Abstract
Hexavalent chromium [Cr(VI)] compounds are redox cycling environmental carcinogens that induce apoptosis as the primary mode of cell death. Defects in apoptosis regulatory mechanisms contribute to carcinogenesis induced by Cr(VI). Activation of apoptosis signaling pathways is tightly linked with the generation of reactive oxygen species (ROS). Likewise, ROS have been implicated in the regulation of Cr(VI)-induced apoptosis and carcinogenicity; however, its role in Cr(VI)-induced apoptosis and the underlying mechanism are largely unknown. We report that ROS, specifically superoxide anion (), mediates Cr(VI)-induced apoptosis of human lung epithelial H460 cells. H460 ρ0 cells that lack mitochondrial DNA demonstrated a significant decrease in ROS production and apoptotic response to Cr(VI), indicating the involvement of mitochondrial ROS in Cr(VI)-induced apoptosis. In agreement with this observation, we found that Cr(VI) induces apoptosis mainly through the mitochondrial death pathway via caspase-9 activation, which is negatively regulated by the antiapoptotic protein Bcl-2. Furthermore, induced apoptosis in response to Cr(VI) exposure by downregulating and degrading Bcl-2 protein through the ubiquitin–proteasomal pathway. This study reveals a novel mechanism linking with Bcl-2 stability and provides a new dimension to ROS-mediated Bcl-2 downregulation and apoptosis induction.
Introduction
Several metals, including chromium, nickel, cobalt, cadmium and silica, have been classified as human carcinogens (1). Although the mechanisms of their carcinogenesis are not clear yet, it is generally believed that generation of reactive oxygen species (ROS) and abnormal regulation of apoptosis play a critical role in neoplastic development in response to these metals. We used hexavalent chromium [Cr(VI)] as a representative metal carcinogen in our study. Cr(VI) compounds have been classified as group I human carcinogens by the International Agency of Research in Cancer in 1990 (2). For more than a century, exposure to Cr(VI) compounds has been associated with the induction of lung cancer in workers employed in stainless steel welding, chrome plating, electroplating, leather tanneries and pigment manufacturing (3–5). Non-occupational exposure to Cr(VI) compounds occurs from cigarette smoke, automobile emissions, areas of landfills and hazardous waste disposal sites (3,6). Upon inhalation, chromate particles dissolve to form soluble Cr(VI) anions that enter cells through non-specific anionic transporters and are metabolically reduced to their lower oxidation states such as Cr(V), Cr(IV) and Cr(III) by cellular reducing agents including glutathione and ascorbate (7,8). During this process, molecular oxygen is reduced to superoxide anion (), which is further converted to hydrogen peroxide (H2O2) via dismutation. The resultant intermediates reacts with H2O2 to generate hydroxyl radicals via a Haber–Weiss- or Fenton-like reaction (9). Thus, during the one-electron reduction of Cr(VI), along with the reduced intermediates, a whole spectrum of ROS is generated that causes diverse cytotoxic and genotoxic effects (9). For instance, Cr(VI) has been shown to induce chromosomal aberrations, mutations, transformations in cultured mammalian cells (10,11) and a variety of DNA lesions such as strand breaks, DNA protein cross-links and DNA base modification leading to cell death via apoptosis (12–14).
Apoptosis is a tightly regulated process characterized by shrinkage of the nucleus, blebbing of membranes and condensation or fragmentation of chromatin (15). Caspases are central regulators of the two major apoptosis signaling pathways, viz., the extrinsic or death receptor pathway and the intrinsic or the mitochondrial pathway. Caspase-8 and caspase-9 are the key initiator caspases of the extrinsic and the intrinsic pathway, respectively, that cleave and activate downstream effector caspases such as caspase-3 leading to apoptosis (16,17). Activated caspase-8 can also stimulate apoptosis via cleavage and activation of cytosolic BH3 interacting domain death agonist (a Bcl-2 family protein) (18–20). The active fragment or truncated BH3 interacting domain death agonist translocates to the mitochondria, inducing cytochrome c release, which sequentially activates caspases-9 and -3 leading to cell death by apoptosis. The proto-oncogene Bcl-2 is one of the major regulators of the mitochondrial apoptotic pathway and its expression levels regulate the antiapoptotic function of Bcl-2 protein. Bcl-2 resides in the outer mitochondrial wall and regulates apoptosis by controlling mitochondrial permeability and the release of cytochrome c, thus, inhibiting apoptosis (21,22). Additionally, in vitro and in vivo studies suggest that Bcl-2 can also block apoptosis through regulation of cellular antioxidant defense mechanisms or by suppressing production of free radicals thus acting as an antioxidant (23–25). The stability and expression levels of Bcl-2 protein can be regulated by different reactive species including nitric oxide and H2O2 through various mechanisms including dimerization, phosphorylation, degradation and posttranslational modification (26–28). Therefore, ROS can mediate apoptosis by regulating the expression of various apoptosis regulatory proteins including Bcl-2. Even though evidences implicate ROS in the regulation of Cr(VI)-induced apoptosis and carcinogenicity (29–31), the specific ROS involved, its cellular source and relationship with the well-studied caspase activation has not been explored.
The objective of this study was to evaluate the role of ROS and characterize the signaling pathways mediated by ROS in Cr(VI)-induced apoptosis. We report that generation of ROS induces apoptotic cell death in response to Cr(VI) exposure. was found to be the key player in Cr(VI)-induced apoptosis that exerted its proapoptotic effect by causing downregulation and degradation of Bcl-2 protein through the ubiquitin–proteasomal pathway, thus inducing apoptosis through the mitochondrial pathway. Furthermore, overexpression of Bcl-2 significantly inhibited Cr(VI)-induced apoptosis. Therefore, we reveal a novel antioxidant mechanism involving Bcl-2-mediated inhibition of by which the protein exerts its antiapoptotic effect. This study forms the basis of the mechanisms involved in the development of apoptosis-resistant phenotype in response to Cr(VI) and can be exploited further to understand the molecular mechanisms involved in general metal carcinogenesis.
