Abstract
Guanylyl cyclase C (GCC), the receptor for diarrheagenic bacterial heat-stable enterotoxins (STs), inhibits colorectal cancer cell proliferation by co-opting Ca2+ as the intracellular messenger. Similarly, extracellular Ca2+ (Ca2+o) opposes proliferation and induces terminal differentiation in intestinal epithelial cells. In that context, human colon cancer cells develop a phenotype characterized by insensitivity to cytostasis imposed by Ca2+o. Here, preconditioning with ST, mediated by GCC signaling through cyclic nucleotide-gated channels, restored Ca2+o-dependent cytostasis, reflecting posttranscriptional regulation of calcium-sensing receptors (CaRs). ST-induced GCC signaling deployed CaRs to the surface of human colon cancer cells, whereas elimination of GCC signaling in mice nearly abolished CaR expression in enterocytes. Moreover, ST-induced Ca2+o-dependent cytostasis was abrogated by CaR-specific antisense oligonucleotides. Importantly, following ST preconditioning, newly expressed CaRs at the cell surface represented tumor cell receptor targets for antiproliferative signaling by CaR agonists. Since expression of the endogenous paracrine hormones for GCC is uniformly lost early in carcinogenesis, these observations offer a mechanistic explanation for the Ca2+o-resistant phenotype of colon cancer cells. Restoration of antitumorigenic CaR signaling by GCC ligand replacement therapy represents a previously unrecognized paradigm for the prevention and treatment of human colorectal cancer employing dietary Ca2+ supplementation.
Introduction
Colorectal cancer is most prevalent in the developed world, representing the second leading cause of cancer-related mortality (1,2). Although the epidemiology of this disease is poorly understood, there is an unexplained inverse relationship between the worldwide incidence of colorectal cancer and enterotoxigenic Escherichia coli infections (3,4). Enterotoxigenic E.coli produces heat-stable enterotoxins (STs), exogenous ligands for the intestine-specific receptor guanylyl cyclase C (GCC) (5) and a principle cause of secretory diarrhea in endemic populations and animal herds (6–8). Regions of the world with the highest incidence of enterotoxigenic E.coli-associated diarrhea exhibit the lowest incidence of colon cancer (4). In that context, activation of GCC inhibits human colon carcinoma cell proliferation (4,9,10) and adenoma formation in mice (3).
Reduced expression of the endogenous paracrine hormones for GCC, guanylin and uroguanylin, represents an early mutational event in colorectal carcinogenesis (11–13). GCC signaling through its second messenger cGMP promotes fluid and electrolyte secretion (5), opposes cell cycle progression and proliferation (4,9) and regulates migration, differentiation and apoptosis along the crypt–villus axis (14,15). Importantly, targeted GCC deletion (GCC−/−) in mice increased intestinal tumorigenesis induced by the carcinogen azoxymethane or adenomatous polyposis coli (APC) mutations by corrupting homeostatic crypt proliferation and genomic integrity (16). These observations suggest a model in which colorectal cancer is a disease of hormone insufficiency where dysregulation of GCC signaling, following loss of guanylin and uroguanylin, promotes tumorigenesis by disrupting mucosal homeostasis (16). However, beyond production of cGMP, molecular mechanisms by which GCC regulates processes underlying carcinogenesis remain undefined.
Like GCC, extracellular Ca2+ (Ca2+o) opposes proliferation and promotes differentiation of intestinal mucosa cells (17–20). Moreover, Ca2+ supplementation abrogates intestinal hyperproliferation and tumor formation induced by a western-style diet in APCMin/+ mice (21). In part, these antitumorigenic effects may reflect GCC-induced activation of cyclic nucleotide-gated (CNG) channels, inducing cytostasis through Ca2+o influx (4). Notably, Ca2+o in the colonic lumen may increase to ≥20 mM that activates calcium-sensing receptors (CaRs) (19,22), G protein-coupled receptors (23) expressed in apical membranes of colonocytes (24), an event resulting in reduced proliferation and tumorigenesis through inhibition of β-catenin/Tcf-4 signaling, and increased cell maturation through p21 and p27 activities (25,26). Conversely, Ca2+o supports colorectal cancer cell proliferation by capacitative entry through store-operated Ca2+ channels (27,28), which opposes antiproliferative GCC signaling through CNG and Ca2+o entry (28). Thus, opposing mechanisms regulating proliferative balance by Ca2+o comprise functional units reciprocally orchestrated in colon cancer cells (28).
Here, the functional relationship between GCC and Ca2+o was explored in human colon carcinoma cells preconditioned with ST, in the context of a dynamic range of Ca2+o, and in GCC−/− mice. These studies revealed a previously unappreciated role for GCC in regulating CaR signaling, offering a novel paradigm for the prevention and treatment of human colorectal cancer employing hormone replacement therapy with GCC ligands in combination with oral Ca2+ supplementation.
Materials and methods
Tumor cell proliferation
Proliferation of cancer cells was quantified in 96 wells per plate by [methyl-3H]thymidine (0.2 μCi/well) incorporation into DNA (9). Cells were pulse labeled (3 h) with 3H-thymidine at the end of 24 h periods of proliferation induced by 10 mM L-glutamine. Following 3H-thymidine labeling, media was aspirated, cells were incubated for 15 min with ice-cold 10% trichloroacetic acid and rinsed sequentially with 10% trichloroacetic acid and 100% methanol. The acid-insoluble material containing 3H-labeled DNA was solubilized in 100 μl of 0.2 N NaOH, 80 μl aliquots were dissolved in 1 ml ScintiVerse and radioactivity quantified in a Packard β-scintillation spectrometer. All experiments were conducted on exponentially growing tumor cells.
