multiple, tightly packed hair follicles (Supplementary Figures S5 and S6). Mutant follicles were associated with sebaceous glands, expressed hair follicle matrix and outer root sheath markers (Figure S6), and produced functional hair shafts (Figure S5). Induced Krt5-rtTA tetO-Cre Ctnnb1(Ex3)fl/+ footpads did not display an obvious increase in the number of sweat glands, but contained ectopic, abnormal hair follicles developing from the epidermis (Figure S5H-K), suggesting that stabilized β-catenin may preferentially induce hair follicle development in this context.
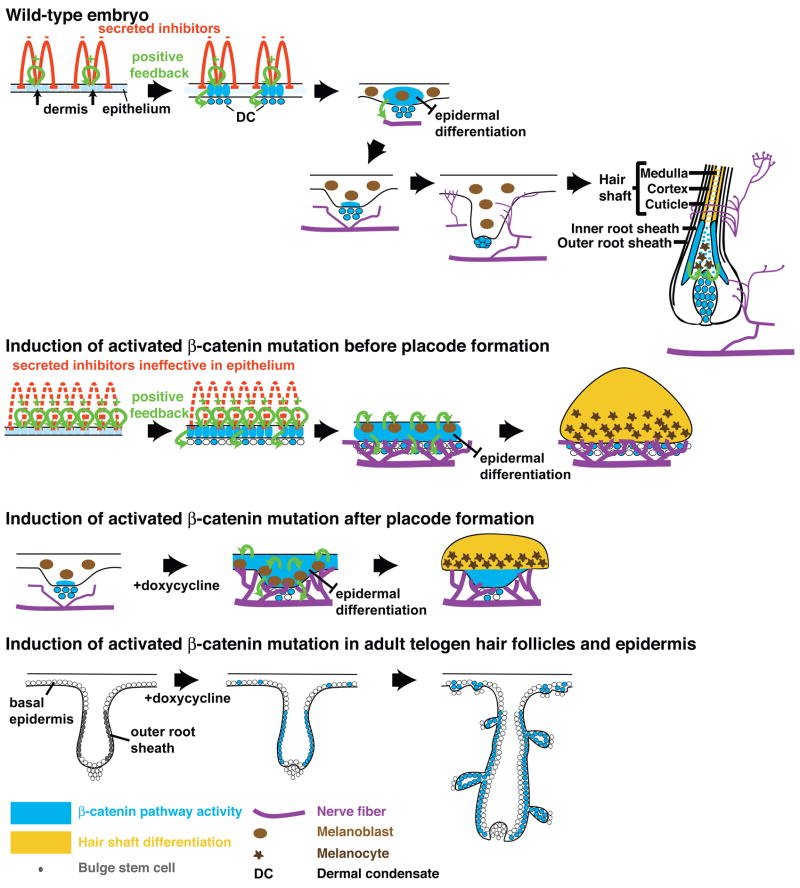
These data indicate that the stabilized β-catenin mutation produces distinct phenotypes in embryonic and adult skin (Figure 7). Nuclear localized β-catenin was detected prominently in adult induced hair follicles (Supplementary Figure S6D′-F′), but was present less uniformly than in induced embryonic ectoderm (Figure 1L), possibly accounting in part for the different phenotypic outcomes. Semi-quantitative PCR analysis of floxed and recombined floxed Ctnnb1 alleles revealed that the efficiency of recombination in induced adult epidermis is similar to, or slightly greater than, that in embryonic surface ectoderm, (Figure S5L), suggesting that differential β-catenin accumulation is not primarily due to differences in recombination efficiency. Instead, stage and context dependent activation of the beta-catenin promoter, positive feedback signaling in embryonic and hair follicle epithelium, and/or inhibitory effects in established, adult epidermis may affect β-catenin expression and function in different subsets of cells. Lack of an embryonic skin phenotype in previously described KRT14-ΔNβcat transgenic mice (Gat et al., 1998) could be due to β-catenin-mediated downregulation of the KRT14 promoter, in contrast to positive feedback regulation of epithelial β-catenin mRNA levels by stabilized β-catenin (Liu et al., 2007), or to subtle activity differences in the ΔNβcat and exon 3 deleted β-catenin mutant proteins.
