Abstract
The C. elegans FGF receptor, EGL-15, is alternatively-spliced to yield two major isoforms that differ in their extracellular domains. The EGL-15(5A) isoform is necessary for the gonadal chemoattraction of the migrating sex myoblasts (SMs), while the EGL-15(5B) isoform is required for viability. Here we show that 5A is predominantly expressed in the M lineage, which gives rise to the migrating SMs and their sex muscle descendants, while 5B is predominantly expressed in the hypodermis. Tissue-specific expression, however, explains only part of the functional differences between these two receptor isoforms. 5A can carry out the reciprocal essential function of 5B when expressed in the hypodermis, but 5B is incapable of carrying out SM chemoattraction. Our data, therefore, indicate that the structural differences in these two isoforms contribute to their functional differences. Two lines of evidence indicate that the 5B isoform also plays a role in SM migration, implicating it in the repulsion that is observed when the chemoattraction is compromised. Thus, structural differences in the extracellular domains of these two isoforms can specify either attraction to or repulsion from the gonad.
Keywords: alternative splicing, chemoattraction, EGL-15, FGF receptor, sex myoblast
INTRODUCTION
During metazoan development, cells acquire functional positions either as a natural consequence of their birth position or by cell migration. The cells that migrate are often guided along their routes by attractive and/or repulsive cues, many of which also serve to guide axon outgrowth (Bagri and Tessier-Lavigne, 2002). Although their paths can be quite intricate, there is a relatively small set of molecules that serve as these cues, including growth factors, netrins, ephrins and semaphorins. Interestingly, many of these components can function either in attraction or repulsion (Bagri and Tessier-Lavigne, 2002).
In C. elegans hermaphrodites, the migrations of the sex myoblasts (SMs) are also controlled by both attractive and repulsive mechanisms (Stern and Horvitz, 1991; Thomas et al., 1990). The SMs are a pair of bilaterally symmetric cells that are born in the posterior of the animal. After their birth, the SMs undergo anteriorly-directed migrations to flank the precise center of the developing gonad (Sulston and Horvitz, 1977); subsequently, they divide and differentiate into the muscles required for egg laying. A variety of mechanisms control sex myoblast migration (Huang and Stern, 2004; Kokel et al., 1998). The mechanism responsible for their precise positioning is a gonad-dependent attraction (GDA), in which the fibroblast growth factor (FGF) chemoattractant, EGL-17, guides the SMs toward the center of the gonad (Branda and Stern, 2000). Mutations that eliminate EGL-17 do not merely disrupt the GDA, but also result in a gonad-dependent repulsion (GDR) of the SMs (Stern and Horvitz, 1991). A specific class of mutations affecting the C. elegans FGF receptor (FGFR), EGL-15, behave similarly (Goodman et al., 2003). These mutations suggest that, in addition to a chemoattractive mechanism, there is also a repulsive mechanism that is revealed when the chemoattraction is no longer operational. SM migration can also occur in the absence of all gonadal cues. In the absence of the GDA and the GDR, the gonad- independent mechanism allows the SMs to migrate anteriorly and assume a broad distribution of final positions just posterior to the center of the gonad (Thomas et al., 1990; Branda and Stern, 2000).
Sex myoblasts also respond to dorsal-ventral signals that help to confine the SMs to the ventral side of the animal (Branda and Stern, 2000). Both the EGL-17 chemoattractant and the C. elegans roundabout (ROBO) orthologue, SAX-3 (Hao et al., 2001), maintain the SMs ventrally (Branda and Stern, 2000). The normal chemoattractive activity of EGL-17, arising from ventral cellular sources, prevents the SMs from wandering dorsally. However, even in the absence of SM chemoattraction, the SMs are maintained ventrally by the action of the SAX-3 receptor, which presumably enables dorsal sources of its ligand, SLIT, to keep the SMs in ventral positions.
In addition to SM migration, EGL-15 plays a role in numerous processes, including an essential, fluid homeostasis function. EGL-15 regulates fluid homeostasis by acting in the hypodermis (Huang and Stern, 2004), activating well-characterized signal transduction pathways (Borland et al., 2001). Several phenotypes have been associated with this role of EGL-15: hyperactivity of EGL-15 results in a Clear (Clr) phenotype (Kokel et al., 1998), while complete loss of function of EGL-15, or the LET-756 FGF ligand, results in an early larval arrest (DeVore et al., 1995; Roubin et al., 1999).
The domain architecture of EGL-15 resembles other FGFRs, but it also has some unique features as well. Similar to mammalian FGF receptors, EGL-15 has three extracellular Ig domains, an acid box, and an intracellular tyrosine kinase domain. Unique to EGL-15, however, is a small peptide insert that lies between the first Ig domain and the acid box, termed the EGL-15-specific insert (Goodman et al., 2003). Alternative splicing of the EGL-15-specific insert leads to two receptor isoforms, EGL-15(5A) and EGL-15(5B). The different EGL-15(5A) and EGL-15(5B) isoforms, along with their ligands, EGL-17 and LET-756, carry out different functions (Bulow et al., 2004; Goodman et al., 2003; Huang and Stern, 2004; Kuroyanagi et al., 2006). EGL-15(5B) is required for the essential function of EGL-15, while EGL-15(5A) is required for SM chemoattraction.
While the role of the 5A isoform in SM chemoattraction is clear, here we further investigate the broad role of EGL-15 in SM migration. Hypomorphic mutations that affect both EGL-15 isoforms have complex effects on SM positioning, suggesting the possibility that EGL-15 is involved in not only the GDA, but possibly also in other SM migration mechanisms as well. Here we investigate the basis of the specificity of the 5A isoform for SM chemoattraction and the 5B isoform for fluid homeostasis. In addition, we show that EGL-15 is involved in both the attractive and repulsive mechanisms that guide the migrating SMs.
MATERIALS AND METHODS
Genetic and transgenic manipulations
All strains were derived from C. elegans var. Bristol, strain N2 using standard genetic protocols and standard genetic manipulations (Herman, 1988). Transgenic arrays were generated using standard germline transformation techniques. Rescue assays and SM scoring were performed as described (Goodman et al., 2003). Laser microsurgery was used to ablate the precursor cells of the somatic gonad (Z1 and Z4) in newly-hatched L1 animals as previously described (Thomas et al., 1990).