Tumor cell toxicity
Cytotoxicity, including occurrence of apoptosis or necrosis, was assessed by flow cytometry (9). Cancer cells (∼106 cells per well in six-well plates) were treated (24 h) with the indicated reagents. Then, cells were placed in suspension by trypsinization, pelleted by centrifugation, washed with phosphate-buffered saline and fixed in ice-cold 75% ethanol (30 min). After another wash with phosphate-buffered saline, cells were resuspended in the staining solution (50 μg/ml propidium iodide, 100 μg/ml RNase A, 1 mM ethylenediaminetetraacetic acid and 0.1% Triton X-100) and analyzed on a Coulter EPICS XL-MCL flow cytometer. Distribution in different phases of the cell cycle was analyzed using WinMDI software (version 2.8) provided by Joseph Trotter, Scripps Research Institute (La Jolla, CA). Twenty thousand cells, cleared from doublets, were analyzed from each sample.
Cyclic GMP assay
GCC-induced intracellular cGMP accumulation was assessed after treating cancer cells in triplicate with ST (15 min in six well per plate) employing Eagle’s minimal essential medium supplemented with 2 mM L-glutamine. Reactions were terminated by adding ice-cold 100% ethanol, each well was washed twice with ice-cold 100% ethanol and supernates separated from pellets by centrifugation (12 000g, 15 min at 4°C). Supernates containing cGMP were evaporated in a Savant SVC-100H concentrator (Thermo Electron Corporation, Waltham, MA) and reconstituted with 50 mM sodium acetate (pH 4.0), and cGMP was quantified in each sample in triplicate by radioimmunoassay (4).
Immunoblot analysis
Proteins from total cell lysates, cytosol or membrane extracts (28) prepared in sodium dodecyl sulfate sample buffer were separated by electrophoresis on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred on nitrocellulose membranes and then probed with rabbit polyclonal antibodies directed against CaR (dilution 1:1000) or human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000) or with goat anti-villin antibody [Villin(C-19); 1:1000] in Tris-buffered saline–Tween (5% milk) overnight at 4°C. Then, membranes were probed with horseradish peroxidase-conjugated secondary antibody (dilution 1:5000) for 1 h at room temperature, and specific bands were visualized employing West Pico Chemiluminescent Substrate and subjected to densitometry. Immunoblots, performed under reducing conditions, exhibited only one specific band of ∼125 kDa corresponding to the monomer form of CaR.
Immunostaining analyses
For immunohistochemistry, tumor cells were washed two times with cold phosphate-buffered saline and immediately fixed (30 min in 4% paraformaldehyde) at room temperature followed by quenching with 3% H2O2. CaR or CD104 at the cell surface was visualized employing (overnight at 4°C) rabbit anti-CaR (1:100) or mouse monoclonal anti-human CD104 (1:100), respectively, and the Histostain-plus kit. For immunofluorescence, GCC−/− mice, generated by neomycin-resistant gene insertion on an I-129 background (29) and backcrossed with a C57BL/6 strain for seven or more generations, were employed. All animals were treated in compliance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and under a protocol approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. GCC+/+ and GCC−/− littermates (males 2 months old; N, three per genotype) were killed, the intestine collected, divided into anatomically comparable segments and processed for paraffin-based archiving. Paraffin-embedded tissue sections (5 μm) were rehydrated, heated (two times for 5 min in citrate buffer, pH 6) for antigen retrieval, incubated overnight (4°C) with rabbit anti-CaR (1:100) or goat anti-villin (1:50) followed by incubation for 30 min (room temperature) with Alexa fluor 555 anti-rabbit IgG (for CaR) or Alexa fluor 488 anti-goat IgG (for villin). Digital images were acquired by computers attached to a light microscope or a confocal laser scanning microscope (LSM510, Carl Zeiss, Jena, Germany).
Cell transductions
A 5′ 327 bp CaR fragment containing 8 bp of upstream DNA was cloned into MSCV-puro retroviral vectors in both sense and antisense orientations (30), confirmed by DNA sequencing. Antisense, sense and empty vectors were transfected along with the packaging vector pCL Ampho into HEK 293 cells employing Fugene. Viral supernatants harvested at 48 and 72 h after transfection were used to transduce T84 cells (for 72 h). Then, tumor cells stably transduced with CaR antisense, CaR sense or empty vector were selected employing 5 μg/ml puromycin in Dulbecco’s modified Eagle’s medium/F12.
Statistical analysis
Data are expressed as the mean ± SEM of a representative of at least three experiments performed in triplicate. Data were analyzed employing the unpaired two-tailed Student’s t-test, and significance was assumed for P < 0.05.