Figure 7. Model of the effects of activated β-catenin on ectodermal differentiation.
Wnt/β-catenin signaling (blue) is weakly activated in subsets of wild type epithelial cells in response to dermal signals. Positive feedback signaling enhances pathway activity in placodes (Liu et al., 2008; Liu et al., 2007), activates secreted factors that promote dermal condensation and attract melanoblasts and nerve fibers, and induces expression of secreted Wnt inhibitors that block pathway activation in adjacent cells by a reaction-diffusion mechanism. Epithelial signaling reappears after birth in hair shaft precursor cells that attract pigment deposition. In the constitutive mutant, elevated epithelial β-catenin overrides the requirement for a dermal message, and stimulates enhanced positive feedback signaling, dermal condensation, and expression of secreted Wnt inhibitors that reduce Wnt/β-catenin signaling in dermal cells, but not in epithelial cells expressing activated mutant β-catenin. The imbalance between positive and negative signals leads to expanded adoption of placode fate, broad suppression of epidermal differentiation, enhanced innervation, precocious pigmentation and differentiation towards hair shaft. Induced mutation in embryonic epithelium after the placode stage blocks follicle downgrowth, stimulates hair shaft differentiation, and attracts pigment deposition and innervation. Induced mutation in adult telogen skin induces abnormal growth of existing follicles, and formation of ectopic follicles from outer root sheath and epidermis. Diagrams are not to scale.
Discussion
We demonstrate here that an activating mutation in epithelial β-catenin had dramatic effects on embryonic surface ectoderm. Hair follicle placodes were expanded, but failed to develop normally, instead precociously expressing hair shaft keratins (Figure 7). Mutant embryonic skin was prematurely pigmented and innervated, and epidermal stratification was disrupted, resulting in total failure of epidermal barrier function. Our data support a model in which Wnt/β-catenin signaling plays multiple roles in the embryonic surface ectoderm, promoting placode and hair shaft fate, activating signals that attract nerve fibers and enhance pigmentation, and blocking squamous differentiation.
While these experiments rely on a gain of function approach, many of the genes displaying differential expression in our microarray profiling experiments are known from previous studies to be expressed in developing hair follicles, and/or are suppressed in embryonic skin expressing the secreted Wnt inhibitor DKK1 or lacking β-catenin (Andl et al., 2002; Huelsken et al., 2001). Furthermore, we were able to verify placodal expression in wild-type embryos and decreased expression in Wnt-inhibited skin for Sp5, a gene not previously associated with embryonic hair follicle development.
Controlled β-catenin signaling is essential for periodic pattern formation in the surface ectoderm
Placode induction occurs at least two days earlier in gain of function β-catenin mutants than in wild-type ectoderm, indicating that stabilized β-catenin overrides the requirement for an initiating dermal message. Uncontrolled ectodermal β-catenin signaling induces formation of a broadly condensed dermis associated with widespread acquisition of dermal condensate markers. Thus dermal condensates can be either initiated, or stabilized and expanded, by signaling from the epithelium. These data suggest a model in which the final positioning of hair follicles is not determined by a fixed pattern of dermal condensates, but rather by interplay of epithelial and dermal signals. Consistent with this model, stable dermal condensates are not observed in mice lacking epithelial Eda signaling (Schmidt-Ullrich et al., 2006).
Our results further indicate that controlled downregulation of β-catenin signaling is required to generate a regular array of placode and interplacode fate, consistent with a reaction diffusion model for establishment of the placodal array (Jiang et al., 2004), involving Wnt/β-catenin as the primary placode promoting signal, and predicting an important function in placode spacing for secreted Wnt inhibitory factors whose expression is promoted within placodes by β-catenin. Microarray analysis revealed increased expression in gain of function mutant β-catenin skin of several secreted Wnt inhibitors, including Dkk4, Dkk1 and Wif1, consistent with prior reports and mathematical modeling analyses (Bazzi et al., 2007; Maini et al., 2006; Sick et al., 2006; Stark et al., 2007). Secreted Wnt inhibitors act at the level of Wnt receptors and/or co-receptors and so would be ineffective at modulating the activity of epithelial gain of function mutant β-catenin. Thus the balance between activating and inhibitory signals is shifted in favor of placode formation in stabilized β-catenin mutant ectoderm, resulting in expanded adoption of placode fate (Figure 7).
