Abstract
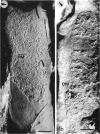
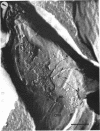
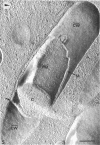
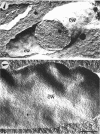
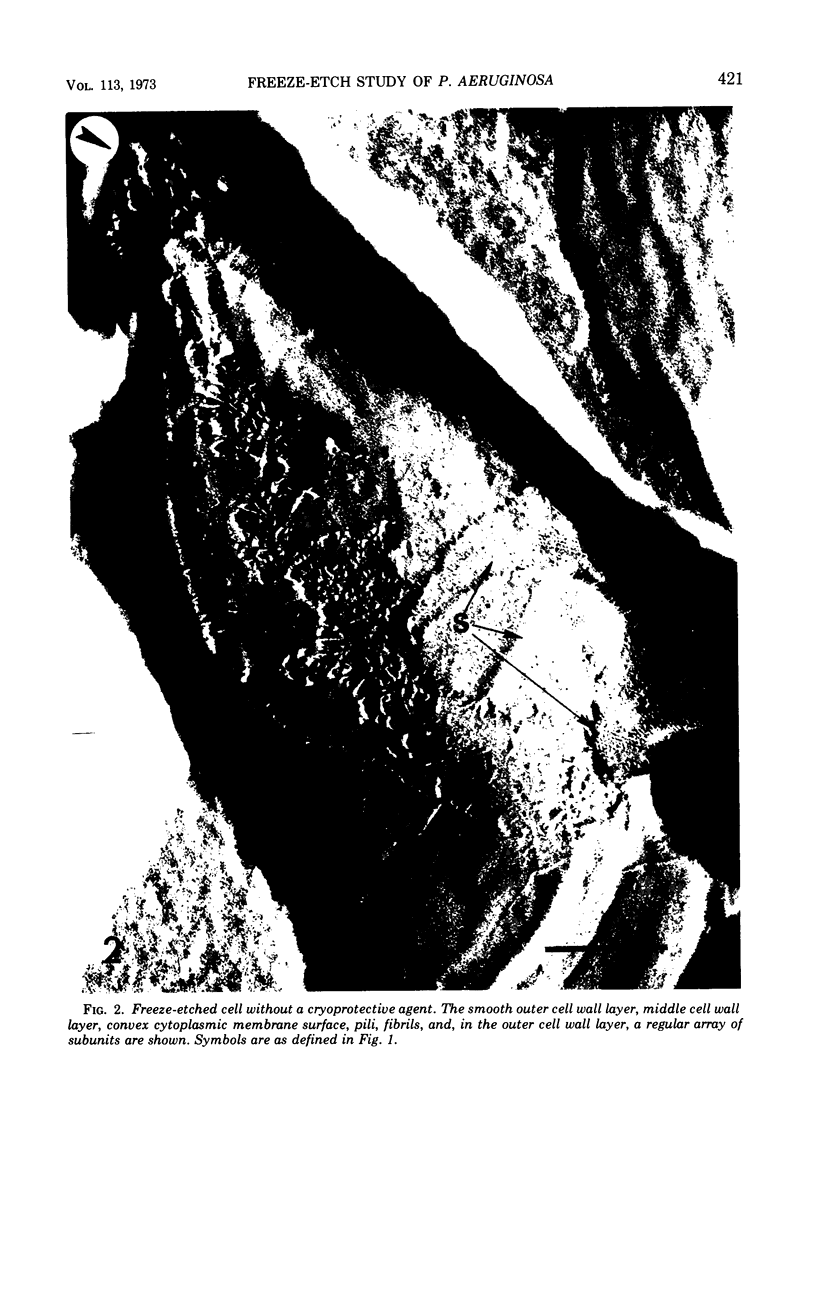
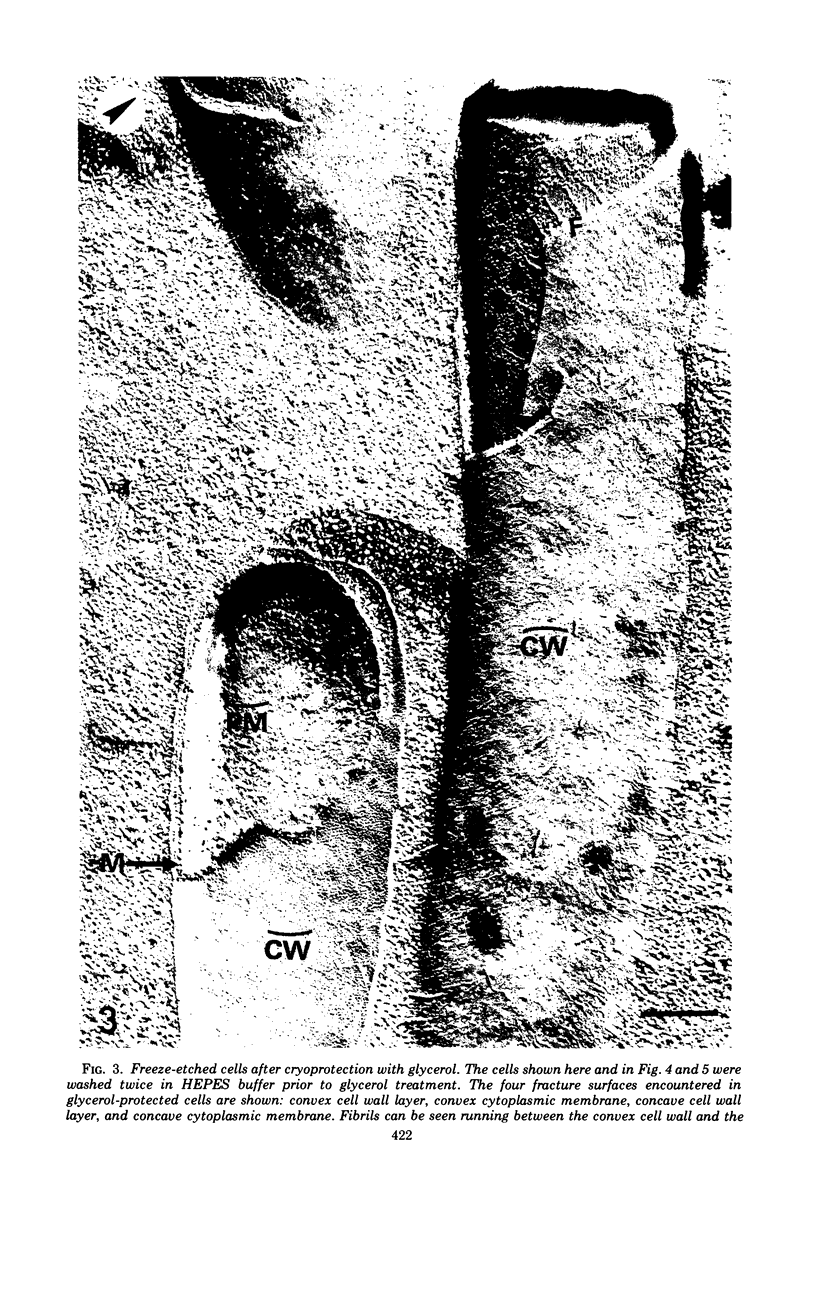
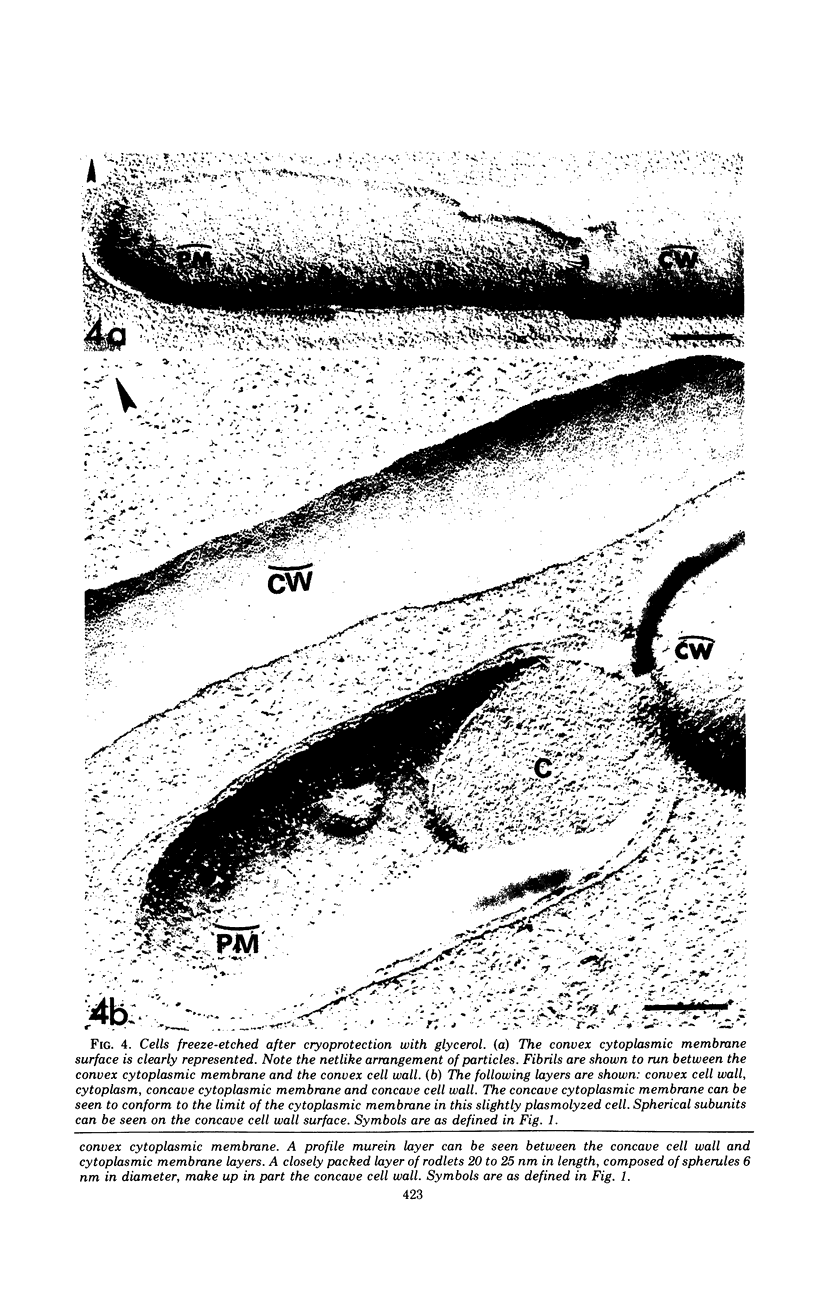
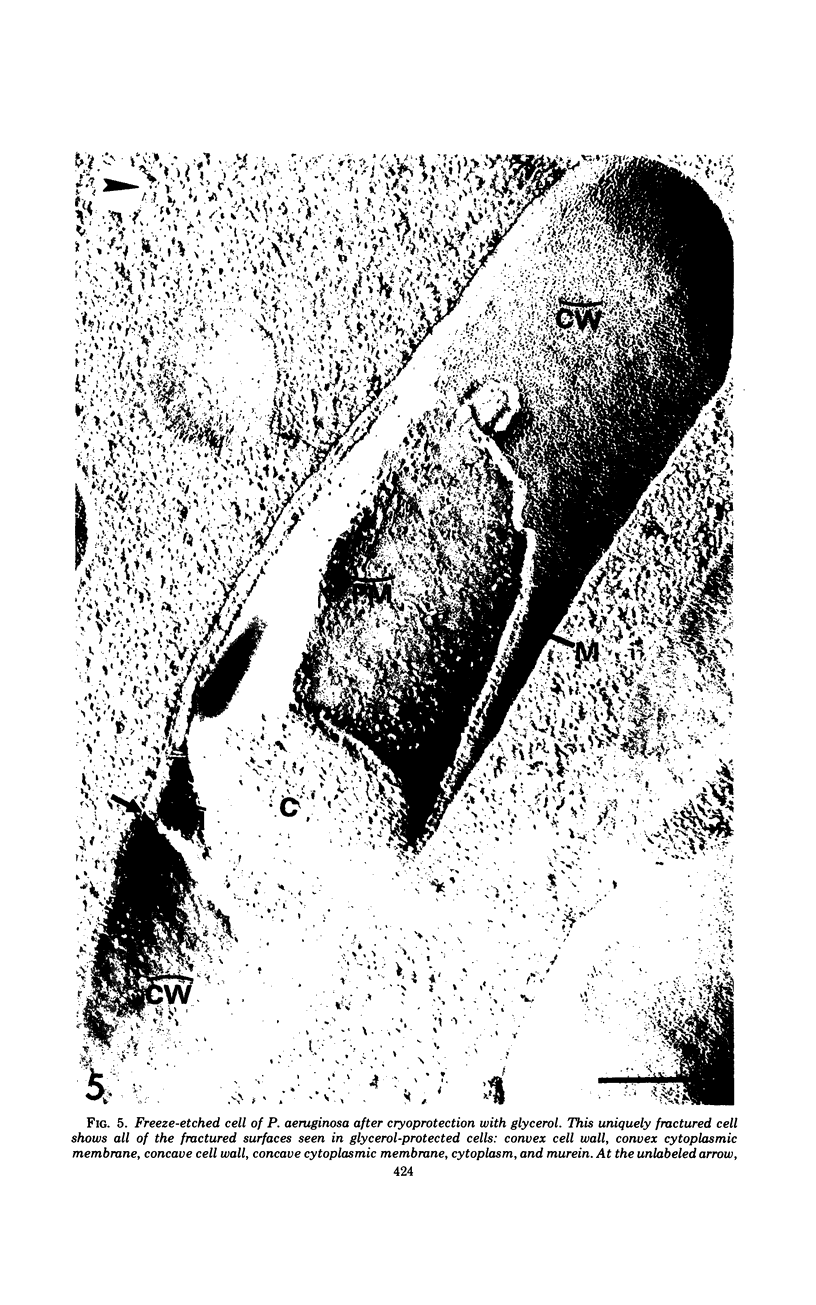
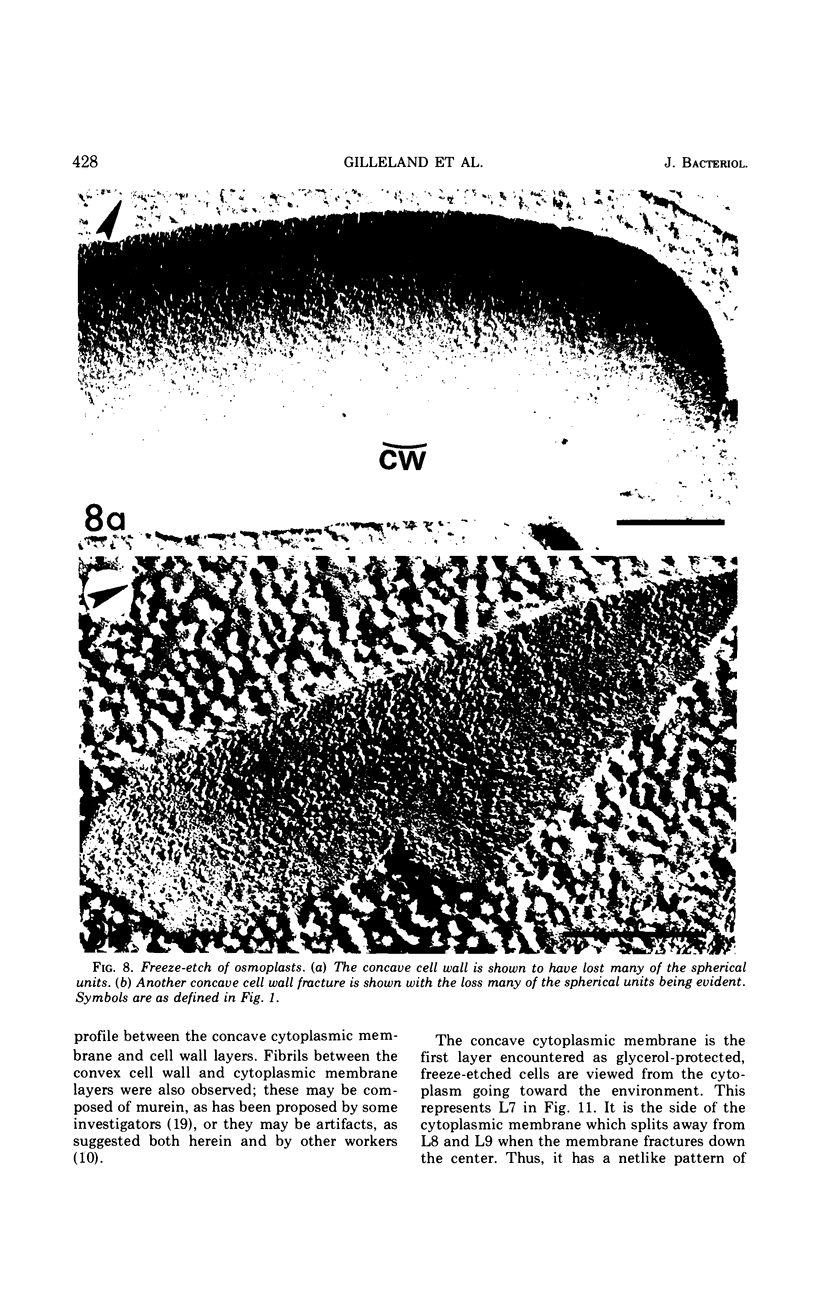
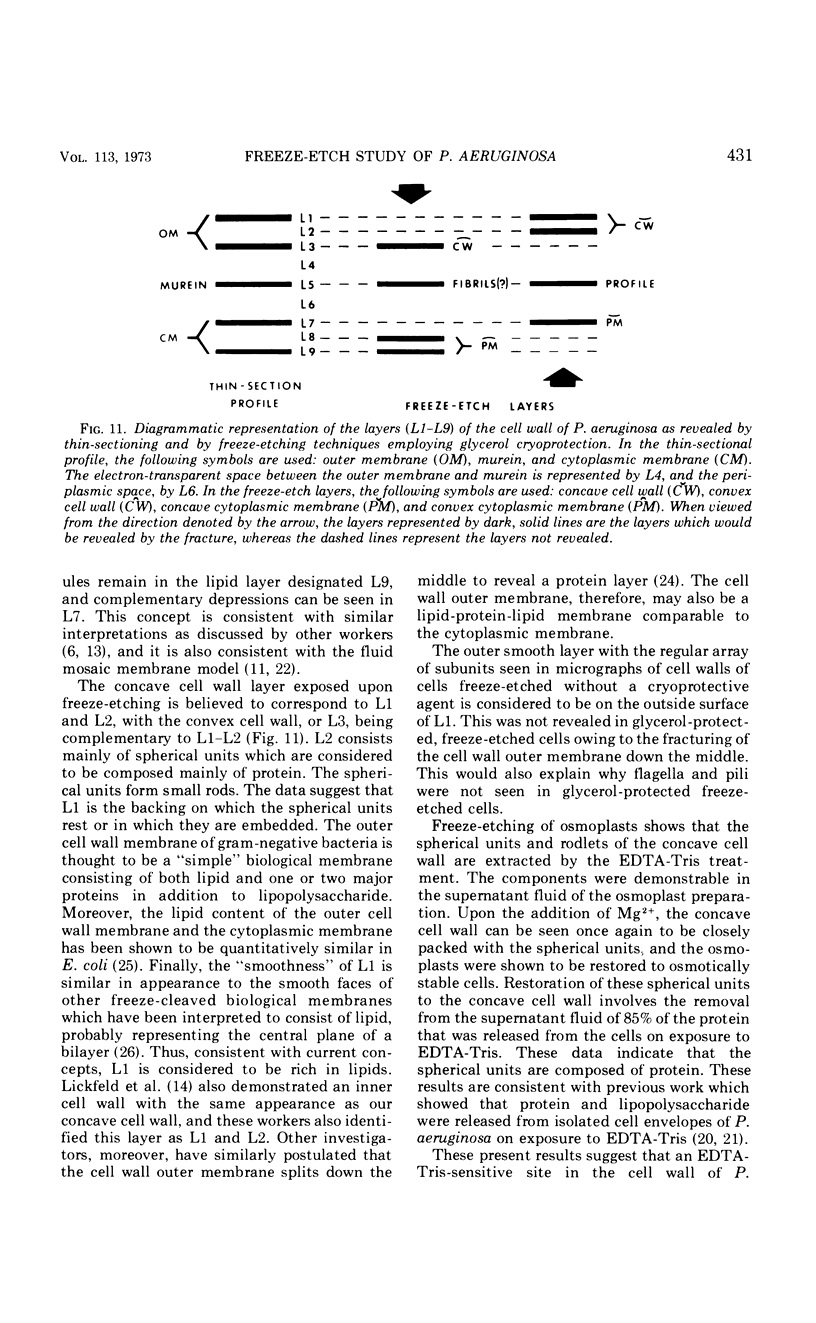
A freeze-etch study of normal cells of Pseudomonas aeruginosa and of cells after incubation with ethylenediaminetetraacetate (EDTA) and tris(hydroxymethyl)aminomethane (Tris) was performed. When cells were