Materials and methods
Chemicals and reagents
N-acetyl cysteine, 6-anilinoquinoline-5,8-quinone (LY83583), H2O2, xanthine, xanthine oxidase, rotenone (ROT), diphenylene iodonium (DPI), lactacystin (LAC), ethidium bromide, sodium pyruvate, uridine and sodium dichromate (Na2Cr2O7.2H2O) [Cr(VI)] were obtained from Sigma Chemical (St Louis, MO). Caspase substrates IETD amino-4-methylcoumarin (AMC) and LEHD-AMC, caspase inhibitors IETD-CHO and LEHD-CHO and pan-caspase inhibitor (zVAD.FMK) were from Alexis Biochemicals (San Diego, CA). Cell-permeable superoxide dismutase (SOD) mimetic, Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), was purchased from Calbiochem (La Jolla, CA) and catalase was from Roche Diagnostics (Indianapolis, IN). The oxidative probes, dichlorofluorescein (DCF) diacetate and dihydroethidium (DHE), and the apoptosis dye Hoechst 33342 were from Molecular Probes (Eugene, OR). Antibodies for Bcl-2, peroxidase-labeled secondary antibodies, anti-myc agarose beads and protein A-agarose were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for ubiquitin and β-actin were from Sigma (St Louis, MO) and the transfecting agent Lipofectamine 2000 was from Invitrogen (Carlsbad, CA).
Cell culture
The human lung epithelial cancer cell line NCI-H460 was obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 medium (Sigma) containing 5% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin in a 5% CO2 environment at 37°C. Cells were passaged at preconfluent densities using a solution containing 0.05% trypsin and 0.5 mM ethylenediaminetetraacetic acid (Invitrogen).
Derivation of ρ0 cells
H460 ρ0 cells were prepared as described previously (32). NCI-H460 cells were cultured in the presence of ethidium bromide (100 ng/ml) to inhibit mitochondrial DNA replication for >20 generations until use. ρ0 cells were maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml uridine and 100 μg/ml pyruvate in a 5% CO2 environment at 37°C.
Caspase assay
Caspase activity was determined by fluorometric assay using the enzyme substrate IETD-AMC for caspase-8 and LEHD-AMC for caspase-9, which is specifically cleaved by the respective enzymes at the Asp residue to release the fluorescent group, AMC. Cell extracts containing 20 μg of protein were incubated with 100 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid containing 10% sucrose, 10 mM dithiothreitol, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate and 50 μM caspase substrate in a total reaction volume of 0.25 ml. The reaction mixture was incubated for 60 min at 37°C and quantified fluorometrically at the excitation and emission wavelengths of 380 and 460 nm, respectively, using RF5301PC spectrofluorometer (Shimadzu, Kyoto, Japan).
Apoptosis assay
After specific treatments, apoptosis was determined by incubating the cells with 10 μg/ml Hoechst 33342 nuclear stain for 30 min at 37°C and scoring the percentage of cells having intensely condensed chromatin and/or fragmented nuclei by fluorescence microscopy (Axiovert 100; Carl Zeiss, Gottingen, Germany) using Pixera software.
ROS detection
Intracellular peroxide and production was determined by flow cytometry using DCF diacetate and DHE, respectively. Cells (1 × 106/ml) were incubated with the fluorescent probes (10 μM) for 30 min at 37°C, after which the cells were washed, resuspended in phosphate-buffered saline and analyzed for DCF (494/519 nm) and DHE fluorescence (535/617 nm) using FACS Calibur (Becton-Dickinson, Rutherford, NJ). The median fluorescence intensity was quantitated by CellQuest software (Becton-Dickinson) analysis of the recorded histograms.
Plasmids and stable transfection
Stable transfectants of Bcl-2, glutathione peroxidase (GPx) and SOD were generated by culturing H460 cells in six-well plates until they reached 80% confluence. One microgram of cytomegalovirus-neo vector and 15 μl of Lipofectamine 2000 reagent with 2 μg of Bcl-2, GPx, SOD1 or control pcDNA3 plasmid were used to transfect the cells in the absence of serum. After 10 h, the medium was replaced with culture medium containing 5% fetal bovine serum. Approximately 36 h after the beginning of the transfection, the cells were digested with 0.03% trypsin, and the cell suspensions were plated onto 75 ml culture flasks and cultured for 24–28 days with G418 selection (400 μg/ml). Stable transfectants were identified by western blot analysis and were cultured in G418-free RPMI 1640 medium for at least two passages before each experiment.
Western blotting
After specific treatments, cells were incubated in lysis buffer containing 20 mM Tris–HCl (pH 7.5), 1% Triton X-100, 150 mM NaCl, 10% glycerol, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 100 mM phenylmethylsulfonyl fluoride and a protease inhibitor mixture (Roche Molecular Biochemicals, Basel, Switzerland) for 20 min on ice. After insoluble debris was precipitated by centrifugation at 14 000g for 15 min at 4°C, the supernatants were collected and assayed for protein content using bicinchoninic acid method (Pierce Biotechnology, Rockford, IL). Equal amount of protein per sample (15 μg) was resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a 0.45 μm nitrocellulose membrane (Pierce Biotechnology). The transferred membranes were blocked for 1 h in 5% non-fat dry milk in tris buffered saline with tween 20 (25 mM Tris–HCl pH 7.4, 125 mM NaCl and 0.05% Tween-20) and incubated with the appropriate primary antibodies and horseradish peroxidase-conjugated isotype-specific secondary antibodies. The immune complexes were detected by chemiluminescence (Supersignal® West Pico, Pierce) and quantified by imaging densitometry, using UN-SCAN-IT automated digitizing software (Silk Scientific Corp., Orem, UT). Mean densitometry data from independent experiments were normalized to the control. The data were presented as mean ± SD and analyzed by Student’s t-test.