The precocious development of placodes, the increase in their density, and their formation in normally hairless areas, suggests that the broad distribution of placodes in mutant embryos is due at least in part to a switch in fate of the embryonic surface ectoderm, rather than being wholly accounted for by expansion of cells normally destined to adopt placode fate. Deletion of the intracellular Wnt pathway inhibitor Apc produces a milder phenotype (Kuraguchi et al., 2006), possibly be due in part to the central, integrating role of β-catenin in the Wnt/β-catenin signaling pathway and compensation of Apc functions by other pathway inhibitors.
β-catenin signaling activates the hair shaft differentiation program and indirectly enhances innervation and pigmentation
Our phenotypic analyses at successive developmental stages indicate that β-catenin first promotes ectodermal placode fate, and indirectly enhances attraction of nerve fibers to the skin. Rather than proliferating, penetrating the dermis, and developing into a mature multi-layered hair follicle, mutant placodes precociously express hair shaft markers and exhibit pigmentation. The predominance of hair shaft differentiation is consistent with the hypothesis that, at certain developmental stages, β-catenin acts as a specific activator of hair shaft fate (Figure 7). Our data further suggest that β-catenin signaling directly or indirectly suppresses expression of Gata3, a transcription factor required for normal development of the inner root sheath.
Our results indicate that Sp5 is a direct transcriptional target of β-catenin in epithelial cells, and may suppress expression of a subset of epidermal differentiation genes including Krt10 and involucrin, contributing to the adoption of placode rather than stratified epidermal fate. Sp1 binding sequences in the involucrin promoter are required for normal expression levels in vivo (Crish et al., 2006), and Sp5 competitively suppresses Sp1 target genes in other developmental systems (Fujimura et al., 2007), providing a possible mechanism for its effects on involucrin expression. Sp5 is expressed in wild-type placodes, suggesting a role in normal placode development. Sp5-null mice show no overt phenotype (Harrison et al., 2000); however we speculate that Sp5 may act redundantly with Sp8 or other related genes in the skin. Activated β-catenin appears to directly or indirectly inhibit Sp1 transcript accumulation (Figure 6A), which could provide an additional mechanism by which epidermal differentiation gene expression is suppressed in placodes.
While the activating β-catenin mutation promotes hair follicle fate in the skin, previous analyses of KRT14-Cre Ctnnb1(Ex3)fl/+ mutant embryos revealed enhanced and accelerated development of fungiform taste papillae and taste buds in the tongue, and ectopic tooth development in the maxilla and mandible (Jarvinen et al., 2006; Liu et al., 2008; Liu et al., 2007). Thus the effects of stabilized ectodermal β-catenin exhibit a remarkable regional specificity. These observations suggest the existence of pre-existing regional signals that differ between the skin, and oral epithelia of the tongue and jaws, and determine the precise nature of the cellular responses downstream of β-catenin.
Supplementary Material
Acknowledgments
We thank Elaine Fuchs for TOPGAL reporter mice, Adam Glick for K5-rtTA mice, Don Baldwin for Affymetrix array analysis, Qian-Chun Yu for TEM, Jean Richa for transgenic mouse production, Henry Sun, Pierre Coulombe and Lutz Langbein for keratin antibodies, Leroy Ash and Tzvete Dentchev for histology, and Uri Gat and George Cotsarelis for helpful discussions. This work was supported by NIH grants RO1-AR47709, R01-DE015342, RO1-HD053829 (S. E. M.) and RO1-AR45973 (A. A. D.).
Footnotes
Note: While this manuscript was under revision Nähri et al. (Development 135, 1019-28, 2008) published data similar to those described here.