Results
Colorectal cancer cells are insensitive to Ca2+o-dependent cytostasis
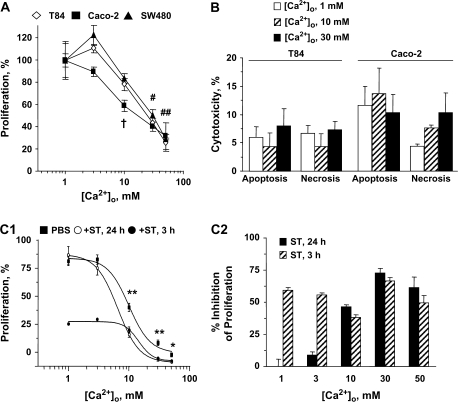
Whereas intestinal epithelial cells along the crypt–villus axis transition from proliferation to differentiation at lumenal Ca2+o ∼2–3 mM (22), neoplastic transformation progressively desensitizes these cells to the cytostatic effects of Ca2+o (20), with ultimate loss of Ca2+o-induced cell cycle arrest (31). Accordingly, Ca2+o induced cytostasis (Figure 1A), but not cytotoxicity (Figure 1B), in moderately and well-differentiated colon cancer cells. However, Ca2+o failed to induce cell cycle arrest and a substantial proliferating cell population (∼20–30%) persisted at 50 mM Ca2+o (Figure 1A), consistent with a phenotype of insensitivity to proliferative restriction by Ca2+o in human colon cancer cells.
Fig. 1.
Bacterial enterotoxin ST potentiates Ca2+o-mediated cytostasis in colorectal cancer cells. (A) Dose responses of cytostasis by Ca2+o in various human colon carcinoma cells. †P < 0.01 versus control for Caco-2 cells; #P < 0.05 and ##P < 0.01 versus respective controls in each of the three cell lines. (B) Absence of cytotoxicity by Ca2+o in human colon cancer cells. Cytotoxicity was assessed as the proportion of tumor cells identified by flow cytometry in the sub-G1 fractions, which correspond to cells undergoing apoptosis or necrosis, of the cell cycle. Data are mean ± SEM from three independent experiments. (C1) Concentration dependence of T84 cell proliferation on Ca2+o in the presence or absence of 1 μM ST. Results in (A) and (C1) are expressed as the percentage of respective control incubations treated with 1 mM Ca2+o. *P < 0.05 and **P < 0.01, comparing respective conditions treated with Ca2+o alone and Ca2+o plus ST for 24 h. In (C2), data obtained in (C1) are expressed as {100 − [(condition treated with ST plus Ca2+o)/(respective control condition treated with Ca2+o alone) × 100]}.
ST potentiates Ca2+o-dependent cytostasis by inducing GCC signaling through CNG channels
Acute (3 h) ST exposure, which restricts cell cycle progression of colon cancer cells by activating GCC signaling (4,9), induced cytostasis that was additive with Ca2+o (Figure 1C1). Indeed, cytostasis induced by acute ST exposure was not altered by Ca2+o over a range of 1–50 mM (Figure 1C2), suggesting that the antiproliferative effects of ST and Ca2+o are mediated by distinct, non-interfering mechanisms. In contrast, ST preconditioning (24 h), which induces desensitization to GCC-dependent cytostasis in colon cancer cells (10), potentiated the inhibition of proliferation by Ca2+o (Figure 1C1). Thus, ST synergistically increased the potency (IC50: Ca2+o alone, 9.91 ± 1.09 mM; ST plus Ca2+o, 6.49 ± 1.13 mM) and efficacy (mean percentage of proliferation: Ca2+o alone, 61.30 ± 1.84; ST plus Ca2+o, 49.47 ± 2.14) of Ca2+o to inhibit colon cancer cell proliferation (P < 0.001; Figure 1C1). Importantly, while failing to inhibit cell proliferation, reflecting desensitization to cGMP signaling (10) (see conditions at 1 mM Ca2+o in Figure 1C1), ST preconditioning revealed a novel antiproliferative mechanism that required ≥3 mM Ca2+o (Figure 1C2).
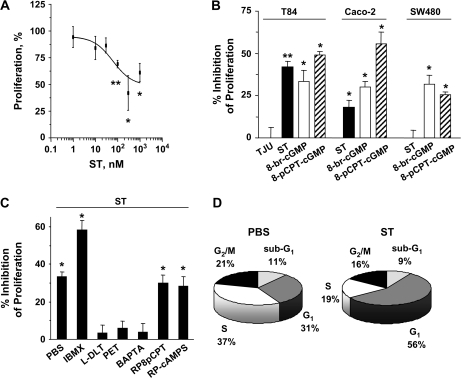
Potentiation of Ca2+o-induced cytostasis by ST preconditioning was mediated by GCC since ST, but not an inactive analog, inhibited (IC50, 62.22 ± 3.21 nM; Figure 2A) proliferation of human colon carcinoma cells expressing GCC, but not of cells lacking GCC (32) (Figure 2B). Similarly, the effects of ST preconditioning were greater in T84 cells, which exhibits the highest GCC expression (32), compared with Caco-2 cells (Figure 2B). Further, two membrane-permeant cGMP analogs mimicked (Figure 2B), whereas an inhibitor of cyclic nucleotide-hydrolyzing phosphodiesterases enhanced (Figure 2C), the effects of ST preconditioning on tumor cell proliferation. Conversely, two inhibitors of CNG channels and an intracellular Ca2+ chelator, but not inhibitors of cGMP- or cAMP-dependent protein kinase, blocked ST-mediated potentiation of Ca2+o-dependent cytostasis (Figure 2C). Notably, ST did not induce cytotoxicity in colon cancer cells exposed to high Ca2+o (Figure 2D). Rather, ST delayed the progression of these tumor cells through the cell cycle (Figure 2D), consistent with the notion that GCC agonists are cytostatic agents for colon cancer (4,9,10). Together, these observations suggest that in colon cancer cells induction of Ca2+o-dependent cytostasis by ST preconditioning is mediated by cGMP-dependent activation of CNG channels and Ca2+o entry, the same effector mechanism mediating GCC-induced cell cycle delay (4).