Immunoprecipitation
Stable H460 cells overexpressing Bcl-2 were washed after treatments with ice-cold phosphate-buffered saline and lysed in lysis buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 0.2% NP40, 100 mM phenylmethylsulfonyl fluoride and a commercial protease inhibitor mixture) at 4°C for 20 min. After centrifugation at 14000g for 15 min at 4°C, the supernatants were collected and protein content was determined by bicinchoninic acid protein assay. Cleared lysates were normalized and 60 μg proteins were incubated with 8 μl of anti-myc agarose bead (Santa Cruz Biotechnology) diluted with 12 μl protein A-agarose for 4 h at 4°C. The immune complexes were washed three times with 500 μl lysis buffer, resuspended in 2× Laemmli sample buffer and boiled at 95°C for 5 min. The immune complexes were separated by 10% dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by western blot as described above.
Results
Effect of Cr(VI) and ROS modulators on cellular ROS levels
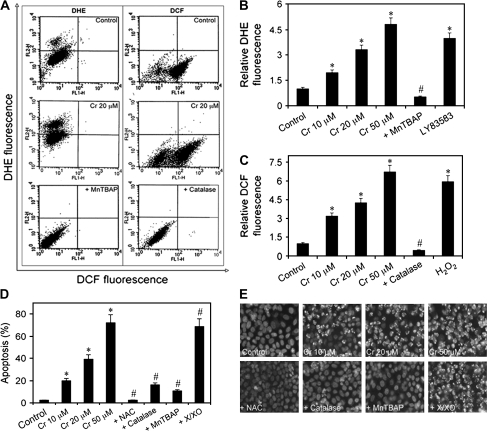
In the cellular system, Cr(VI) is metabolically reduced to its lower oxidation states, generating ROS in this process (7,8,33). We first quantified the induction of cellular ROS production in response to Cr(VI) exposure by flow cytometry in treated human lung epithelial H460 cells using the fluorescent probes DCF and DHE, which detect hydroperoxide and , respectively. Cr(VI)-induced hydroperoxide and production in a dose-dependent manner, as indicated by the increase in DCF and DHE fluorescence intensities and ROS modulators used in combination with Cr(VI) verified these results (Figure 1 A–C). DHE signal was increased by Cr(VI) and LY83583 (donor) and inhibited by the addition of the SOD mimetic MnTBAP ( scavenger). Similarly, DCF signal was increased by Cr(VI) and H2O2 and was inhibited by catalase (H2O2 scavenger).
Fig. 1.
Effects of Cr(VI) and ROS modulators on ROS levels and apoptosis in human lung epithelial H460 cells. (A, B and C) Subconfluent (90%) monolayers of H460 cells were treated with varying doses of Cr(VI) (0–50 μM) or were pretreated with MnTBAP (100 μM) or catalase (1000 U/μl) for 0.5 h followed by Cr(VI) (20 μM) treatment and were analyzed for and H2O2 production by flow cytometry using DHE and DCF diacetate fluorescent probes, respectively. Plots show relative DHE or DCF fluorescence intensity over non-treated control determined at the peak response time of 1 h after Cr(VI) treatment. LY83583 (10 μM) and H2O2 (0.1 mM) were used as positive controls for DHE and DCF measurements, respectively. (D) Cells were exposed to Cr(VI) (0–50 μM) for 12 h or pretreated with MnTBAP (100 μM), catalase (1000 U/μl), N-acetyl cysteine (NAC) (10 mM) or xanthine/xanthine oxidase (X/XO) (1 μM/2 mU/ml) for 1 h followed by Cr(VI) (20 μM) treatment for 12 h and analyzed for apoptosis by Hoechst 33342 assay. (E) Fluorescence micrographs of treated cells stained with the Hoechst dye. Apoptotic cells exhibited shrunken and fragmented nuclei with bright nuclear fluorescence. Values are mean ± SD (n ≥ 3). *P < 0.05 versus non-treated control, #P < 0.05 versus 20 μM Cr(VI)-treated control.
ROS regulates Cr(VI)-induced apoptosis
Cr(VI) compounds induce apoptosis as the primary mode of cell death (34). Therefore, we characterized the apoptotic response to Cr(VI) treatment in human lung epithelial H460 cells. Cells were treated with different doses of Cr(VI) (0–50 μM), and apoptosis was determined after 12 h by Hoechst 33342 assay. Cr(VI) treatment caused a dose-dependent increase in cell apoptosis over control (Figure 1D and E). To test whether the apoptosis-inducing effect of Cr(VI) is associated with ROS production, cells were treated with Cr(VI) in the presence or absence of various ROS modulators, including N-acetyl cysteine (a general antioxidant), catalase (H2O2 scavenger), MnTBAP ( scavenger) and xanthine/xanthine oxidase ( donor), and apoptosis was determined by Hoechst assay. All the tested antioxidants inhibited apoptosis induced by Cr(VI) (Figure 1D and E), indicating that multiple ROS are involved in the apoptotic process. The potent inhibitory effects of catalase and MnTBAP further indicate that H2O2 and play an important role in the process.
GPx and SOD overexpression inhibits Cr(VI)-induced apoptosis and ROS generation
To confirm the role of ROS in Cr(VI)-induced apoptosis, cells were stably transfected with antioxidant enzymes GPx, SOD or control plasmid (Cont), and their effects on ROS generation and apoptosis were determined. Enzyme overexpression was confirmed by western blot analysis. Transfected cells showed an increase in antioxidant enzyme expression as compared with the mock-transfected control (Figure 2A). Apoptosis assay showed a decrease in apoptosis in GPx- and SOD-transfected cells as compared with the mock-transfected cells (Figure 2B). The effect was more prominent in cells overexpressing SOD, with a dose as high as 100 μM of Cr(VI) did not induce apoptosis. This suggested that although H2O2 is involved in Cr(VI)-induced apoptosis, may be the major regulator of Cr(VI)-induced apoptosis. Flow cytometric analysis showed a substantial reduction in Cr(VI)-induced peroxide generation in GPx-transfected cells and production in SOD-transfected cells as compared with mock-transfected cells verifying the specificity of the stable cells (Figure 2C and D).
