Abstract
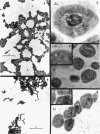
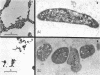
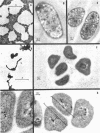
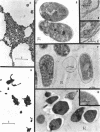
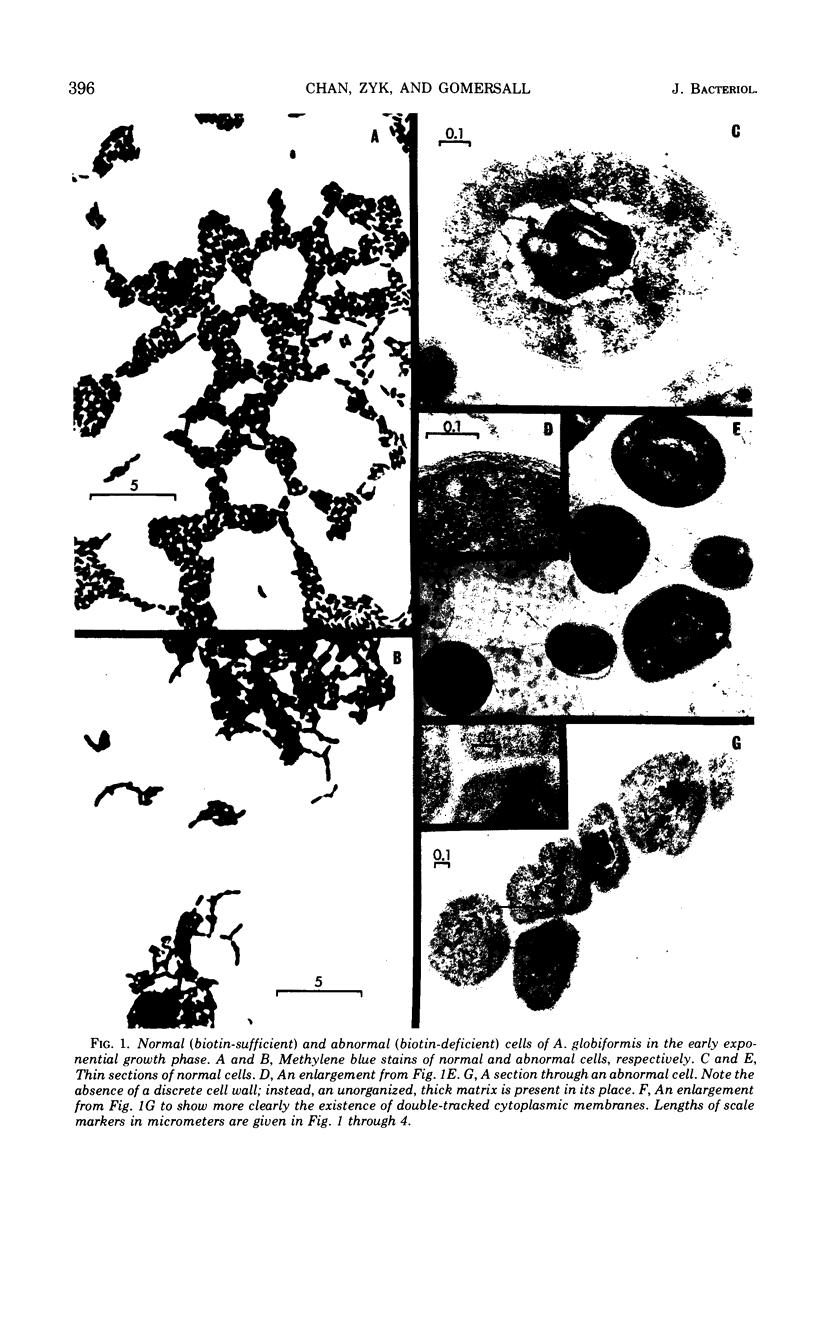
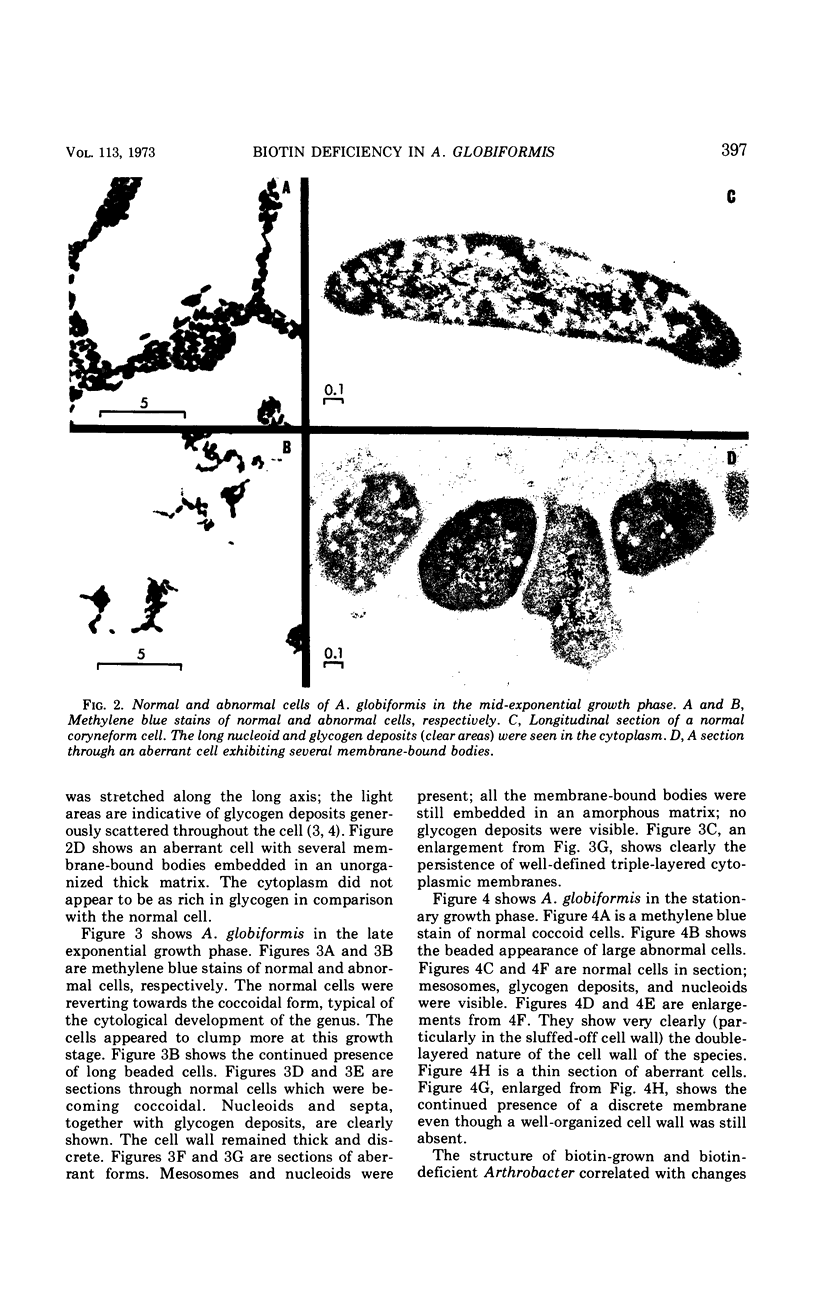
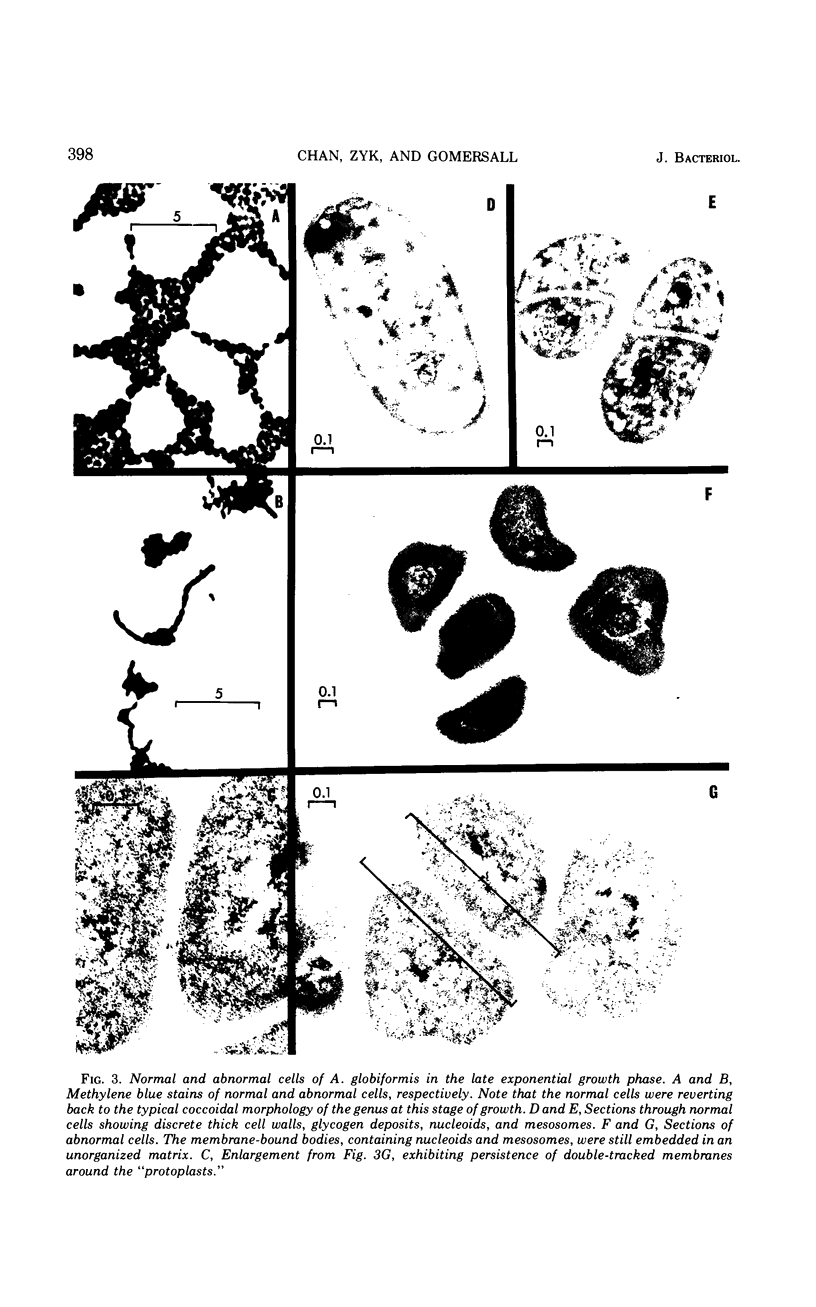
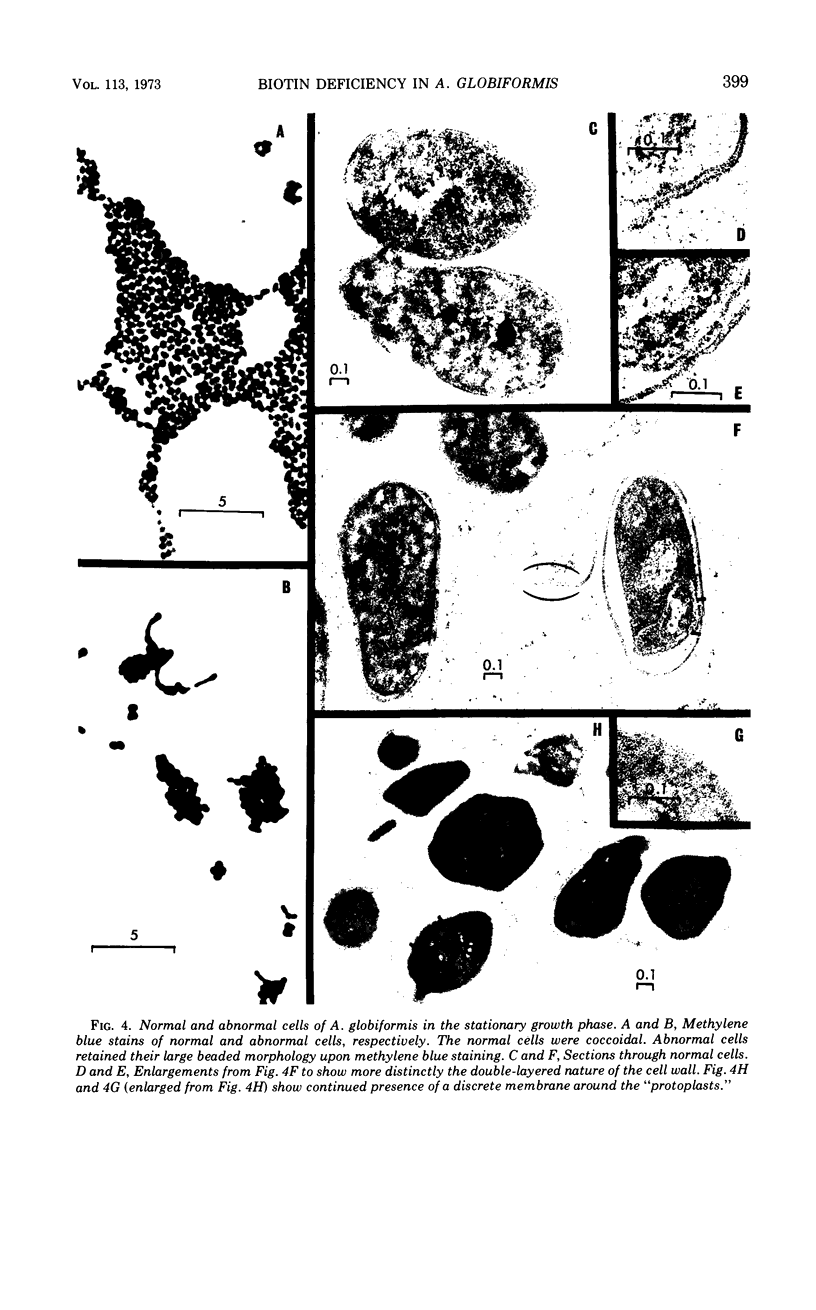
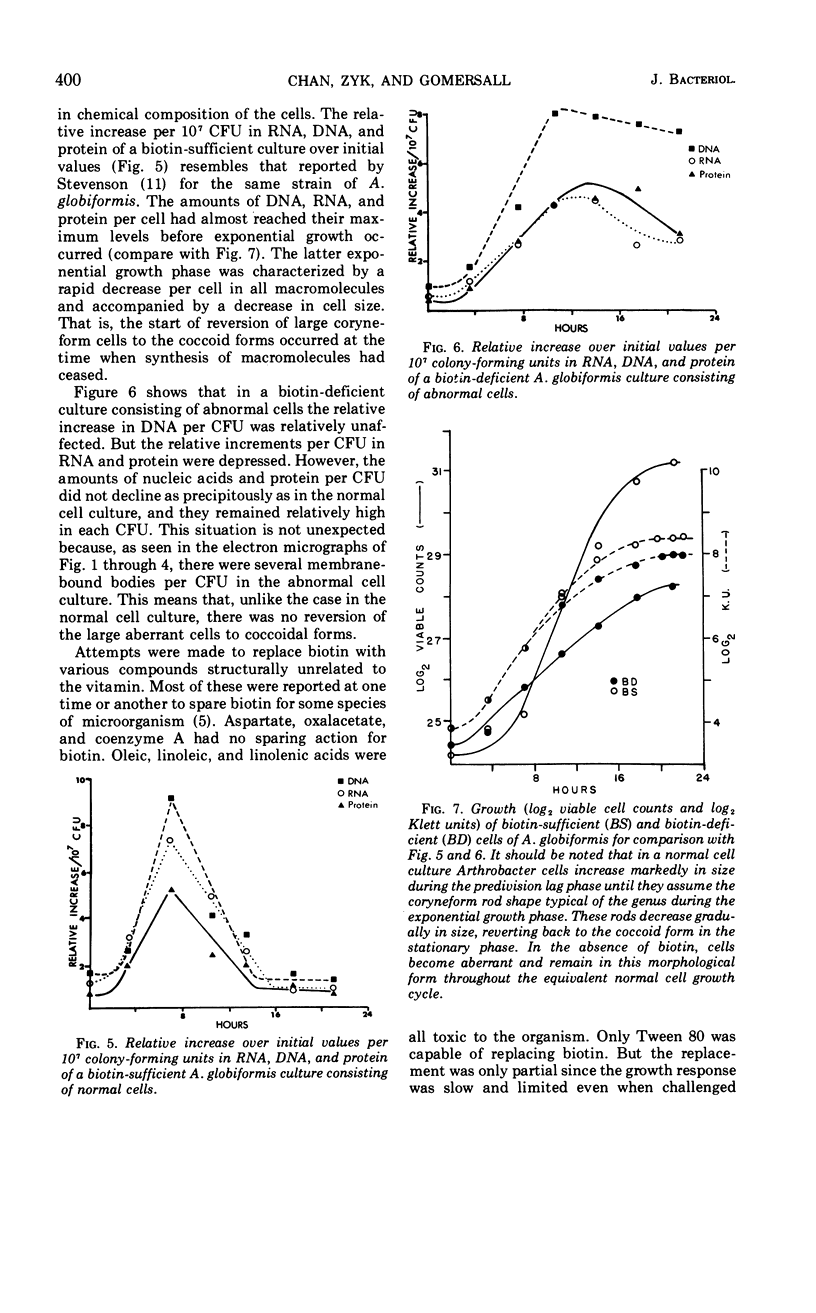
The development of aberrant cell forms of Arthrobacter globiformis 425 due to biotin deficiency was followed by