Fig. 2.
ST potentiates Ca2+o-mediated cytostasis by inducing GCC signaling through CNG. (A) Dose response of ST-induced potentiation of antiproliferation by Ca2+o in T84 cells. (B) ST (1 μM) actions on Ca2+o are mediated by GCC and cGMP in colorectal cancer cells. TJU, 1 μM of the inactive ST analog Thomas Jefferson University 1–103; 8-br-cGMP (5 mM) and 8-pCPT-cGMP (0.5 mM), membrane-permeant cGMP analogs. (C) CNG channel and Ca2+o entry are the proximal molecular effectors of enhanced Ca2+o cytostasis by ST (1 μM) in T84 cells. IBMX (50 μM), the general phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine; L-DLT (L-cis-diltiazem, 10 μM) and PET (8-br-PET-cGMPS, 50 μM), two inhibitors of CNG channels; BAPTA (2 μM), the intracellular Ca2+ chelator BAPTA-AM; RP8pCPT (50 μM), the cGMP-dependent protein kinase inhibitor Rp-8-pCPT-cGMP; Rp-cAMPS (50 μM), an inhibitor of cAMP-dependent protein kinase. Inhibitors were employed at concentrations that completely inhibit their target enzymes (4). Results in (A) are expressed as the percentage of respective control incubations treated with 10 mM Ca2+o, whereas in (B) and (C) are expressed as in Figure 1C2. *P < 0.05 and **P < 0.01 versus respective parallel control conditions treated with 10 mM Ca2+o. (D) ST (1 μM) induces cytostasis, but not cytotoxicity, in synchronized T84 cells exposed to 10 mM Ca2+o. The percentage of tumor cells in each phase of the cell cycle, including the sub-G1 fraction of apoptotic/necrotic cells, was quantified by flow cytometry. Data are from a representative experiment.
GCC regulates the function of CaRs in normal and malignant colonocytes
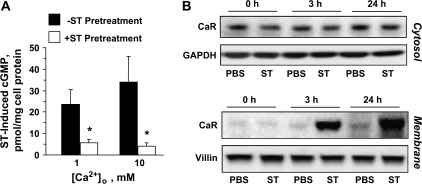
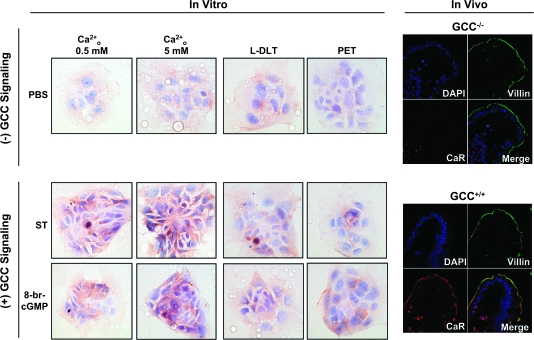
Increased antiproliferative effects of Ca2+o following ST preconditioning did not reflect enhanced GCC-dependent cytostasis by Ca2+o because increased Ca2+o did not enhance cytostasis induced by GCC signaling (+ST for 3 h in Figure 1C2) and did not prevent GCC-mediated desensitization in cGMP signaling (10) (Figure 3A). Rather, ST preconditioning enhanced Ca2+o-dependent cytostasis by coupling increased Ca2+o with intracellular signaling mechanisms induced by cGMP signaling (Figure 2A–C). Ca2+o induces cytostasis in colonocytes, in part, by activating CaR signaling (22), a mechanism typically lost during neoplastic transformation (26,33). ST preconditioning (≥3 h) augmented the complement of total cellular (supplementary Figure 1A is available at Carcinogenesis Online) and membrane-bound CaRs (Figure 3B) without significantly altering cytosolic CaRs (Figure 3B) or CaR messenger RNA levels (data not shown). Further, examination of specific cell fractions (supplementary Figure 1B is available at Carcinogenesis Online) revealed that ST treatment (3 h) induces ∼80-fold increase of CaR at tumor cell membranes (supplementary Figure 1C is available at Carcinogenesis Online), reflecting translocation of ∼60% of the total complement of CaR protein in cancer cells from the cytosol to the membrane compartment (supplementary Figure 1D is available at Carcinogenesis Online). Importantly, GCC signal deprivation, either in ligand-free tumor cells (Figure 4, in vitro panels on the left showing CaR surface staining of T84 cells) or in enterocytes of GCC−/− mice (Figure 4, in vivo panels on the right), reduced CaRs in cell membranes. Induction of GCC signaling with bacterial enterotoxin in tumor cells or endogenous paracrine hormonal circuitry in enterocytes of GCC+/+ mice, in turn, increased the complement of CaRs at the cell surface (Figure 4). Effects of ST preconditioning on CaR staining at the surface of cancer cells were selective (supplementary Figure 2 is available at Carcinogenesis Online), independent of elevated Ca2+o but dependent upon cGMP signaling through CNG (Figure 4). Moreover, compared with GCC+/+ mice, CaR expression in brush-border membranes of GCC−/− enterocytes was uniformly attenuated along the entire rostrocaudal axis of the intestine in a GCC-specific fashion since the expression of a marker for absorptive cells, villin, remained unchanged in the same anatomical locations (Figure 4, in vivo panels showing representative sections from the jejunum). Thus, GCC signaling physiologically regulates posttranscriptional expression of CaRs in enterocytes, and ST preconditioning induces cell surface translocation of CaR receptors in human colon cancer cells.