Fig. 2.
Effects of GPx and SOD overexpression on Cr(VI)-induced apoptosis and ROS generation. (A) H460 cells were stably transfected with GPx, SOD or control pcDNA3 plasmid (Cont) as described under Materials and Methods. Cell lysates were analyzed for GPx and SOD expression by western blotting. β-Actin was used as a loading control. (B) Transfected cells were treated with Cr(VI) (1–100 μM) for 12 h and analyzed for apoptosis by Hoechst 33342 assay. (C) GPx- and mock-transfected cells were treated with Cr(VI) (0–100 μM) and analyzed for DCF fluorescence at 1 h posttreatment. (D) SOD- and mock-transfected cells were treated with Cr(VI) (0–100 μM) and analyzed for DHE fluorescence at 1 h posttreatment. Plots show relative fluorescence intensity over non-treated control. Values are mean ± SD (n ≥ 3).
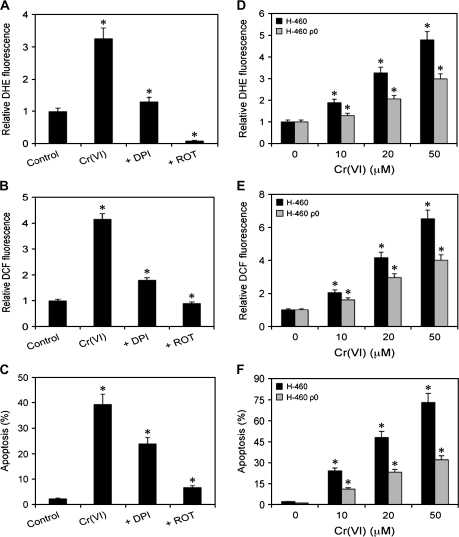
Mitochondrial ROS may be responsible for Cr(VI)-induced apoptosis
To determine the cellular source of ROS involved in Cr(VI)-induced apoptosis, cells were treated with Cr(VI) in the presence or absence of DPI or ROT, and their effects on apoptosis and ROS generation were examined. DPI and ROT are widely used in the literature as the specific indicators of cytosolic and mitochondrial ROS, respectively, since DPI is a specific inhibitor of reduced nicotinamide adenine dinucleotide phosphate oxidase (35,36), and ROT is a mitochondrial electron transport chain interrupter (36,37). The results show that both DPI and ROT inhibited Cr(VI)-induced ROS generation and apoptosis, with the effect of ROT being more dominant (Figure 3A–C). These results indicate that even though cytosolic ROS is involved in Cr(VI)-induced apoptosis, mitochondria may be the major source of ROS involved in the apoptotic process. To further confirm the role of mitochondrial ROS in Cr(VI)-induced apoptosis, we used H460 ρ0 cells that have dysfunctional mitochondrial electron transport and are less capable of producing mitochondrial ROS, and compared the effect of Cr(VI) on ROS production and apoptosis in H460 and H460 ρ0 cells. It was observed that the induction of and hydroperoxide by Cr(VI) in H460 cells was significantly decreased in a dose-dependent manner in H460 ρ0 cells as assessed by DHE and DCF fluorescence (Figure 3D and E). Further, Cr(VI)-induced apoptosis was reduced by half in H460 ρ0 cells as compared with H460 cells (Figure 3F), indicating that mitochondrial functional electron transport is important in this process.
Fig. 3.
Cellular source of Cr(VI)-induced ROS generation. (A and B) Subconfluent (90%) monolayers of H460 cells were either left untreated or pretreated with 5 μM of DPI or ROT for 0.5 h, followed by Cr(VI) treatment (20 μM) and analyzed for DHE and DCF fluorescence intensities after 1 h. (C) Cells were similarly treated with Cr(VI) and DPI or ROT and analyzed for apoptosis after 12 h by Hoechst assay. (D and E) H460 and H460 ρ0 cells were treated with Cr(VI) (0–50 μM) for 1 h and analyzed for DHE and DCF fluorescence intensities. Plots show relative fluorescence intensity over non-treated control. (F) Cells were similarly treated and analyzed for apoptosis after 12 h. Values are mean ± SD (n ≥ 3). *P < 0.05 versus non-treated control.
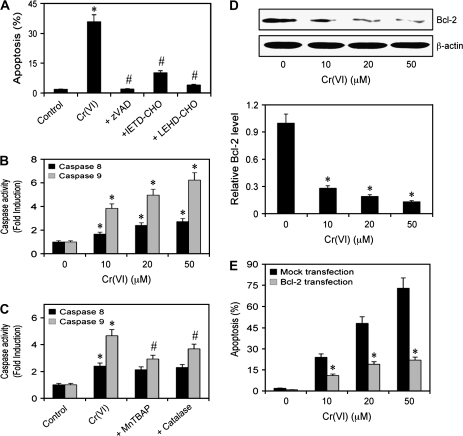
Caspase activation in response to Cr(VI) exposure
To characterize the signaling pathways involved in Cr(VI)-induced apoptosis, H460 cells were treated with zVAD.FMK (pan-caspase inhibitor), IETD-CHO (caspase-8 inhibitor) or LEHD-CHO (caspase-9 inhibitor) along with Cr(VI). Figure 4A shows that the pan-caspase inhibitor blocked Cr(VI)-induced apoptosis reducing it to control levels, indicating that Cr(VI) induces apoptosis through the classical caspase-dependent pathways. Both caspase-8 and -9 inhibitors significantly blocked Cr(VI)-induced apoptosis; however, the effect of caspase-9 inhibitor was more pronounced. This was further verified by caspase activity assay, which showed that Cr(VI) induced both caspase-8 and caspase-9 activation with the latter effect being more dominant (Figure 4B). Since caspase-8 and caspase-9 are the key marker proteins of the death receptor and mitochondrial pathway, respectively, this suggests that the mitochondrial pathway is the major apoptotic pathway induced in response to Cr(VI) exposure. Additionally, to establish a correlation between Cr(VI)-induced ROS and apoptosis, we tested the effect of ROS scavengers, catalase and MnTBAP on caspase-8 and -9 activity. We observed that the antioxidants significantly blocked Cr(VI)-induced caspase-9 activation but had minimal effect on caspase-8 activation (Figure 4C). These observations confirmed our earlier data that mitochondria play a critical role in Cr(VI)-induced apoptosis.