Determination of sex myoblast positions
The final positions of sex myoblasts were determined with respect to the underlying hypodermal Pn.p cells as previously described (Thomas et al., 1990; Goodman et al., 2003). SM distributions for each strain are depicted using box-and-whisker plots (Moore and McCabe, 1993) aligned to a schematic representation of the Pn.p cell metric. This representation of SMs depicts the overall range of SMs as well as their general distribution and median position. Briefly, each set of SMs is ordered according to anteroposterior position and divided into quartiles. The “box” includes the positions of SMs within the two central quartiles. An additional vertical line within the box indicates the median SM position. A quartile length (1Q) is determined based on the range of positions covered by the 2Q box length. Bars (“whiskers”) of up to 1.5Q length extend from the edges of the box to additional data points. As whisker length does not extend beyond the range of data points, these bars may be shorter than a 1.5 Q length, or even absent. Data points beyond the edge of the bars (“outliers”) are indicated by individual hash marks. SM distributions
Plasmid Construction
NH#112 [egl-15(A+B+)] contains the wild-type egl-15 genomic rescuing fragment. NH#872 [egl-15(A−B+)] and NH#873 [egl-15(A+B−)] are NH#112 derivatives that eliminate the normal expression of either the EGL-15(5A) or the EGL-15(5B) isoform, respectively, without altering the expression pattern of the non-mutated isoform (Goodman et al., 2003). NH#1130 [egl-15(Δ5)] is another NH#112 derivative in which exon 4 was fused directly to exon 6, thereby eliminating the EGL-15-specific insert from both extracellular isoforms. NH#1159 [egl-15(4A6)] was generated from NH#112 by replacing genomic DNA spanning from exon 4 through exon 6 with the corresponding exon 5A-containing cDNA, thereby forcing expression of the 5A transcript at all sites of egl-15 transcription. NH#1160 [egl-15(4B6)] was generated similarly to NH#1159 using an exon 5B-containing cDNA, thereby forcing the expression of the 5B transcript at all sites of egl-15 transcription. NH#1173, Prol-6::egl-15, fuses the rol-6 promoter region (from −976 to −1) to the genomic coding sequence of egl-15 at an inserted NcoI site overlapping the initiating ATGs of rol-6 and egl-15. All PCR-derived fragments were confirmed by sequencing. A summary table of plasmids and alleles described in this work and further details of their construction can be found in the online supplementary material.
Transgenes
Transgenic rescue assays were performed as described (Goodman et al., 2003). Transgenes that have the normal expression pattern for only one of the two EGL-15 isoforms contained egl-15 constructs in which nonsense mutations specifically eliminate one of the two isoforms (Goodman et al., 2003). The sites of observable EGL-15 expression from these arrays are likely to reflect the endogenous expression pattern, since the arrays rescue the corresponding specific EGL-15 function (Goodman et al., 2003). ayIs6[Ptwist::GFP] animals express GFP in all the undifferentiated cells in the M lineage (Harfe et al., 1998). ayIs29[egl-15(WT)] was isolated as a spontaneous integrant of an extrachromosomal array carrying the wild-type egl-15 genomic rescuing fragment, NH#112 (5 ng/µl), the Pmyo-2::GFP transformation marker, pJKL449.1 (5 ng/µl), and pGEM-5Z (70 ng/µl). All other integrated arrays were isolated using a standard UV-TMP protocol (Huang, 2004). ayIs39[egl-15(A−B+)] and ayIs40[egl-15(A+B−)] are integrated arrays that were isolated from extrachromosomal arrays generated with the same mixture of DNAs, using NH#872 or NH#873, respectively, as the source of egl-15 DNA. Extrachromosomal arrays that alter the normal expression pattern for the two EGL-15 isoforms, 4A6 (NH#1159) and 4B6 (NH#1160), as well as the extrachromosomal arrays containing the egl-15 construct that lacked both the 5A and 5B domains, Δ5 (NH#1130), were injected using the same concentrations of egl-15 DNA, the transformation marker, and pGEM-5Z. ayIs30–ayIs33 and ayIs36 are integrated arrays carrying the Prol-6::egl-15 plasmid (NH#1173, 5 ng/µl), the Pmyo-2::GFP transformation marker (pJKL449.1, 5 ng/µl) and sheared genomic DNA (50 ng/µl).
Mosaic analysis
The ayEx86 array used for the mosaic analysis contained NH#112 [egl-15(+), 75 ng/ul], c33C3 [ncl-1(+), 150 ng/ul], and col-19::GFP [50 ng/µl]. This array rescues the Ncl phenotype of ncl-1(e1865) mutants such that cells carrying the array are easily distinguishable from those that have lost it (Hedgecock and Herman, 1995; see Suppl. Material for a list of cells scored). This array also rescues the SM migration defects of egl-15 mutants. egl-15(n1458) (A−B+) or egl-15(n1456) (A−B−) mutant animals carrying this array appear wild type and have correctly positioned SMs; progeny which have lost the array in the P0 zygote have either posteriorly-positioned SMs or an L1 larval arrest phenotype, respectively. col-19::GFP served as the co-injection marker and is expressed in adult hypodermal cells.
Immunohistochemistry
Animals were synchronized in early L1 by hatching in the absence of food and then grown to the appropriate developmental stage by culturing on seeded plates at 15°C. Developmental stage was confirmed by examining Ptwist::GFP expression in the M lineage as well as other landmarks such as the divisions of the intestinal nuclei and the extent of gonadal development. Animals were harvested and stained following a protocol modified from Finney and Ruvkun (1990). Two affinity purified α-EGL-15 primary antibodies (Pop, which recognizes the acid box, and Crackle, which recognizes the carboxy-terminal domain) were used at a 1:10 dilution; Alexa Fluor 546-conjugated anti-rabbit antibody (Molecular Probes) was used as a secondary antibody at a 1:250 dilution. Double-staining experiments were performed by following the above protocol, applying the α-EGL-15 primary antibody, the Alexa Fluor 546-conjugated anti-rabbit antibody, and then repeating the staining protocol using an α-GFP monoclonal antibody (JL-8, Clontech) at a 1:10 dilution, and a goat α-mouse FITC-conjugated secondary antibody (Jackson ImmunoLab) at a 1:50 dilution.
Photomicroscopy
All photomicrographs were collected using a Hamamatsu C4742-95 digital camera mounted on a Zeiss Axioskop microscope that contains an internal 0.63x reduction lens. Images were collected as a z-series of 0.5µm sections, merged into a single composite image, and false colored using OpenLab software. Images were then cropped and scaled using Adobe Photoshop.
RESULTS
In vivo distribution of EGL-15
It was important to establish the sites of expression of the two major isoforms of EGL-15 in order to understand the basis of the specificity of the 5A and 5B EGL-15 isoforms for their respective functions, and to determine whether EGL-15 affects SM migration mechanisms beyond the requirement of the 5A isoform for SM chemoattraction. Previous analyses revealed that egl-15 is expressed both in the hypodermis (Bulow et al., 2004; Huang and Stern, 2004) and in the early M lineage and SM-derived vulval muscles (Harfe et al., 1998; Sasson and Stern, 2004). In addition, it was recently shown that alternative splicing is responsible for a bias of expression of the egl-15(5A) transcript in vulval and body wall muscles and egl-15(5B) in the hypodermis (Kuroyanagi et al., 2006). However, expression of EGL-15 has only rarely been observed in the SMs (Burdine et al., 1998) and never in the SMs at the time of their migration, the crucial time for EGL-15 to control SM migration guidance.