Fig. 3.
ST induces CaR translocation in colon cancer cells. (A) Intracellular cGMP accumulation induced by ST (1 μM for 15 min) in T84 cells pretreated with ST (1 μM for 24 h) or the vehicle control in the presence of the indicated Ca2+o concentration. *P < 0.05 versus respective control conditions not pretreated with ST. (B) Time course of ST (1 μM) effects on cytosol (upper panel) or membrane (lower panel) CaR expression in T84 cells. Phosphate-buffered saline (PBS), the vehicle control; GAPDH and villin, the loading controls.
Fig. 4.
Ligand-dependent GCC signaling upregulates surface CaR receptors in normal and transformed intestinal epithelial cells. Membranes of non-permeabilized T84 cells (in vitro) were stained (brown) with specific rabbit polyclonal anti-CaR in the presence of low (0.5 mM; left column) or high (5 mM; right three columns) Ca2+o (magnification ×40). In blue, counterstaining of nuclei with hematoxylin. Tumor cells were treated for 24 h with phosphate-buffered saline (PBS) (vehicle control; upper row), ST (1 μM; middle row) or 8-br-cGMP (5 mM; lower row) in the absence (left two columns) or presence (right two columns) of the indicated CNG inhibitors (see Figure 1 legend for keys). Also, epithelial cells in villus tips from the jejunum of GCC+/+ and GCC−/− mice were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue, nuclei) and specific antibodies against villin (green, enterocyte brush-border membranes) and CaR (red) and subjected to confocal microscopy (magnification ×63).
ST preconditioning, with restoration of CaR signaling, is a novel cytostatic therapeutic strategy in colon cancer cells
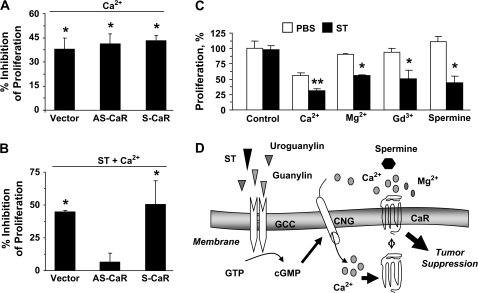
To explore whether posttranscriptional regulation of CaRs mediates Ca2+o-dependent antiproliferation induced by ST preconditioning, CaR expression in colon cancer cells was eliminated employing specific antisense constructs (30) (supplementary Figure 3 is available at Carcinogenesis Online; ∼70% inhibition of CaR expression by densitometry, normalized to the respective GAPDH, in CaR antisense-transfected cells compared with CaR sense controls). Whereas the response of tumor cells to Ca2+o was not altered by CaR antisense (Figure 5A), reflecting lack of effective CaR signaling in colon cancer cells, CaR antisense eliminated the response to Ca2+o of cancer cells preconditioned with ST (Figure 5B). Further, ST preconditioning of tumor cells produced a novel sensitivity of DNA synthesis to acute inhibition by 10 mM, but not 1 mM, Ca2+o (supplementary Figure 4 is available at Carcinogenesis Online), consistent with membrane translocation of low-affinity CaRs that typically sense 3–10 mM Ca2+o (34). Moreover, beyond Ca2+o, other CaR agonists, including Mg2+, Gd3+ and spermine, selectively inhibited proliferation of cancer cells preconditioned by ST (Figure 5C). Thus, ST preconditioning restores the sensitivity of human colon cancer cells to Ca2+o-dependent antiproliferation through CaRs, suggesting GCC-induced CaRs as previously unrecognized therapeutic targets for cytostatic strategies in patients with colorectal cancer.
Fig. 5.
CaR induced by ST is a novel therapeutic target in human colon cancer cells. (A and B) The proliferation of T84 cells stably expressing the empty vector (vector, control), the CaR antisense vector (AS-CaR) or the CaR sense vector (S-CaR) was examined. Cells were treated for 24 h with Ca2+o (5 mM) alone (A) or ST (1 μM) plus Ca2+o (5 mM) (B). Results are expressed as in Figure 1C2; the controls were low (0.5 mM; A) or high (5 mM; B) Ca2+o. *P < 0.05 versus parallel control conditions. (C) T84 cell proliferation exposed to CaR agonists was measured as the percentage of respective control incubations treated with 0.5 mM Ca2+o (first two columns on the left). CaR agonists were added to tumor incubations for 24 h in the presence of the vehicle control (PBS) or ST (1 μM), and include Ca2+, 10 mM; Mg2+, 20 mM; Gd3+, 100 μM and spermine, 100 μM. CaR agonists are used at concentrations that maximally induce CaR signaling (34,40). *P < 0.05 and **P < 0.01 versus respective control [phosphate-buffered saline (PBS)] conditions. (D) Proposed molecular mechanism for restoration of CaR signaling by GCC associated with colorectal cancer suppression (see Discussion for description).