Fig. 4.
Caspase activation and Bcl-2 expression in response to Cr(VI). (A) Subconfluent (90%) monolayer of H460 cells were either left untreated or pretreated with zVAD-FMK (10 μM), IETD-CHO (2 μM) or LEHD-CHO (2 μM) for 1 h followed by Cr(VI) treatment (20 μM) and analyzed for apoptosis after 12 h. (B) Caspase activity assays of cells treated with Cr(VI) (0–50 μM) for 12 h. Cell lysates (20 μg protein) were prepared and analyzed for caspase-8 and -9 activity using specific fluorescent substrates IETD-AMC and LEHD-AMC, respectively. (C) Subconfluent (90%) monolayer of H460 cells were either left untreated or pretreated with MnTBAP (100 μM) or catalase (1000 U/ml) for 1 h followed by Cr(VI) treatment (20 μM) for 12 h. Caspase activity was measured as mentioned above. (D) Dose effect of Cr(VI) on Bcl-2 expression. Cells were treated with varying doses of Cr(VI) (0–50 μM) for 12 h and cell lysates were prepared and analyzed for Bcl-2 expression by western blotting. Blots were reprobed with β-actin antibody to confirm equal loading of samples. Immunoblot signals were quantified by densitometry and mean data from independent experiments were normalized to the result obtained in non-treated cells (control). (E) H460 cells were stably transfected with Bcl-2 or control pcDNA3 plasmid. Transfected cells were treated with Cr(VI) (0–50 μM) for 12 h and analyzed for apoptosis. Plots are mean ± SD (n = 4). *P < 0.05 versus non-treated or mock-transfected controls. #P < 0.05 versus 20 μM Cr(VI)-treated control.
Bcl-2 expression levels in response to Cr(VI) exposure
To provide a mechanistic insight into the regulation of Cr(VI)-induced apoptosis, we determined the expression level of Bcl-2, a key antiapoptotic protein of the mitochondrial death pathway, in response to Cr(VI) treatment by western blotting. Figure 4D shows that treatment of the cells with Cr(VI) caused a dose-dependent decrease in Bcl-2 expression level. To confirm the role of Bcl-2 in Cr(VI)-induced apoptosis, we stably transfected H460 cells with Bcl-2 or control plasmid and determined apoptosis in response to Cr(VI) by Hoechst 33342 assay. Figure 4E shows that overexpression of Bcl-2 significantly inhibited Cr(VI)-induced apoptosis over a wide concentration range as compared with the vector-transfected control. These results indicate the role of Bcl-2 as a negative regulator of Cr(VI)-induced apoptosis and further support the role of the mitochondrial death pathway in Cr(VI)-induced cell death.
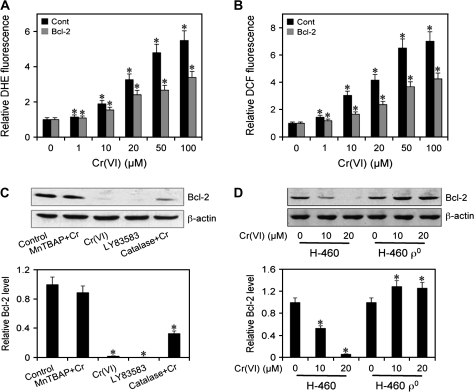
Correlation between ROS and Bcl-2
H460 cells overexpressing Bcl-2 or control plasmid (Cont) were treated with varying doses of Cr(VI) (0–100 μM) and ROS generation was determined. Figure 5A and B shows that overexpression of Bcl-2 significantly inhibited Cr(VI)-induced ROS generation, as assessed by relative DCF and DHE fluorescence in comparison with vector-transfected control. This result indicates a direct correlation between Bcl-2 and ROS as well as an antioxidant role of Bcl-2 in Cr(VI)-induced apoptosis. Further, cells were treated with Cr(VI) in the presence or absence of ROS modulators, and their effect on Bcl-2 expression was determined. Figure 5C shows that treatment of the cells with MnTBAP ( scavenger) strongly inhibited Cr(VI)-induced Bcl-2 downregulation, whereas catalase (H2O2 inhibitor) treatment showed weak inhibitory effect. LY83583 ( donor) downregulated Bcl-2 independently confirming the role of in Bcl-2 downregulation. Analysis of Bcl-2 expression in H460 ρ0 cells further shows that unlike in H460 cells, Cr(VI) was unable to downregulate the Bcl-2 expression (Figure 5D), indicating that mitochondrial ROS may play a key role in this process.
Fig. 5.
Correlation between Cr(VI)-induced ROS generation and Bcl-2 expression. (A) and (B) H460 cells were stably transfected with Bcl-2 or control pcDNA3 plasmid. Transfected cells were treated with Cr(VI) (0–100 μM) for 1 h and analyzed for DHE and DCF fluorescence intensities. Plots show relative fluorescence intensity over non-treated control. (C) H460 cells were either left untreated or pretreated with MnTBAP (100 μM) or catalase (1000 U/μl) for 1 h and then treated with Cr(VI) (20 μM) for 12 h. Cells were also treated with LY83583 (10 μM) for 12 h as a positive control. Cell lysates were prepared and analyzed for Bcl-2 expression by western blotting. (D) H460 and H460 ρ0 cells were treated with Cr(VI) (10 and 20 μM) for 12 h and Bcl-2 expression was determined. Blots were reprobed with β-actin antibody to confirm equal loading of samples. Densitometry was performed to determine the relative expression of Bcl-2 in treated cells compared with non-treated cells. Plots are mean ± SD (n = 4). *P < 0.05 versus non-treated control.