References
- Allen M, Grachtchouk M, Sheng H, Grachtchouk V, Wang A, Wei L, Liu J, Ramirez A, Metzger D, Chambon P, et al. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163:2173–8. doi: 10.1016/S0002-9440(10)63574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–68. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–9. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT Signals Are Required for the Initiation of Hair Follicle Development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Fantauzzo KA, Richardson GD, Jahoda CA, Christiano AM. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Dev Biol. 2007;305:498–507. doi: 10.1016/j.ydbio.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–29. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Crish JF, Gopalakrishnan R, Bone F, Gilliam AC, Eckert RL. The distal and proximal regulatory regions of the involucrin gene promoter have distinct functions and are required for in vivo involucrin expression. J Invest Dermatol. 2006;126:305–14. doi: 10.1038/sj.jid.5700019. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–42. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N, Vacik T, Machon O, Vlcek C, Scalabrin S, Speth M, Diep D, Krauss S, Kozmik Z. Wnt-mediated down-regulation of Sp1 target genes by a transcriptional repressor Sp5. J Biol Chem. 2007;282:1225–37. doi: 10.1074/jbc.M605851200. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends in Genetics. 1992;8:55–60. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Houzelstein D, Dunwoodie SL, Beddington RS. Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev Biol. 2000;227:358–72. doi: 10.1006/dbio.2000.9878. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Hutchin ME, Kariapper MS, Grachtchouk M, Wang A, Wei L, Cummings D, Liu J, Michael LE, Glick A, Dlugosz AA. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005;19:214–23. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–32. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci U S A. 2006;103:11958–63. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, Lin CM, Jung HS, Chuong CM. Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormone models. Int J Dev Biol. 2004;48:117–35. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SA, Jackson IJ. MGF (KIT ligand) is a chemokinetic factor for melanoblast migration into hair follicles. Dev Biol. 2000;225:424–36. doi: 10.1006/dbio.2000.9856. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML, Fuchs E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–22. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, Rodriguez-Leon J, Kato S, Belmonte JC. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004;131:4763–74. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, Kucherlapati M, Maas RL, Kucherlapati R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel-Wunder S, Bockler D, Schirmacher P, Schweizer J. Novel type I hair keratins K39 and K40 are the last to be expressed in differentiation of the hair: completion of the human hair keratin catalog. J Invest Dermatol. 2007;127:1532–5. doi: 10.1038/sj.jid.5700734. [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–24. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, Andl T, Taketo MM, Dlugosz AA, Moon RT, et al. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–12. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–99. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lynch MH, O’Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Biol. 1986;103:2593–606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini PK, Baker RE, Chuong CM. Developmental biology. The Turing model comes of molecular age. Science. 2006;314:1397–8. doi: 10.1126/science.1136396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML, Millar SE. The mammary bud as a skin appendage: unique and shared aspects of development. J Mammary Gland Biol Neoplasia. 2006;11:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Morasso MI, Mahon KA, Sargent TD. A Xenopus distal-less gene in transgenic mice: conserved regulation in distal limb epidermis and other sites of epithelial-mesenchymal interaction. Proc Natl Acad Sci U S A. 1995;92:3968–72. doi: 10.1073/pnas.92.9.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta -catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003 doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Peters EM, Botchkarev VA, Muller-Rover S, Moll I, Rice FL, Paus R. Developmental timing of hair follicle and dorsal skin innervation in mice. J Comp Neurol. 2002;448:28–52. doi: 10.1002/cne.10212. [DOI] [PubMed] [Google Scholar]
- Rao C, Foernzler D, Loftus SK, Liu S, McPherson JD, Jungers KA, Apte SS, Pavan WJ, Beier DR. A defect in a novel ADAMTS family member is the cause of the belted white-spotting mutation. Development. 2003;130:4665–72. doi: 10.1242/dev.00668. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–28. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–61. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133:1045–57. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–50. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–31. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–68. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Stark J, Andl T, Millar SE. Hairy math: insights into hair-follicle spacing and orientation. Cell. 2007;128:17–20. doi: 10.1016/j.cell.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nakamura Y, Obama K, Furukawa Y. Identification of SP5 as a downstream gene of the beta-catenin/Tcf pathway and its enhanced expression in human colon cancer. Int J Oncol. 2005;27:1483–7. [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–51. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Weiner L, Han R, Scicchitano BM, Li J, Hasegawa K, Grossi M, Lee D, Brissette JL. Dedicated epithelial recipient cells determine pigmentation patterns. Cell. 2007;130:932–42. doi: 10.1016/j.cell.2007.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.