Discussion
Enterotoxins STs (18 amino acid long) and endogenous paracrine hormones guanylin and uroguanylin (15/16 amino acid long) are structurally homologous peptides (35,36), which specifically bind to and activate GCC at enterocyte brush-border membranes inducing cGMP-dependent protein kinase-dependent ion channel currents (36). In this way, endogenous hormones regulate body fluid homeostasis, whereas STs, principle diarrheagenic agents maximally activating that pathway, permit enterotoxigenic bacteria to propagate in the environment (7,36–38). Of significance, GCC−/− mice exhibit increased proliferation, migration and apoptosis and decreased differentiation along the crypt–villus axis (14) and are more susceptible to intestinal tumorigenesis induced by azoxymethane or APC mutations (16). Moreover, expression of guanylin and uroguanylin is uniformly lost early in intestinal carcinogenesis (11–13), loss of guanylin expression resulted in crypt hyperproliferation (15) and administration of GCC ligands inhibits intestinal tumor formation in APCMin/+ mice (3) and cell cycle progression of colon cancer cells (9). Thus beyond fluid homeostasis, GCC controls proliferation and tumorigenesis in intestine.
Mechanisms mediating antiproliferation by GCC appear diverse. GCC signaling through unidentified effectors engages colonic cell quality control mechanisms assuring genomic integrity and tumor suppression (16). Also, GCC- and cGMP-dependent Ca2+ influx through CNGs induces colon cancer cell cytostasis by reducing nuclear rates of DNA synthesis (4,9). Here, a previously unappreciated mechanism contributing to tumor suppression by GCC is described (Figure 5D). ST preconditioning induces cGMP-mediated CNG signaling and synthesis and cell surface delivery of CaRs. Increased CaRs at the plasma membrane, in turn, provide new tumor-specific targets for antiproliferative signaling by CaR agonists, including Ca2+o, Mg2+, Gd3+ and spermine. Although mediated by the same proximal effector, Ca2+o influx through CNG, inhibition of DNA synthesis (4) and activation of CaR signaling (Figure 5D) represent two distinct, non-overlapping GCC-dependent antiproliferative mechanisms, distinguished by acute (minutes) (9) and chronic (≥3 h Figure 3B) kinetics, respectively. They may represent sequential temporal arms of an integrated antiproliferative strategy in which acute cytostasis, silenced by phosphodiesterase-dependent desensitization of cGMP signaling (10), is propagated by CaR-dependent signaling to ensure enduring colon tumor cytostasis imposed by GCC. Interestingly, CaR signaling also promotes phosphodiesterase-dependent hydrolysis of cGMP (39), a mechanism that may contribute to desensitize cGMP-mediated inhibition of DNA synthesis and represents a negative feedback loop for cGMP-induced CaR.
First discovered in parathyroid cells where it senses blood Ca2+ levels and regulates parathyroid hormone release (40), CaRs are expressed in many cell types, including osteoclasts, neurons and hematopoietic cells (23,41,42). In colonocytes, CaRs sense 1–10 mM Ca2+o in fecal water by interacting with the N-terminal extracellular domain, inducing intracellular signaling through heterotrimeric G proteins (23,43). One emerging function of CaRs in colonocytes is the regulation of proliferation and differentiation and its putative role as a tumor suppressor (22,24,44). Although the CaR gene is not lost or mutated in colon cancer (33), CaR expression is inexplicably reduced during disease progression (25,26,33). The present finding that GCC regulates the posttranscriptional expression of CaRs in normal and malignant intestinal cells offers a mechanistic explanation for that observation since GCC signaling is silenced early during colorectal tumorigenesis following loss of ligand expression (11–13). Accordingly, elimination of GCC signaling in mice abolished the expression of CaRs in enterocytes (Figure 4). Moreover, CaRs are primarily expressed in the apex and central regions of human crypts (26) where expression of endogenous GCC ligands and cGMP levels are greatest (45,46), suggesting that CaR signaling may be conditionally regulated along the crypt–villus axis by GCC- and cGMP-dependent mechanisms. In this model, the common abilities of CaRs and GCC to promote the transition from proliferation to differentiation and inhibit intestinal carcinogenesis may represent convergent, rather than parallel, signaling pathways. Finally, although they remain to be fully characterized, GCC effects on CaRs, including induction of translation, posttranslational processing or trafficking to cell membranes, represent a previously unrecognized mechanism of transcriptionally independent regulation of CaR surface expression and signaling (47).
Beyond CaR, Ca2+o opposes tumorigenesis by forming benign insoluble complexes with toxic ionized fatty acids and bile acids (48) and promoting cis-dimerization of E-cadherin molecules underlying cell–cell adhesion (49) and growth suppression (50). This latter mechanism, which occurs at Ca2+o > 0.5 mM (49) and induces the function of p27 (50), may explain, in part, the cytostatic effects of high Ca2+o in colon cancer cells not exposed to ST observed here. Indeed, these cells were not affected by CaR antisense delivery (Figure 5A) or application of CaR agonists (Figure 5C), indicating that in the absence of GCC activation CaRs are functionally silent in colon cancer cells. Loss of GCC (11–13) and CaR (25,26,33) signaling, in turn, may underlie the resistant phenotype of colon cancer cells to Ca2+o-induced cytostasis (20,31). These observations are significant since levels of 3–10 mM Ca2+o, required for significant CaR activation (34), are typically achieved in the fecal colonic water as a result of dietary Ca2+ intake, absorption and secretion (19,22). Moreover, allosteric CaR activators present in the intestinal lumen such as L-amino acids (23) may further increase the CaR sensitivity for Ca2+o. Importantly, CaRs sense other polyvalent cations (i.e. Gd3+, Mg2+, Ni2+ and polylysine), including polyamines (spermine, spermidine and putrescine) endogenously produced by colonic bacteria (23), suggesting that GCC-induced CaRs may subserve diverse tumor suppressor pathways in intestine.