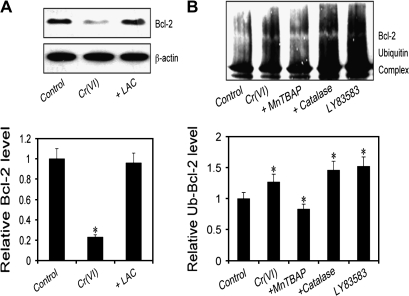
Superoxide mediates Cr(VI)-induced ubiquitination of Bcl-2
Previous studies have shown that Bcl-2 is downregulated primarily through the proteasomal degradation pathway (26–28). We tested whether this pathway is involved in the downregulation of Bcl-2 by Cr(VI). Cells were treated with Cr(VI) in the presence or absence of LAC, a highly specific proteasome inhibitor, and its effect on Bcl-2 expression was determined. Figure 6A shows that LAC completely inhibited Bcl-2 downregulation induced by Cr(VI), indicating that proteasomal degradation is a key mechanism involved in Cr(VI)-induced Bcl-2 downregulation. We further analyzed the effect of ROS on Cr(VI)-induced Bcl-2 ubiquitination by immunoprecipitation. Cells overexpressing myc-Bcl-2 were treated with Cr(VI) in the presence or absence of ROS modulators. Cell lysates were prepared and immunoprecipitated using anti-myc antibody. The resulting immune complexes were analyzed for ubiquitination by western blot using anti-ubiquitin antibody. The results show that Cr(VI)-induced ubiquitination of Bcl-2 and MnTBAP ( scavenger) inhibited this effect, whereas catalase (H2O2 inhibitor) had no inhibitory effect (Figure 6B), LY83583 ( donor) also induced Bcl-2 ubiquitination, supporting the role of as a primary ROS involved in Cr(VI)-induced Bcl-2 degradation via the ubiquitination pathway.
Fig. 6.
Effect of ROS on ubiquitination of Bcl-2. (A) H460 cells were either left untreated or pretreated with LAC (10 μM) for 0.5 h, followed by Cr(VI) treatment (20 μM) for 12 h. Cell lysates were prepared and analyzed for Bcl-2 expression by western blotting. (B) H460 cells stably transfected with myc-Bcl-2 were pretreated with MnTBAP (100 μM) or catalase (1000 U/μl) for 1 h and then treated with Cr(VI) (20 μM) for 3 h in the presence of LAC (10 μM) to prevent proteasome-mediated Bcl-2 degradation. Cells were also treated with LY83583 (10 μM) as a positive control. Cell lysates were immunoprecipitated with anti-myc antibody and the immune complexes were analyzed for ubiquitin by western blotting. Analysis of ubiquitin was performed at 3 h post-Cr(VI) treatment where ubiquitination was found to be maximal. Densitometry was performed to determine the relative expression of Bcl-2 in treated cells compared with non-treated cells. Data are mean ± SD. (n = 4). *P < 0.05 versus non-treated control.
Discussion
ROS produced during Cr(VI) exposure plays an important role in its cytotoxicity (30,31). In the present study, we confirmed the role of ROS in Cr(VI)-induced apoptosis of human lung cancer epithelial H460 cells and identified the underlying mechanism. Depending on various factors including the cell line, dose and exposure time, Cr(VI)-treated cells exhibit apoptotic features. For H460 cells, Cr(VI) treatment ranging from 1–100 μM for 12 h induced apoptotic cell death, with a dose of 100 μM killing 90% of the cells (data not shown). Therefore, we used a dose range of 10–50 μM as it provided the best apoptotic response. We observed that Cr(VI)-induced ROS production in cells was responsible for Cr(VI)-induced apoptosis (Figure 1). This was verified in the presence of antioxidants such as MnTBAP and catalase that effectively inhibited apoptosis induced by Cr(VI), indicating the role of and H2O2 in this process. Furthermore, Cr(VI)-induced apoptosis in a classical caspase-dependent manner as zVAD.FMK completely blocked apoptosis induced by Cr(VI) (Figure 4A). Apoptosis may be initiated through the stimulation of death receptors located on the cell surface or through the intrinsic pathway involving the release of apoptotic signals from mitochondria. We observed that both apoptotic pathways were induced by Cr(VI) exposure. However, the mitochondrial pathway was more dominant compared with the death receptor pathway, as indicated by the greater increase in caspase-9 activation by Cr(VI) and more potent inhibitory effect of caspase-9 inhibitor on Cr(VI)-induced apoptosis (Figure 4A and B). It is possible that the two death pathways activated by Cr(VI) may be linked as demonstrated with other apoptogens. Therefore, activated caspase-8 induces apoptosis at least in part through caspase-9 pathway via BH3 interacting domain death agonist cleavage (16–19). The significant inhibition of caspase-9 activation by antioxidants, MnTBAP and catalase, indicates an important role of ROS in Cr(VI)-induced apoptosis (Figure 4C). In addition, we also observed that mitochondria was the major source of ROS involved in Cr(VI)-induced apoptosis. Cr(VI)-induced ROS generation and apoptosis were significantly inhibited by DPI and ROT, with the effect of the latter being more dominant (Figure 3A–C). Since ROT inhibits mitochondrial ROS and DPI inhibits cellular reduced nicotinamide adenine dinucleotide phosphate oxidase (35–37), this data indicate that total ROS produced in the cell is important in Cr(VI)-induced apoptosis, but mitochondrial ROS may play a major role. This was further confirmed in H460 ρ0 cells that are prepared by long-term exposure to ethidium bromide that leads to the depletion of critical respiratory chain subunits encoded by mitochondrial DNA (38). Therefore, these cells have impaired ROS generating capability and oxidative phosphorylation (39). We observed significantly decreased ROS production and apoptosis in H460 ρ0 cells as compared with H460 cells (Figure 3D–F). Since ρ0 cells have dysfunctional mitochondria, this observation indicates an important role of mitochondrial electron transport chain and ROS in Cr(VI)-induced apoptosis (40,41). All these observations establish an important role of mitochondria in Cr(VI)-induced apoptosis and are consistent with another report that suggested that apoptotic doses of Cr(VI) caused mitochondrial instability (42).