To establish the sites of expression of the two major isoforms of EGL-15, α–EGL-15 antibodies were usend in a series of systematic immunofluorescence studies. Since endogenous levels of EGL-15 are below the threshold of detection, we performed these studies on strains that express increased levels of EGL-15 under the control of its own promoter from integrated transgenic arrays. These arrays can rescue the appropriate functions of EGL-15 and do not cause any observable phenotypes characteristic of gross over-expression. ayIs29 [egl-15(A+B+)] contains the wild-type egl-15 genomic rescuing fragment and expresses both isoforms of EGL-15. Similar arrays express either the EGL-15(5A) isoform (ayIs40) or the EGL-15(5B) isoform (ayIs39). To aid in the identification of M-derived cells, these strains also contain the reporter construct ayIs6[Phlh-8::GFP] (Harfe et al., 1998).
Examination of ayIs29; ayIs6 animals at various developmental stages revealed that EGL-15 is expressed in both the hypodermis and in portions of the M lineage, consistent with what has previously been reported (Fig 1. A, D, G) (Bulow et al., 2004; Huang and Stern, 2004; Sasson and Stern, 2004). However, expression of EGL-15 within the SM during the time of its migration (early L2 stage) remained difficult to observe. To understand the specific requirement for each of the EGL-15 isoforms better, we looked at the tissue-specific distribution of each of the isoforms using α–{EGL-15 antibodies and the arrays described above designed to express only one of the two EGL-15 isoforms. These studies revealed that expression of the 5A isoform is predominantly localized to the M lineage, while the hypodermis is the major site of EGL-15(5B) expression (Fig. 1). Moreover, expression analysis of these isoforms across development revealed that 5A is present in the M lineage at all stages (Fig. S1). These data suggest that the tissue-specific expression patterns for the 5A and 5B isoforms may be important in specifying the specific functions of EGL-15.
Fig. 1.
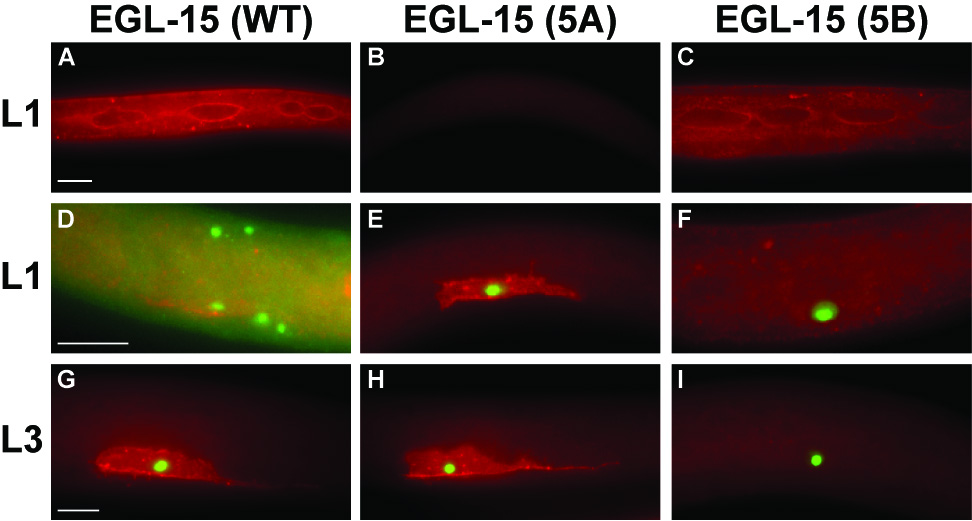
EGL-15 isoform-specific expression patterns. EGL-15(5B) is primarily expressed in the hypodermis, EGL-15(5A) is primarily expressed in the M lineage. (A,D,G) ayIs29[egl-15(WT)]; ayIs6, (B,E,H) ayIs40[egl-15(5A+B−)]; ayIs6, and (C,F,I) ayIs39[egl-15(5A−B+)]; ayIs6 animals. α-GFP staining in ayIs6[Ptwist::GFP]-containing animals identifies the SM and other undifferentiated cells in the M lineage. (A,B,C) α-EGL-15 staining in the hypodermis of L1 animals. Staining in the syncytial hypodermis contrasts with the lack of staining in the seam cells, with increased staining intensity at the hyp-seam cell junction. In the absence of transgenic 5B, no expression is seen in the hypodermis (B). (D, E, F) EGL-15 expression in the migrating sex myoblast at the end of the L1 stage. EGL-15 is expressed in the GFP+ SM. In the absence of transgenic 5A, no expression is seen in the SM (F, I). (G, H, I) EGL-15 expression in the undivided SM at the L3 stage. All animals are oriented anterior left and ventral down. Red, α-EGL-15 (Pop) staining. Green, α-GFP staining. Scale bars, 10µm.
The 5A specific insert is unique in its ability to mediate SM chemoattraction
Previous isoform-specific constructs (NH#872, NH#873) eliminated either the 5A or the 5B isoforms from the wild-type egl-15 rescuing fragment, but did not alter the normal expression pattern for the remaining isoform (Goodman et al., 2003). Thus, those constructs tested which EGL-15 functions could be performed by the normal expression pattern of the remaining isoform. To test whether structural differences in the two isoforms also specified their functions, we tested whether individual isoforms could carry out each EGL-15 function if expressed in the cellular site of action corresponding to that function. In other words, we wanted to test whether driving expression of the 5A isoform in the hypodermis could substitute for the 5B isoform in mediating the essential function of EGL-15, and whether expression of the 5B isoform in the SM could replace the 5A isoform in mediating SM chemoattraction. Two constructs, EGL-15(4A6) (expressing 5A) and EGL-15(4B6)(expressing 5B), were built that eliminate the alternative splicing of exon 5. These constructs are predicted to express a single isoform in all places where both EGL-15 isoforms are normally expressed. Immunofluorescence and western blot analysis of representative lines from each strain confirmed the expression of these constructs (Fig. S2).