Strategies for cancer control include chemoprevention and chemotherapy. In contrast to its endogenous ligands, GCC is universally overexpressed in colorectal tumors (51,52), and oral administration of GCC ligands is a novel targeted approach to prevent tumor initiation and arrest disease progression in patients (3,4,9). Limitations to these therapies include adverse effects associated with the use of diarrheagenic bacterial enterotoxins (7,37,38). Conversely, dietary Ca2+ supplementation has been proposed as a chemoprevention strategy against colon cancer (53). However, caution has been suggested based on the reduced efficacy of Ca2+o to inhibit intestinal cell proliferation following neoplastic transformation probably reflecting reduced CaR expression (33). In that context, dietary Ca2+o could potentially promote growth of Ca2+o-insensitive tumor cells while suppressing proliferation of normal adjacent cells, which retain Ca2+o sensitivity (22). The present observations that GCC preconditioning restores antitumorigenic CaR signaling in human colon carcinoma cells may offer a solution to the limitations of Ca2+o supplementation strategies. Oral Ca2+o therapy, in turn, may prevent adverse effects of GCC-targeted strategies because activation of CaR opposes secretory diarrhea by bacterial enterotoxins (39). Taken together, these data suggest that combinatorial therapies including dietary Ca2+o supplementation and GCC ligand replacement represent a previously unrecognized paradigm for the prevention and treatment of human colorectal cancer.
Supplementary material
Supplementary Figures 1–4 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (CA75123, CA95026); Targeted Diagnostic and Therapeutics to S.A.W.; Pennsylvania Department of Health and Prevent Cancer Foundation to G.M.P.; National Institutes of Health Institutional Training Award (5T32 CA09662) to W.J.L.
Supplementary Material
Acknowledgments
S.A.W. is the Samuel M.V. Hamilton Endowed Professor of Thomas Jefferson University. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
adenomatous polyposis coli
- Ca2+o
extracellular Ca2+
- CaR
calcium-sensing receptor
- CNG
cyclic nucleotide-gated
- GCC
guanylyl cyclase C
- ST
heat-stable enterotoxin
References
- 1.Jemal A, et al. Cancer statistics, 2004. CA Cancer J. Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Descriptive Epidemiology Group. GLOBOCAN 2002. Lion: International Agency for Research on Cancer; 2004. [Google Scholar]
- 3.Shailubhai K, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60:5151–5157. [PubMed] [Google Scholar]
- 4.Pitari GM, et al. Bacterial enterotoxins are associated with resistance to colon cancer. Proc. Natl Acad. Sci. USA. 2003;100:2695–2699. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz S, et al. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JM, et al. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978;271:755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- 7.Guarino A, et al. Small and large intestinal guanylate cyclase activity in children: effect of age and stimulation by Escherichia coli heat-stable enterotoxin. Pediatr. Res. 1987;21:551–555. doi: 10.1203/00006450-198706000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Giannella RA. Escherichia coli heat-stable enterotoxins, guanylins, and their receptors: what are they and what do they do? J. Lab. Clin. Med. 1995;125:173–181. [PubMed] [Google Scholar]
- 9.Pitari GM, et al. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc. Natl Acad. Sci. USA. 2001;98:7846–7851. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitari GM, et al. Interruption of homologous desensitization in cyclic guanosine 3′,5′-monophosphate signaling restores colon cancer cytostasis by bacterial enterotoxins. Cancer Res. 2005;65:11129–11135. doi: 10.1158/0008-5472.CAN-05-2381. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MB, et al. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab. Invest. 1998;78:101–108. [PubMed] [Google Scholar]
- 12.Notterman DA, et al. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124–3130. [PubMed] [Google Scholar]
- 13.Birkenkamp-Demtroder K, et al. Gene expression in colorectal cancer. Cancer Res. 2002;62:4352–4363. [PubMed] [Google Scholar]
- 14.Li P, et al. Homeostatic regulation of the crypt-to-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am. J. Pathol. 2007;171:1847–1858. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinbrecher KA, et al. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am. J. Pathol. 2002;161:2169–2178. doi: 10.1016/S0002-9440(10)64494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Lipkin M, et al. Calcium and the prevention of colon cancer. J. Cell. Biochem. 1995;22(suppl):65–73. doi: 10.1002/jcb.240590810. [DOI] [PubMed] [Google Scholar]
- 18.Lipkin M. Preclinical and early human studies of calcium and colon cancer prevention. Ann. N. Y. Acad. Sci. 1999;889:120–127. doi: 10.1111/j.1749-6632.1999.tb08729.x. [DOI] [PubMed] [Google Scholar]
- 19.Whitfield JF. Calcium signals and cancer. Crit. Rev. Oncog. 1992;3:55–90. [PubMed] [Google Scholar]