Induction of proapoptotic proteins does not necessarily lead to apoptosis induction (43,44), indicating that inhibitors of both apoptotic pathways exist and are important. Bcl-2 is a key regulator of the mitochondrial pathway that prevents apoptosis by preserving mitochondrial permeability transition (45). We observed that overexpression of Bcl-2 strongly inhibited Cr(VI)-induced apoptosis (Figure 4E), further supporting the role of mitochondria in Cr(VI)-induced cell death. Exposure of the cells to Cr(VI) caused downregulation of Bcl-2 (Figure 4D) that was dependent as cotreatment of the cells with the scavenger MnTBAP completely inhibited this downregulation (Figure 5C). In contrast, the H2O2 scavenger catalase failed to inhibit this effect, indicating that H2O2 has a minimal role in Cr(VI)-induced Bcl-2 downregulation. H460 cells overexpressing GPx and SOD also showed reduced apoptotic responses to Cr(VI) treatment (Figure 2). This effect was more pronounced in SOD-overexpressing cells suggesting that even though both and H2O2 are important, may be the major ROS involved in Cr(VI)-induced apoptosis. Treatment with donor LY83583 also downregulated Bcl-2 indicating a general correlation between and Bcl-2. It was further observed that Bcl-2 was upregulated in H460 ρ0 cells when treated with Cr(VI) as compared with H460 cells (Figure 5D). Therefore, mitochondrial may play a critical role in maintaining Bcl-2 stability since H460 ρ0 cells have a dysfunctional electron transport chain and produce significantly less ROS, especially , which is the main product of the mitochondrial respiratory chain.
The antiapoptotic function of Bcl-2 is closely associated with its expression levels, which is controlled by various mechanisms. Posttranslational modifications, such as ubiquitination and phosphorylation, have emerged as important regulators of Bcl-2 function (26,46). The ability of the proteasome inhibitor LAC to inhibit Bcl-2 downregulation in response to Cr(VI) treatment strongly supports the role of the proteasomal pathway in Bcl-2 regulation (Figure 6A). To further explore the mechanism involved, we tested the effect of ROS modulators on Bcl-2 ubiquitination. We observed that scavenger MnTBAP inhibited Bcl-2 ubiquitination induced by Cr(VI), whereas H2O2 scavenger catalase had minimal effect (Figure 6B). This result indicated that mediates the degradation and downregulation of Bcl-2 by inducing its ubiquitination in response to Cr(VI) exposure. This was contrary to the reports that indicated H2O2 to be the major ROS involved in Bcl-2 ubiquitination induced by other test agents (15,26). However, treatment with donor LY83583 also induced ubiquitination of Bcl-2 indicating that plays an important role in maintaining Bcl-2 stability in lung epithelial cells. It is plausible that the type of reactive species involved in mediating Bcl-2 stability in various biological systems differs according to the variables involved.
In summary, our data provide evidence that the mechanism by which Cr(VI) induces apoptosis in human lung epithelial H460 cells involves rapid generation of ROS, with playing a major role. Although total cellular ROS is involved, mitochondria may be the major source of ROS involved in Cr(VI)-induced apoptosis. Furthermore, Cr(VI)-induced apoptosis is mainly mediated through the mitochondrion-dependent caspase-9 activation and is negatively regulated by the antiapoptotic Bcl-2 protein. Cr(VI) induces downregulation of Bcl-2 through a process that involves -mediated ubiquitin–proteasomal degradation. may represent a common regulator of Bcl-2 function that controls apoptotic cell death induced by various physiologic and pathologic stimuli. Recent evidences suggest that Bcl-2 protein inhibits apoptosis by suppressing free radicals generation or by regulating cellular antioxidant defense mechanisms (23–25). Since Bcl-2 overexpression significantly blocks ROS-mediated Cr(VI)-induced apoptosis (Figure 5A and B), it is plausible that Bcl-2 may be acting as an antioxidant in response to Cr(VI) exposure. By linking Bcl-2 and , we document a novel mechanism that forms the basis for differential susceptibility of cells to apoptotic cell death. This study provides new mechanistic insights into the interaction of Bcl-2 with that may be exploited in the treatment of cancer and related apoptosis disorders.
