Abstract
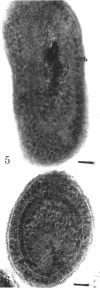
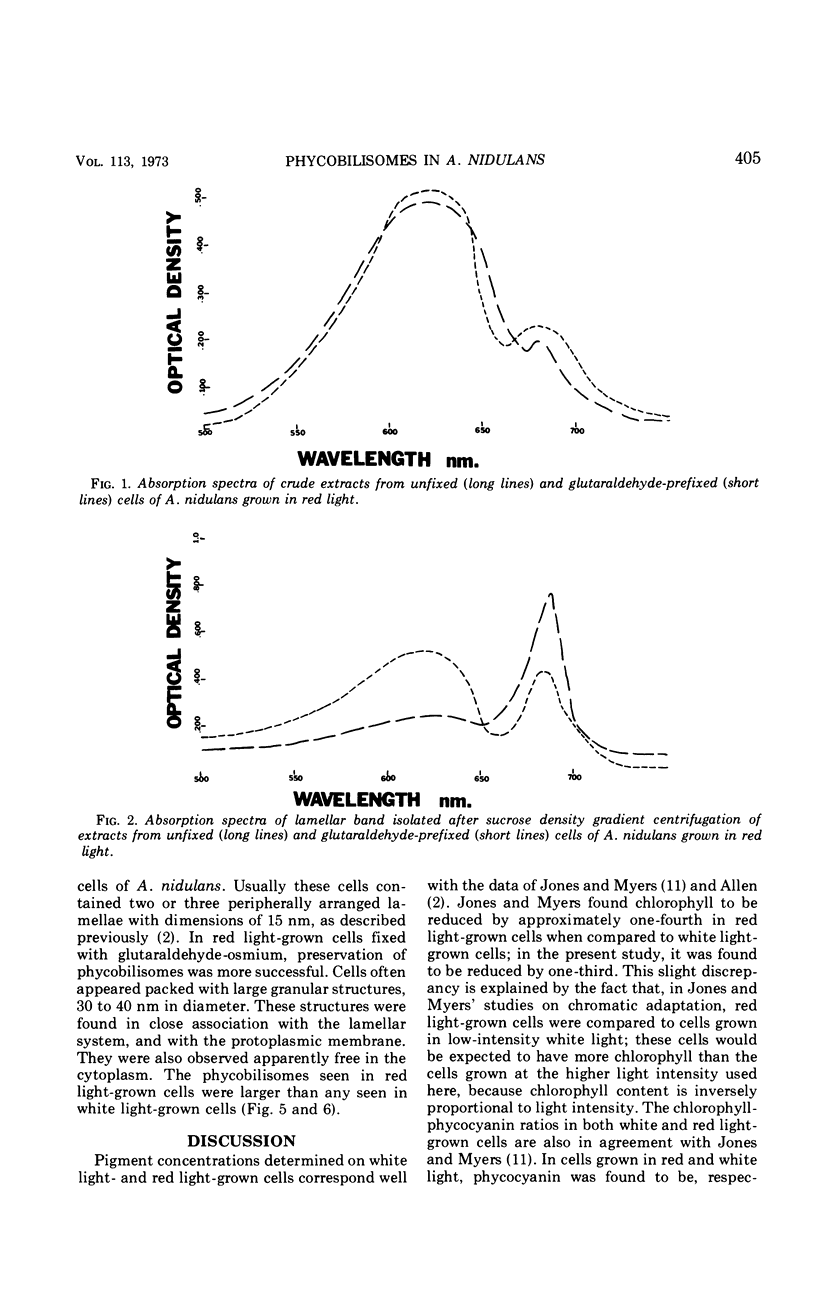
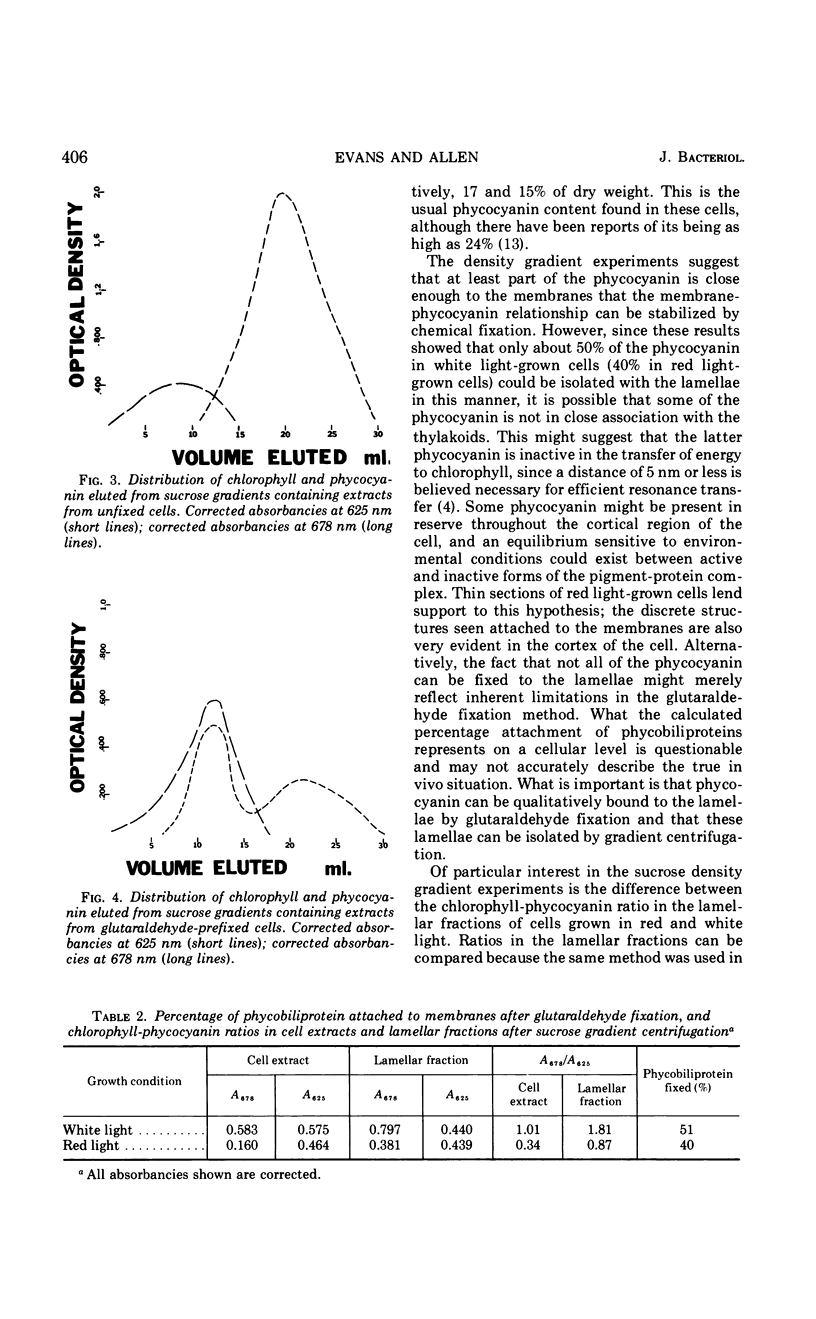
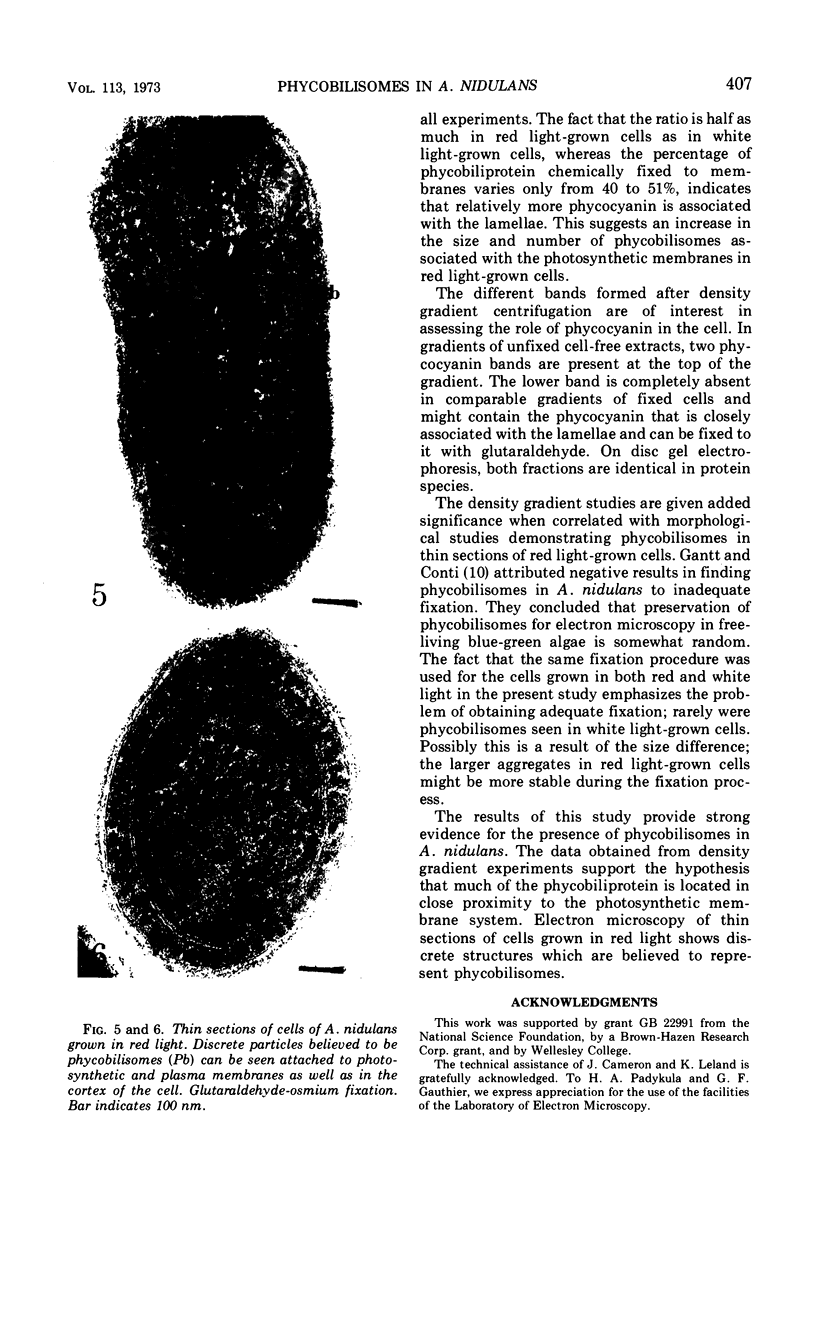
Phycobilisomes were demonstrated in Anacystis nidulans by chemical and morphological studies on cells grown in red light. These cells showed a marked reduction in the chlorophyll-phycocyanin ratio owing to a decreased chlorophyll content. Granular structures of approximately 35 nm were observed throughout red light-grown cells, but were most distinct in the peripheral region. The presence of phycobilisomes in cells grown in red light as well as in cells grown in white light is supported by experiments