- 20.Buras RR, et al. The effect of extracellular calcium on colonocytes: evidence for differential responsiveness based upon degree of cell differentiation. Cell Prolif. 1995;28:245–262. doi: 10.1111/j.1365-2184.1995.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Newmark HL, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield JF, et al. Calcium-cell cycle regulator, differentiator, killer, chemopreventor, and maybe, tumor promoter. J. Cell. Biochem. 1995;22(suppl):74–91. [PubMed] [Google Scholar]
- 23.Hofer AM, et al. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 24.Kallay E, et al. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect. Prev. 2000;24:127–136. [PubMed] [Google Scholar]
- 25.Chakrabarty S, et al. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 26.Chakrabarty S, et al. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3) Cancer Res. 2005;65:493–498. [PubMed] [Google Scholar]
- 27.Weiss H, et al. Inhibition of store-operated calcium entry contributes to the anti-proliferative effect of non-steroidal anti-inflammatory drugs in human colon cancer cells. Int. J. Cancer. 2001;92:877–882. doi: 10.1002/ijc.1280. [DOI] [PubMed] [Google Scholar]
- 28.Kazerounian S, et al. Proliferative signaling by store-operated calcium channels opposes colon cancer cell cytostasis induced by bacterial enterotoxins. J. Pharmacol. Exp. Ther. 2005;314:1013–1022. doi: 10.1124/jpet.105.089052. [DOI] [PubMed] [Google Scholar]
- 29.Schulz S, et al. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J. Clin. Invest. 1997;100:1590–1595. doi: 10.1172/JCI119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamauchi M, et al. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol. Endocrinol. Metab. 2005;288:E608–E616. doi: 10.1152/ajpendo.00229.2004. [DOI] [PubMed] [Google Scholar]
- 31.Buset M, et al. Inhibition of human colonic epithelial cell proliferation in vivo and in vitro by calcium. Cancer Res. 1986;46:5426–5430. [PubMed] [Google Scholar]
- 32.Waldman SA, et al. Heterogeneity of guanylyl cyclase C expressed by human colorectal cancer cell lines in vitro. Cancer Epidemiol. Biomarkers Prev. 1998;7:505–514. [PubMed] [Google Scholar]
- 33.Kallay E, et al. Molecular and functional characterization of the extracellular calcium-sensing receptor in human colon cancer cells. Oncol. Res. 2003;13:551–559. doi: 10.3727/000000003108748072. [DOI] [PubMed] [Google Scholar]
- 34.Garrett JE, et al. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J. Biol. Chem. 1995;270:12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 35.Forte LR. Guanylin regulatory peptides: structures, biological activities mediated by cyclic GMP and pathobiology. Regul. Pept. 1999;81:25–39. doi: 10.1016/s0167-0115(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 36.Lucas KA, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 37.Acosta-Martinez F, et al. Escherichia coli heat-stable enterotoxin in feces and intestines of calves with diarrhea. Am. J. Vet. Res. 1980;41:1143–1149. [PubMed] [Google Scholar]
- 38.Haberberger RL, Jr, et al. Enteritis due to multiresistant enteroadherent Escherichia coli. Lancet. 1991;337:235–236. doi: 10.1016/0140-6736(91)92197-a. [DOI] [PubMed] [Google Scholar]
- 39.Geibel J, et al. Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc. Natl Acad. Sci. USA. 2006;103:9390–9397. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown EM, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 41.House MG, et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J. Bone Miner. Res. 1997;12:1959–1970. doi: 10.1359/jbmr.1997.12.12.1959. [DOI] [PubMed] [Google Scholar]
- 42.Chattopadhyay N, et al. Cellular “sensing” of extracellular calcium (Ca(2+)(o)): emerging roles in regulating diverse physiological functions. Cell. Signal. 2000;12:361–366. doi: 10.1016/s0898-6568(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 43.Gama L, et al. Ca2+-sensing receptors in intestinal epithelium. Am. J. Physiol. 1997;273:C1168–C1175. doi: 10.1152/ajpcell.1997.273.4.C1168. [DOI] [PubMed] [Google Scholar]
- 44.Lamprecht SA, et al. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat. Rev. Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 45.Cohen MB, et al. Immunohistochemical localization of guanylin in the rat small intestine and colon. Biochem. Biophys. Res. Commun. 1995;209:803–808. doi: 10.1006/bbrc.1995.1571. [DOI] [PubMed] [Google Scholar]
- 46.Whitaker TL, et al. Uroguanylin and guanylin: distinct but overlapping patterns of messenger RNA expression in mouse intestine. Gastroenterology. 1997;113:1000–1006. doi: 10.1016/s0016-5085(97)70197-5. [DOI] [PubMed] [Google Scholar]
- 47.Bouschet T, et al. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J. Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newmark HL, et al. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J. Natl Cancer Inst. 1984;72:1323–1325. [PubMed] [Google Scholar]
- 49.Pertz O, et al. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.St Croix B, et al. E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J. Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witek ME, et al. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin. Cancer Res. 2005;11:8549–8556. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 52.Schulz S, et al. A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase C in patients with colorectal cancer. Clin. Cancer Res. 2006;12:4545–4552. doi: 10.1158/1078-0432.CCR-06-0865. [DOI] [PubMed] [Google Scholar]
- 53.Cho E, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J. Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.