As expected, the 4B6 transgene rescues viability in egl-15(null) animals (5/5 lines). Interestingly, the 4A6 transgene rescues equally well (7/7 lines). Thus, 5A can play the role that 5B normally plays when expressed where 5B is required for function. These data demonstrate that tissue-specific expression is an important specificity factor for the essential function. Moreover, the ability of both constructs to rescue the essential function allowed us to observe the SM distributions in these transgenic animals and the effects of either having only 5A or 5B in the SMs. As expected, 4A6 animals display a normal SM distribution (Fig. 2A, B). By contrast, 4B6 arrays were unable to rescue the SM migration defects (Fig. 2C) despite being functional in the hypodermis and expressed in the M lineage (Fig. S2A). These data indicate that tissue-specific expression can account for only a portion of the isoform-specific functions of EGL-15. Sequence-specific information must be encoded by the 5A insert, since the 5A peptide is absolutely required for normal SM positioning and cannot be replaced by the 5B peptide to mediate SM chemoattraction.
Fig. 2. Expression of EGL-15 isoforms lacking the 5A domain is not sufficient to mediate SM chemoattraction.
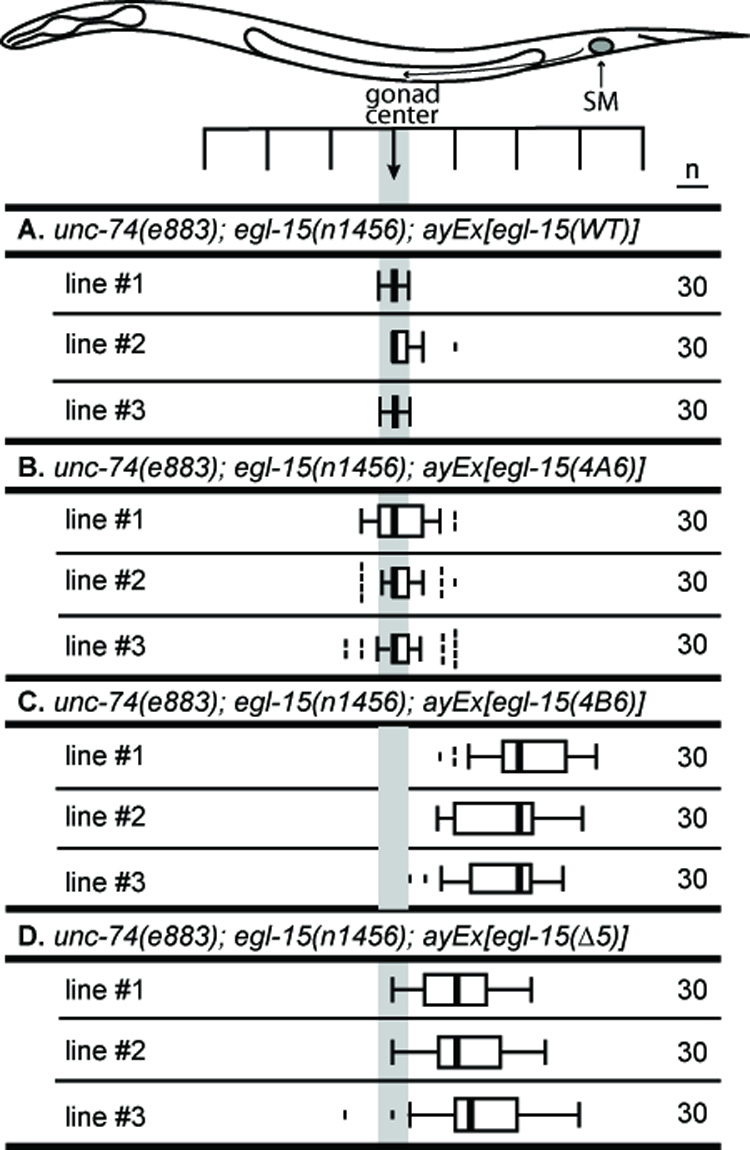
All transgenes rescue the larval arrest phenotype of egl-15(n1456)(A−B−). Transgenes with wild-type EGL-15 (NH#112, A+B+) (A), and EGL-15(4A6) (NH#1159, expresses 5A) (B) restore SM attraction. EGL-15(4B6) (NH#1160, expresses 5B) transgenic animals have posteriorly displaced sex myoblasts (C). (D) EGL-15(Δ5) cannot rescue the SM chemoattraction function.
In addition to these isoform-specific constructs, we also made a construct, termed EGL-15(Δ5), that removes both the 5B and 5A domains and creates a form of EGL-15 that resembles canonical FGFRs that lack these domains. Transgenic animals with Δ5 arrays are viable (5/5 lines), but have posteriorly-displaced SMs (Fig. 2D). Thus, similar to 4B6, Δ5 can rescue the essential function but not the SM chemoattraction function of EGL-15. Thus, in contrast to 5A, the 5B insert is unlikely to encode specific information, since replacement of the 5B insert with 5A sequence or completely removing the exon 5 peptide can restore viability to egl-15(null) animals.
EGL-15(5A) and EGL-15(5B) act in the sex myoblast to mediate opposing mechanisms
To establish how SM migration is affected when SMs completely lack EGL-15, we sought to eliminate all expression of EGL-15 in the SMs. This required us to circumvent the EGL-15 requirement for viability. Two experimental approaches were used to maintain EGL-15 expression specifically in the major hypodermis where it is necessary for the essential function in an otherwise egl-15(null) mutant background: mosaic analysis and tissue-specific expression of egl-15 in the hypodermis.
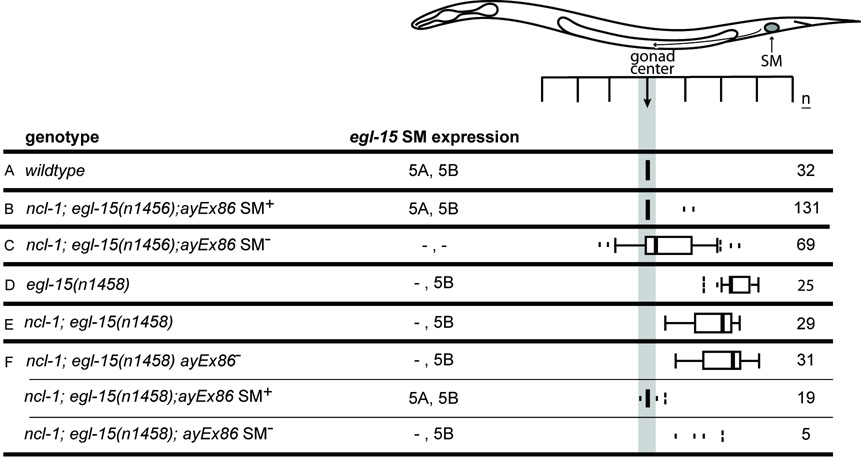
For the first approach, we performed a mosaic analysis of egl-15 using an extrachromosomal array (ayEx86) containing both egl-15(+) and the lineage marker ncl-1(+) in an egl-15(null) background. Animals remain viable due to having the array in a subset of cells that include those necessary for viability, but can lose the array in the lineage giving rise to the SMs, resulting in the complete loss of all EGL-15 isoforms in the migrating SMs. Mosaic animals that maintained the array in the M lineage had precisely centered SMs (Fig. 3B). By contrast, mosaic animals that lost the array in the M lineage had SMs that were not precisely positioned, indicating a loss of the gonad-dependent attraction (GDA; Fig. 3C). Thus, these results indicate that EGL-15 is required in the migrating SMs for their chemoattractive response. Mosaic losses in three particular animals specifically implicate the SM as the necessary site of EGL-15. In these three animals, one SM was non-Ncl, indicating that it maintained the array and was EGL-15(+), while the other SM was Ncl, and thus had lost the array and was EGL-15(−). In all three of these animals, the SMs that contained the array were found correctly positioned at P6.p, while the SMs that had lost the array were found at P6.pp, P7.p-P8.p, and P8.p. Taken together, these results strongly indicate that EGL-15 is both expressed and required in the SMs for chemoattraction to guide them to their precise final positions.