electron microscopy of ultrathin sections. Upon comparison with normal cell growth, aberrant cells developed in the early logarithmic growth phase. Several membrane-bound bodies were embedded in a thick matrix, showing that cell division was impaired. Mesosomes and cytoplasmic membranes were still present in the abnormal cell although the normal cell wall was absent. This condition persisted throughout the growth cycle. This pattern of morphological development was correlated with changes in macromolecular composition of the cells. Various structurally unrelated compounds were tested for their ability to replace biotin. These included aspartic acid, oxalacetic acid, coenzyme A, oleic acid, linoleic acid, linolenic acid, and Tween 80. Only Tween 80 was able to spare biotin to a limited degree. However, this sparing action was eliminated in the presence of avidin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAN E. C. MORPHOLOGICAL ABERRATION OF ARTHROBACTER GLOBIFORMIS CELLS DUE TO BIOTIN DEFICIENCY. J Bacteriol. 1964 Mar;87:641–651. doi: 10.1128/jb.87.3.641-651.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J Bacteriol. 1966 Dec;92(6):1748–1764. doi: 10.1128/jb.92.6.1748-1764.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLME T., CEDERGREN B. Demonstration of intracellular polysaccharide in Escherichia coli by electron microscopy and by cytochemical methods. Acta Pathol Microbiol Scand. 1961;51:179–186. doi: 10.1111/j.1699-0463.1961.tb00357.x. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J., Chan E. C. Biotin deficiency in Arthrobacter globiformis: fine structure and effect of physical stress. Can J Microbiol. 1970 Apr;16(4):227–230. doi: 10.1139/m70-042. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L. The fine structure of Arthrobacter pascens and the development of mesosomes during the growth cycle. Can J Microbiol. 1968 Oct;14(10):1029–1034. doi: 10.1139/m68-173. [DOI] [PubMed] [Google Scholar]