Funding
National Institutes of Health (HL0763401).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AMC
amino-4-methylcoumarin
- ROS
reactive oxygen species
superoxide anion
- Cr(VI)
hexavalent chromium
- H2O2
hydrogen peroxide
- SOD
superoxide dismutase
- GPx
glutathione peroxidase
- DHE
dihydroethidium
- DCF
dichlorofluorescein
- MnTBAP
Mn(III)tetrakis (4-benzoic acid) porphyrin
- DPI
diphenylene iodonium
- ROT
rotenone
- LAC
lactacystin
- LY83583
6-anilinoquinoline-5,8-quinone
References
- 1.Wang S, et al. Role of reactive oxygen species and Cr(VI) in Ras-mediated signal transduction. Mol. Cell. Biochem. 2004;255:119–127. doi: 10.1023/b:mcbi.0000007268.42733.53. [DOI] [PubMed] [Google Scholar]
- 2.IARC. Vol. 49. Lyon: IARC; 1990. Chromium, nickel, and welding. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; pp. 49–445. [PMC free article] [PubMed] [Google Scholar]
- 3.Langard S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am. J. Ind. Med. 1990;17:189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- 4.Langard S. Role of chemical species and exposure characteristics in cancer among persons occupationally exposed to chromium compounds. Scand. J. Work Environ. Health. 1993;19(suppl. 1):81–89. [PubMed] [Google Scholar]
- 5.Simonato L, et al. A historical prospective study of European stainless steel, mild steel, and shipyard welders. Br. J. Ind. Med. 1991;48:145–154. doi: 10.1136/oem.48.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien TJ, et al. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Shi X, et al. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health B Crit. Rev. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- 8.Shi XL, et al. Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI) Biochem. Biophys. Res. Commun. 1989;163:627–634. doi: 10.1016/0006-291x(89)92183-9. [DOI] [PubMed] [Google Scholar]
- 9.Shi X, et al. Reaction of Cr(VI) with ascorbate and hydrogen peroxide generates hydroxyl radicals and causes DNA damage: role of a Cr(IV)-mediated Fenton-like reaction. Carcinogenesis. 1994;15:2475–2478. doi: 10.1093/carcin/15.11.2475. [DOI] [PubMed] [Google Scholar]
- 10.De Flora S, et al. Genotoxicity of chromium compounds. A review. Mutat. Res. 1990;238:99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama M. Role of physiological antioxidants in chromium(VI)-induced cellular injury. Free Radic. Biol. Med. 1992;12:397–407. doi: 10.1016/0891-5849(92)90089-y. [DOI] [PubMed] [Google Scholar]
- 12.Luo H, et al. Chromium (IV)-mediated fenton-like reaction causes DNA damage: implication to genotoxicity of chromate. Ann. Clin. Lab Sci. 1996;26:185–191. [PubMed] [Google Scholar]
- 13.Shi XG, et al. Deferoxamine inhibition of Cr(V)-mediated radical generation and deoxyguanine hydroxylation: ESR and HPLC evidence. Arch. Biochem. Biophys. 1992;293:281–286. doi: 10.1016/0003-9861(92)90396-e. [DOI] [PubMed] [Google Scholar]
- 14.Standeven AM, et al. Possible role of glutathione in chromium(VI) metabolism and toxicity in rats. Pharmacol. Toxicol. 1991;68:469–476. doi: 10.1111/j.1600-0773.1991.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang JH, et al. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-kappaB activation. J. Biol. Chem. 2004;279:38779–38786. doi: 10.1074/jbc.M406371200. [DOI] [PubMed] [Google Scholar]
- 16.Danial NN, et al. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson DW, et al. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 18.Boldin MP, et al. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 19.Chinnaiyan AM, et al. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 20.Muzio M, et al. An induced proximity model for caspase-8 activation. J. Biol. Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 21.Deveraux QL, et al. Endogenous inhibitors of caspases. J. Clin. Immunol. 1999;19:388–398. doi: 10.1023/a:1020502800208. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, et al. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Amstad PA, et al. BCL-2 is involved in preventing oxidant-induced cell death and in decreasing oxygen radical production. Redox. Rep. 2001;6:351–362. doi: 10.1179/135100001101536535. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanov MB, et al. Oxidative stress is attenuated in mice overexpressing BCL-2. Neurosci. Lett. 1999;262:33–36. doi: 10.1016/s0304-3940(99)00047-6. [DOI] [PubMed] [Google Scholar]
- 25.Hockenbery DM, et al. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 26.Breitschopf K, et al. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol. Cell. Biol. 2000;20:1886–1896. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chanvorachote P, et al. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 28.Azad N, et al. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J. Biol. Chem. 2006;281:34124–34134. doi: 10.1074/jbc.M602551200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, et al. Cr (VI) induces cell growth arrest through hydrogen peroxide-mediated reactions. Mol. Cell. Biochem. 2001;222:77–83. [PubMed] [Google Scholar]
- 30.Bagchi D, et al. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 2001;222:149–158. [PubMed] [Google Scholar]
- 31.Ye J, et al. Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J. Biol. Chem. 1999;274:34974–34980. doi: 10.1074/jbc.274.49.34974. [DOI] [PubMed] [Google Scholar]
- 32.Park KS, et al. Depletion of mitochondrial DNA alters glucose metabolism in SK-Hep1 cells. Am. J. Physiol. Endocrinol. Metab. 2001;280:E1007–E1014. doi: 10.1152/ajpendo.2001.280.6.E1007. [DOI] [PubMed] [Google Scholar]
- 33.Shi XG, et al. On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium(V) species. Arch. Biochem. Biophys. 1990;277:342–350. doi: 10.1016/0003-9861(90)90589-q. [DOI] [PubMed] [Google Scholar]
- 34.Carlisle DL, et al. Chromium(VI) induces p53-dependent apoptosis in diploid human lung and mouse dermal fibroblasts. Mol. Carcinog. 2000;28:111–118. doi: 10.1002/1098-2744(200006)28:2<111::aid-mc7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 35.Freeman BA, et al. Biology of disease: free radicals and tissue injury. Lab. Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 36.Irani K, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 37.Chen Q, et al. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 38.King MP, et al. Mitochondria-mediated transformation of human rho(0) cells. Methods Enzymol. 1996;264:313–334. doi: 10.1016/s0076-6879(96)64030-0. [DOI] [PubMed] [Google Scholar]
- 39.Chandel NS, et al. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 1999;454:173–176. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- 40.Cai J, et al. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 41.Panduri V, et al. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L1220–L1227. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- 42.Pritchard DE, et al. Cyclosporin A inhibits chromium(VI)-induced apoptosis and mitochondrial cytochrome c release and restores clonogenic survival in CHO cells. Carcinogenesis. 2000;21:2027–2033. doi: 10.1093/carcin/21.11.2027. [DOI] [PubMed] [Google Scholar]
- 43.Klas C, et al. Activation interferes with the APO-1 pathway in mature human T cells. Int. Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 44.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 45.Fiers W, et al. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 46.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]