Fig. 3. Complete loss of EGL-15 in the SMs is phenotypically different from loss of only EGL-15(5A).
SM distributions in the following populations of animals. (A) wild type. (B,C) Mosaic animals derived from a strain homozygous for a null (5A−B−) allele of egl-15 that is viable due to an egl-15(+) transgene on an extrachromosomal array (genotype: ncl-1(e1865); egl-15(n1456); ayEx86[egl-15(+)]). (B) Mosaic animals that retain the transgene in the SMs show normal SM chemoattraction, with centered SMs. (C) Mosaic animals that have lost the transgene in the SMs and, thus, lack both EGL-15 5A and 5B. These SMs show a centrally-dispersed distribution. (D) egl-15(n1458) animals (A−B+) that lack EGL-15(5A), resulting in posteriorly-displaced SMs. (E) ncl-1(e1865); egl-15(n1458), showing the effect of the ncl-1(e1865) mutation on SM positioning in an egl-15(n1458) (A−B+) background. (F) SM distributions for subsets of the population of animals derived from the ncl-1(e1865); egl-15(n1458); ayEx86[egl-15(+)] mosaic progenitor strain. All progeny still express EGL-15(5B) from the chromosomal egl-15(n1458) (A−B+) alleles. (F, top) Mosaic animals with broad losses of the transgenic array [ayEx86(−)]. SMs still have EGL-15(5B) and are posteriorly-displaced. (F, middle) Mosaic animals that have maintained the wild-type egl-15(+) transgene in lineages that include the SMs [SM+]. SMs have both EGL-15 isoforms and are centered. (F, bottom) Mosaic animals that have losses of the egl-15(+) transgene in a restricted number of lineages that include the SMs [SM−].
Interestingly, the centrally-dispersed distribution of SMs in the mosaic animals (Fig. 3C) is significantly different from the far posterior positions typical of egl-15(A−B+) mutants (Fig. 3D). Although the ncl-1(e1865) mutation has a minor effect on SM positions (Fig. 3E), this does not account for the different distributions. Thus, the loss of 5B in addition to the loss of 5A alters SM migration guidance mechanisms, implicating EGL-15(5B) in some aspect of this process as well. A simple interpretation of these results that is consistent with the centrally-dispersed SM distribution is that 5A is required for SM chemoattraction (the gonad-dependent attraction, or GDA) while 5B is necessary for the gonad-dependent repulsion (GDR). In the absence of both isoforms, the SMs are neither attracted nor repelled, resulting in the centrally-dispersed distribution characteristic of animals lacking both the GDA and the GDR (Branda and Stern, 2000).
By contrast to the above mosaic results, a similar mosaic analysis in a genetic background that instead retains the 5B isoform results in repelled SMs. We conducted this analysis using the same ayEx86 extrachromosomal array in an egl-15(A−B+) background (Fig. 3F). Similar to the results of the mosaic analysis in the egl-15(null) background, mosaic animals that maintain the array in the M lineage have precisely centered SMs (Fig. 3F, middle). Those that lose the array in all scored lineages, including the M lineage, result in a posteriorly-displaced distribution (Fig. 3F, top). Only a few mosaic animals were found that lost the array specifically in the M lineage (since all animals are viable in this background, there is no selection for animals that maintain the array, making this class more difficult to find); the positions of the SMs in these animals were consistent with a posteriorly-displaced distribution (Fig. 3F, bottom). These results confirm that the presence of the 5B isoform can result in posteriorly-displaced SM distributions.
As an alternative approach to observe the positions of SMs that lack all EGL-15 expression, we created a set of integrated transgenic lines in which EGL-15 was expressed tissue-specifically in the major hypodermis using the Prol-6 promoter. In an egl-15(null) background, expression of EGL-15 from the Prol-6 promoter can rescue the essential function of EGL-15, but leaves the SMs completely lacking EGL-15. The advantage of this approach, as opposed to mosaic animals, was the stability of the genotype; these arrays could be crossed into genetic backgrounds expressing the 5B isoform [egl-15(A−B+)] or not [egl-15(A−B−)] to determine the role of 5B in the GDR. If the 5B isoform contributes to the GDR, the SM distribution should be shifted posteriorly in the egl-15(A−B+) background. The stability of the genotype also would allow gonad ablation to test whether the SMs were repelled by the gonad.
SM distributions in these transgenic strains support a role for EGL-15(5B) in the GDR. Extrachromosomal arrays that use the Prol-6 promoter to drive expression of EGL-15 in an egl-15(null) background restore viability; SM distributions in these lines are centrally-dispersed (Fig. 4B), not posteriorly displaced. Arrays generated with other promoters that drive EGL-15 expression in the hypodermis behaved similarly (data not shown). The derived integrated lines also show centrally-dispersed SMs (Fig. 4C–G), although to various extents: ayIs30 is the most posterior distribution, with the others lying more consistently intermediate between the mosaic distribution (Fig. 4A) and the posterior positions typical of egl-15(A−B+) strains. Part of this difference could be due to the slight anterior shift due to the ncl-1(e1865) mutation present in the mosaic strain (see Fig. 3). Importantly, all five of these distributions are displaced posteriorly when placed in an egl-15(A−B+) background, consistent with the 5B isoform contributing to the GDR. SM distributions were determined for two of the integrated transgenic lines in gonad-ablated, egl-15(A−B−) animals (Fig. 4C,F). In these strains that lack all the 5B in the SMs, SM distributions are shifted anteriorly, suggesting that the SMs in these transgenic lines are still partially repelled by the gonad. However, the additional repulsion observed when the 5B isoform is fully restored indicates that robust SM repulsion requires the 5B isoform of EGL-15.
Fig. 4. Characterization of SMs lacking EGL-15 by expressing EGL-15 specifically in the hypodermis.