in which glutaraldehyde was used to stabilize the attachment between the phycobiliprotein and the thylakoids, allowing the isolation of both in the same fraction by sucrose density gradient centrifugation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNOLD W., OPPENHEIMER J. R. Internal conversion in the photosynthetic mechanism of blue-green algae. J Gen Physiol. 1950 Mar;33(4):423–435. doi: 10.1085/jgp.33.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M. Photosynthetic membrane system in Anacystis nidulans. J Bacteriol. 1968 Sep;96(3):836–841. doi: 10.1128/jb.96.3.836-841.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Lefort-Tran M. Fixation of phycobiliproteins to photosynthetic membranes by glutaraldehyde. Arch Mikrobiol. 1970;71(3):245–257. doi: 10.1007/BF00410158. [DOI] [PubMed] [Google Scholar]
- Edwards M. R., Gantt E. Phycobilisomes of the thermophilic blue-green alga Synechococcus lividus. J Cell Biol. 1971 Sep;50(3):896–900. doi: 10.1083/jcb.50.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Granules associated with the chloroplast lamellae of Porphyridium cruentum. J Cell Biol. 1966 Jun;29(3):423–434. doi: 10.1083/jcb.29.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Phycobiliprotein localization in algae. Brookhaven Symp Biol. 1966;19:393–405. [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]