freeze-etched without a cryoprotective agent, a smooth outer cell wall layer, which showed a regular array of subunits, and the presence of flagella and pili were observed. These features were not observed in cells freeze-etched after cryoprotection with glycerol. Four fracture surfaces, which resulted from splitting down the center of the outer wall membrane and of the inner cytoplasmic membrane, were revealed in freeze-etched glycerol-protected cells. The murein layer was seen in profile between the outer cell wall membrane and the cytoplasmic membrane. Spherical units and small rods composed of the spherical units were observed in the inner layer of the outer cell wall membrane. These spherical units appeared to be attached to, or embedded in, the inner face of the outer layer of the outer cell wall membrane. These spherical units were removed from cells on exposure to EDTA-Tris, resulting in cells that were osmotically fragile. The spherical units were detected via electron microscopy of negatively stained preparations in the supernatant fluid of cellular suspensions treated with EDTA-Tris. Upon addition of Mg2+, the spherical units were reaggregated into the inner layer of the outer cell wall membrane and the cells were restored to osmotic stability. The spherical units were shown to consist primarily of protein. These data are thought to represent the first ultrastructural demonstration of reaggregation of cell wall components within a living cell system.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. The role of multivalent cations in the organization and structure of bacterial cell walls. Biochem Biophys Res Commun. 1966 Mar 22;22(6):664–671. doi: 10.1016/0006-291x(66)90198-7. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971 Mar;105(3):1184–1199. doi: 10.1128/jb.105.3.1184-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W., Branton D. Fracture planes in an ice-bilayer model membrane system. Science. 1967 Nov 3;158(3801):655–657. doi: 10.1126/science.158.3801.655. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickfeld K. G., Achterrath M., Hentrich F., Kolehmainen-Seveus L., Persson A. Die Feinstrukturen von Pseudomonas aeruginosa in ihrer Deutung durch die Gefrierätztechnik, Ultramikrotomie und Kryo-Ultramikrotomie. J Ultrastruct Res. 1972 Jan;38(1):27–45. doi: 10.1016/s0022-5320(72)90082-2. [DOI] [PubMed] [Google Scholar]
- Merton P. A. How we control the contraction of our muscles. Sci Am. 1972 May;226(5):30–37. doi: 10.1038/scientificamerican0572-30. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Fracture faces in intact cells and protoplasts of Bacillus stearothermophilus. A study by conventional freeze-etching and freeze-etching of corresponding fracture moieties. Protoplasma. 1970;71(3):295–312. doi: 10.1007/BF01279638. [DOI] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingsheim H. P. Membrane structure and electron microscopy. The significance of physical problems and techniques (freeze etching). Biochim Biophys Acta. 1972 Aug 4;265(3):339–366. doi: 10.1016/0304-4157(72)90013-5. [DOI] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]