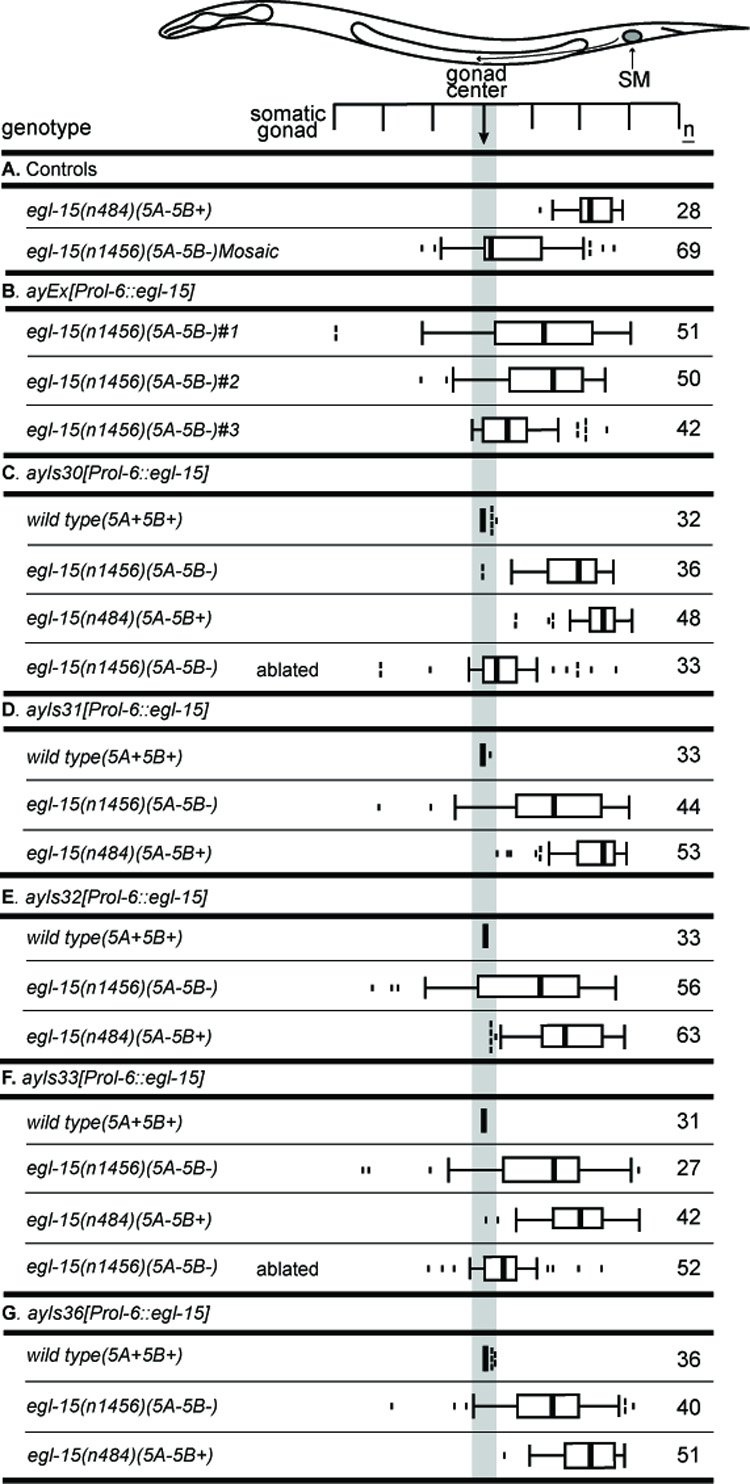
Prol-6::egl-15 transgenes express EGL-15 tissue-specifically in the hypodermis. These transgenes can rescue the essential function of egl-15 without expressing EGL-15 in the SMs. (A) Controls: egl-15(n484) (A−B+) animals without a transgene; and the egl-15(n1456) mosaic animals from Fig. 3C. (B) Three independent extrachromosomal arrays. (C–G) Integrated transgenes (ayIs30-33,36) in various genetic backgrounds. These integrated lines are derived from line #1 in (B). All transgenes behave similarly, despite small differences in SM distributions. Expression in an N2 background shows no observable SM migration defect. Expression in the egl-15(n1456) (A−B−) background results in a broad distribution that is more anterior than that of A−B+ strains, such as egl-15(n484). Expression in an egl-15(n484) (A−B+) background results in the further posteriorization of this distribution. Gonad ablation in egl-15(n1456) (A−B−) animals with Prol-6::egl-15 transgenes anteriorizes the distribution, suggesting some residual repulsion in these strains.
DISCUSSION
Tissue-specific expression patterns determine signaling specificity for the essential function, but the 5A insert is specifically required for SM chemoattraction
FGF receptors are involved in a broad array of processes affecting many aspects of vertebrate development and physiology (for review see Ornitz and Itoh 2001; Eswarakumar et al. 2005), and an increasing number of roles are being found for the C. elegans FGF receptor, EGL-15, as well (Huang and Stern, 2005). Here we have investigated the mechanism of its roles in fluid homeostasis and SM migration. Previous work showed that the two major EGL-15 isoforms, 5A and 5B, are separately responsible for carrying out these two major functions (Goodman et al., 2003). In addition, accumulating evidence indicated that the tissue-specific expression of egl-15 mRNAs might account for this functional specificity.
To understand the mechanistic basis of the functional specificity of EGL-15 5A and 5B, we have looked directly at the cellular distribution of these two EGL-15 isoforms. EGL-15 antibody staining shows that the two major sites of EGL-15 expression are in the M lineage and the major hypodermal syncytium. Transcriptional elements have been identified in the Pegl-15 promoter that enable general expression in both the hypodermis and the M lineage. The e15 enhancer region is an important element for hypodermal expression (Huang and Stern, 2004), and five predicted binding sites for the mesodermal transcription factor HLH-8/CeTwist (Harfe et al., 1998) appear to account for the mesodermal expression of egl-15 in the M lineage: mutating these sites can eliminate reporter gene expression in the M lineage and compromise SM migration guidance (Branda, 2001).
In addition to transcriptional control of egl-15 expression, tissue-specific alternative splicing controls which isoform is expressed at each of these sites. Isoform-specific antibody staining experiments, consistent with previously reported findings (Bulow et al., 2004; Huang and Stern, 2004), demonstrate that expression of the 5A isoform is normally confined to the M lineage, including in the migrating SMs, while the 5B isoform is predominantly expressed in the hypodermis. Thus, evidence now solidly places the 5A isoform in the migrating SMs, where mosaic analysis demonstrates it being necessary for the gonad-dependent attraction mediated by the EGL-17 chemoattractant. The match between the 5A and 5B isoforms and the sites at which they carry out their associated functions suggests that regulation of tissue-specific alternative splicing could, in fact, account for the normal functional specificities of these isoforms.
However, our results show that while tissue-specific expression is the primary regulator of functional specificity for the essential fluid homeostasis function, it does not account for the specificity of the 5A isoform for SM migration guidance. While all forms of EGL-15, including 5B, 5A and Δ5 (a form that mimics the architecture of other FGFRs), can all mediate the essential fluid homeostasis function of EGL-15, only the 5A isoform can mediate SM chemoattraction. 5A is also specifically required for other functions besides SM chemoattraction, such as CAN cell migration and axon position maintenance (Bulow et al., 2004; Fleming et al., 2005). Thus, structural differences in the extracellular domains of the two EGL-15 isoforms are important for their functional specificities, with the 5A isoform specifically required for EGL-15 to serve as a chemoattractant receptor.
The separate sites of action for these two functions enabled us to use a number of methods to observe the effect of complete loss of EGL-15 function on the migrations of the SMs. Normally, severe loss of egl-15 function causes animals to arrest development before SM migration occurs. By generating mosaic animals that had lost all functional copies of egl-15 within the migrating SMs, we were able to confirm that EGL-15 acts cell-autonomously as the EGL-17 receptor within the migrating SMs. EGL-15 has a similar cell autonomous role in CAN cell migration (Fleming et al., 2005). Surprisingly, however, SM distributions in these mosaic animals indicated that EGL-15 also contributes to the gonad-dependent repulsion. Mosaic losses in the lineages giving rise to the migrating SMs result in centrally-dispersed SMs, not the severely posteriorly-displaced SMs characteristic of animals lacking only EGL-15(5A). Thus, these data implicate EGL-15(5B) in SM chemorepulsion.
To confirm the role of EGL-15 5B in chemorepulsion, we expressed an egl-15(+) transgene under the control of a hypodermal-specific promoter, establishing stable lines that lack EGL-15 in the migrating SMs. The SMs in these animals also take on centrally-dispersed final positions. When the 5B isoform is reintroduced into these lines, the SM distributions once again resemble the severely posteriorly-displaced positions typical of animals lacking only EGL-15(5A). Taken together, our data demonstrate that 5B contributes to the gonad-dependent repulsion by acting within the migrating SMs.
Both EGL-15(5A) and EGL-15(5B) function in the migrating sex myoblast to mediate the gonad-dependent attraction and the gonad-dependent repulsion, respectively
We propose a model in which the 5A and 5B isoforms both function in the migrating sex myoblasts but trigger opposite responses. The 5A isoform is clearly expressed in the migrating SMs and can serve as the receptor for the EGL-17 chemoattractant. EGL-17 expression in central cells of the somatic gonad (Branda and Stern, 2000), reinforced by induced expression in the primary vulval precursor cell directly ventral to the center of the gonad (Burdine et al., 1998), both attracts the migrating SMs to their precise final positions and maintains their migrations along the ventral muscle quadrants (Branda and Stern, 2000). In the absence of 5A, a small amount of 5B expression in the SMs is necessary for a gonad-dependent repulsion (GDR) of the SMs that otherwise are moving anteriorly under the guidance of gonad-independent cues. Although 5B is not normally expressed in the SMs at sufficient levels to permit observing it using the same approaches that allow detection of the 5A isoform, traces of 5B expression have been observed in the SMs of rare animals. More importantly, functional evidence clearly implicates 5B in SM migration guidance, and the mosaic analysis implicates it acting within the SM itself. The distinction in the function of 5A and 5B in SM migration guidance appears to be due to extracellular structural differences rather than differences in expression level. For example, the 4B6 construct expresses 5B at high levels in the M lineage and the SMs (Fig. S2A), but results in repulsion, not attraction. It will be interesting to understand how extracellular differences lead to different intracellular responses.
SM chemorepulsion is only observed when the chemoattraction mechanism is compromised, suggesting that a consequence of activating the 5A-mediated chemoattraction is the inhibition of the 5B-mediated repulsion. Importantly, since both the attraction and the repulsion require the same set of gonadal cells (Branda and Stern, 2000; Stern and Horvitz, 1991), this system of attraction and repulsion does not appear to fine-tune the final positioning of the SMs by attraction to one set of cells and repulsion from surrounding non-target cells.
While the 5A isoform acts as a receptor for an instructive chemoattractive cue (Burdine et al., 1998), there is no evidence that the 5B isoform acts instructively as the repellant receptor. The EGL-17 FGF cannot be the repellant, since the repulsion is present even in mutants lacking EGL-17 (Burdine et al., 1997; Goodman et al., 2003). LET-756, the other C. elegans FGF (Roubin et al., 1999), appears to be expressed fairly broadly in the body wall muscles, inconsistent with it being localized in a way to allow it to function as a gonadal repellant (Bulow et al., 2004). A model in which 5B acts permissively in the GDR would allow a non-FGF ligand to be the actual repellant. In such a case, activation of the 5B receptor would allow another receptor to function as the chemorepellant receptor. To date, there is no evidence suggesting the nature of such a chemorepulsion receptor.
It is currently not known what accounts for the difference in the function of the 5A and 5B isoforms, but many types of receptors for migration cues play roles in both attractive and repulsive responses. For example, netrins, ephrins, and semaphorins can all act as either chemoattractants or chemorepellants during axon guidance (Cowan et al., 2005; Culotti and Merz, 1998). In the case of netrins, the different signaling response is dependent upon the presence or absence of the UNC5 co-receptor (Baker et al., 2006; Hong et al., 1999; Manitt and Kennedy, 2002). Similar to the netrins, the switch between attraction and repulsion for the ephrins is also dependent upon the presence or absence of other signaling molecules. In guiding axons from retinal ganglion cells, ephrin binding stimulates the recruitment of Vav2 to the intracellular domain of the Eph receptor. Endocytosis due to transient activation triggers Eph signaling to switch from attraction to repulsion (Cowan et al., 2005). Semaphorins can alternatively stimulate the outgrowth of dendrites and repel growth cones (Fenstermaker et al., 2004) depending upon the presence of other molecules such as guanylate cyclases (Polleux and Ghosh, 2000), neuropilin co-receptors (Wolman et al., 2004), and second messengers such as cAMPs (Terman and Kolodkin, 2004).
Our data now place FGF receptors into this class of guidance molecules that can trigger both attraction and repulsion. FGFs are known to act both as attractants and repellants during vertebrate neuronal development, but the mechanisms that mediate these responses are not understood. For example, FGF8 acts as a chemoattractant to guide trochlear axon growth (Irving and Mason, 2000), while FGF2 stimulates retinal ganglional cell axon extension and chemorepulsion (Weber et al., 2005). Continued investigation of the mechanisms of EGL-15 signal transduction should help shed light on how FGF receptors can mediate either attraction or repulsion.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank members of our lab for critical reading of this manuscript. This work was supported by NIH grant GM50504 (M.J.S.), an Anna Fuller Fellowship (P.H.) and the following NIH training grants: T32 GM07223 (T.L.), T32 GM07499 (C.S.B.), T32 GM07205 (I.E.S.), and T32 DK07259 (S.J.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bagri A, Tessier-Lavigne M. Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Advances in experimental medicine and biology. 2002;515:13–31. [PubMed] [Google Scholar]
- Baker KA, Moore SW, Jarjour AA, Kennedy TE. When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Current opinion in neurobiology. 2006;16:529–534. doi: 10.1016/j.conb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Borland CZ, Schutzman JL, Stern MJ. FGF signaling in the nematode Caenorhabditis elegans. BioEssays. 2001;23:1120–1130. doi: 10.1002/bies.10007. [DOI] [PubMed] [Google Scholar]
- Branda CS. Ph.D. Thesis, Genetics. New Haven: Yale University; 2001. Mechanisms of sex myoblast migration in C. elegans hermaphrodites. [Google Scholar]
- Branda CS, Stern MJ. Mechanisms controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 2000;226:137–151. doi: 10.1006/dbio.2000.9853. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow HE, Boulin T, Hobert O. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron. 2004;42:367–374. doi: 10.1016/s0896-6273(04)00246-6. [DOI] [PubMed] [Google Scholar]
- Burdine RD, Branda CS, Stern MJ. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development. 1998;125:1083–1093. doi: 10.1242/dev.125.6.1083. [DOI] [PubMed] [Google Scholar]
- Burdine RD, Chen EB, Kwok SF, Stern MJ. egl-17 encodes an invertebrate fibroblast growth factor family member required specifically for sex myoblast migration in Caenorhabditis elegans. Proceedings of the National Academy of Sciences, USA. 1997;94:2433–2437. doi: 10.1073/pnas.94.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Culotti JG, Merz DC. DCC and netrins. Current opinion in cell biology. 1998;10:609–613. doi: 10.1016/s0955-0674(98)80036-7. [DOI] [PubMed] [Google Scholar]
- DeVore DL, Horvitz HR, Stern MJ. An FGF receptor signaling pathway is required for the normal cell migrations of the sex myoblasts in C. elegans hermaphrodites. Cell. 1995;83:611–620. doi: 10.1016/0092-8674(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine & growth factor reviews. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fenstermaker V, Chen Y, Ghosh A, Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J Neurobiol. 2004;58:403–412. doi: 10.1002/neu.10304. [DOI] [PubMed] [Google Scholar]
- Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Fleming TC, Wolf FW, Garriga G. Sensitized genetic backgrounds reveal a role for C. elegans FGF EGL-17 as a repellent for migrating CAN neurons. Development. 2005;132:4857–4867. doi: 10.1242/dev.02020. [DOI] [PubMed] [Google Scholar]
- Goodman SJ, Branda CS, Robinson MK, Burdine RD, Stern MJ. Alternative splicing affecting a novel domain in the C. elegans EGL-15 FGF receptor confers functional specificity. Development. 2003;130:3757–3766. doi: 10.1242/dev.00604. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Van Gomes A, Kenyon C, Liu J, Krause M, Fire A. Analysis of a Caenorhabditis elegans Twist homolog identifies conserved and divergent aspects of mesodermal patterning.”. Genes Dev. 1998b;12:2623–2635. doi: 10.1101/gad.12.16.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Herman RK. Genetics. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1988. pp. 17–45. [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Huang P. Ph.D. Thesis, Genetics. New Haven: Yale University; 2004. Regulation of fluid balance by FGF receptor signaling in Caenorhabditis elegans. [Google Scholar]
- Huang P, Stern MJ. FGF signaling functions in the hypodermis to regulate fluid balance in C. elegans. Development. 2004;131:2595–2604. doi: 10.1242/dev.01135. [DOI] [PubMed] [Google Scholar]
- Huang P, Stern MJ. FGF signaling in flies and worms: more and more relevant to vertebrate biology. Cytokine & growth factor reviews. 2005;16:151–158. doi: 10.1016/j.cytogfr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Irving C, Mason I. Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development. 2000;127:177–186. doi: 10.1242/dev.127.1.177. [DOI] [PubMed] [Google Scholar]
- Kokel M, Borland CZ, DeLong L, Horvitz HR, Stern MJ. clr-1 encodes a receptor tyrosine phosphatase that negatively regulates an FGF receptor signaling pathway in Caenorhabditis elegans. Genes & Dev. 1998;12:1425–1437. doi: 10.1101/gad.12.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroyanagi H, Kobayashi T, Mitani S, Hagiwara M. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nature Methods. 2006;3:909–915. doi: 10.1038/nmeth944. [DOI] [PubMed] [Google Scholar]
- Manitt C, Kennedy TE. Where the rubber meets the road: netrin expression and function in developing and adult nervous systems. Prog. Brain Res. 2002;137:425–442. doi: 10.1016/s0079-6123(02)37034-1. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. The EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DS, McCabe GP. Introduction to the practice of statistics. New York: W.H. Freeman and Company; 1993. [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2(3) doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Roubin R, Naert K, Popovici C, Vatcher G, Coulier F, Thierry-Mieg J, Pontarotti P, Birnbaum D, Baillie D, Thierry-Mieg D. let-756, a C. elegans fgf essential for worm development. Oncogene. 1999;18:6741–6747. doi: 10.1038/sj.onc.1203074. [DOI] [PubMed] [Google Scholar]
- Sasson IE, Stern MJ. FGF and PI3 kinase signaling pathways antagonistically modulate sex muscle differentiation in C. elegans. Development. 2004;131:5381–5392. doi: 10.1242/dev.01423. [DOI] [PubMed] [Google Scholar]
- Stern MJ, Horvitz HR. A normally attractive cell interaction is repulsive in two C. elegans mesodermal cell migration mutants. Development. 1991;113:797–803. doi: 10.1242/dev.113.3.797. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Stern MJ, Horvitz HR. Cell interactions coordinate the development of the C. elegans egg-laying system. Cell. 1990;62:1041–1052. doi: 10.1016/0092-8674(90)90382-o. [DOI] [PubMed] [Google Scholar]
- Weber JR, Bell GM, Han MY, Pawson T, Imboden JB. Association of the tyrosine kinase LCK with phospholipase C-gamma 1 after stimulation of the T cell antigen receptor. J. of Exp. Med. 1992;176:373–379. doi: 10.1084/jem.176.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Liu Y, Tawarayama H, Shoji W, Halloran MC. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J Neurosci. 2004;24:8428–8435. doi: 10.1523/JNEUROSCI.2349-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.