Abstract
The identification and functional characterization of Dictyostelium discoideum dynamin A, a protein composed of 853 amino acids that shares up to 44% sequence identity with other dynamin-related proteins, is described. Dynamin A is present during all stages of D. discoideum development and is found predominantly in the cytosolic fraction and in association with endosomal and postlysosomal vacuoles. Overexpression of the protein has no adverse effect on the cells, whereas depletion of dynamin A by gene-targeting techniques leads to multiple and complex phenotypic changes. Cells lacking a functional copy of dymA show alterations of mitochondrial, nuclear, and endosomal morphology and a defect in fluid-phase uptake. They also become multinucleated due to a failure to complete normal cytokinesis. These pleiotropic effects of dynamin A depletion can be rescued by complementation with the cloned gene. Morphological studies using cells producing green fluorescent protein-dynamin A revealed that dynamin A associates with punctate cytoplasmic vesicles. Double labeling with vacuolin, a marker of a postlysosomal compartment in D. discoideum, showed an almost complete colocalization of vacuolin and dynamin A. Our results suggest that that dynamin A is likely to function in membrane trafficking processes along the endo-lysosomal pathway of D. discoideum but not at the plasma membrane.
INTRODUCTION
Dynamins form a protein family within the GTP-binding protein superfamily whose members share considerable sequence identity in the amino-terminal half of the molecule, particularly in the region around the tripartite GTP-binding motif. Large variations in size and sequence are observed in the carboxy-terminal half and are thought to reflect functional differences. Accordingly, members of the dynamin family have been implicated in a wide range of cellular processes, including meiotic spindle pole separation (Yeh et al., 1991), mitochondrial genome maintenance (Jones and Fangman, 1992), cell plate formation in plants (Gu and Verma, 1996), protein trafficking, biogenesis of thylakoid membranes (Park et al., 1998), and various forms of endocytosis. The best characterized function of a dynamin family member, that in clathrin-mediated endocytosis, was deduced from observations in Drosophila and mammalian cells. Initial studies of D. melanogaster shibire mutants (Kosaka and Ikeda, 1983a,b) and later mammalian cells expressing mutant forms of dynamin (Damke et al., 1994) showed an increase in the presence of invaginations at the plasma membrane. Dynamin-1 localizes to endocytic clathrin-coated pits and self-assembles into rings at the neck of clathrin-coated buds. This process is thought to be a key step in the mechanism by which vesicles are ‘pinched off’ at the plasma membrane as the dynamin rings constrict (Hinshaw and Schmid, 1995; Takei et al., 1995).
Many questions remain about the structure and function of dynamins, particularly in regard to a potential role in the organization of the cytoskeleton and the regulation and maintenance of cell shape (Urrutia et al., 1997). The present study attempted to identify a dynamin homologue in Dictyostelium discoideum and to elucidate its function in this genetically tractable, unicellular organism. These highly motile cells have proven to be a useful system in which to investigate the molecular mechanisms regulating protein and vesicular trafficking along the biosynthetic and endosomal pathways. There is good evidence that many of the components involved in secretion, protein sorting, and endocytosis are well conserved between D. discoideum and mammalian cells (Buczynski et al., 1997).
We report here the identification, cloning, and characterization of the dynamin-like protein dynamin A from D. discoideum. A PCR-based approach was used to identify dymA, the gene encoding dynamin A. The functional importance of dynamin A was examined by gene replacement and overexpression of the cloned gene in D. discoideum. Disruption of dymA causes major changes in the morphology and behavior of D. discoideum cells. The mutant cells are large and multinucleated and grow more slowly than wild-type cells. Cells lacking a functional copy of dymA show a marked reduction in cell polarity, display aberrant motile behavior, and frequently fail to complete cytokinesis. A role of dynamin A in endocytosis is suggested by the observations that, compared with control cells, the uptake of fluid-phase markers is twofold reduced in dymA− cells and the transit time of the fluid-phase markers is twofold increased. Analysis by electron microscopy revealed extensive areas with small vesicular profiles in dymA− cells and changes in the morphology of mitochondria and the cell nucleus. A causal relationship between the lack of dynamin A and these phenotypic changes was confirmed by complementation of the dymA− cells with the cloned gene. Recovery of dymA expression was accompanied by stable reversion to the wild-type phenotype.
MATERIALS AND METHODS
Strains, Media, and Buffers
D. discoideum wild-type and mutant strains were grown on plastic culture dishes or in conical flasks in HL-5c medium. Media for growth of dymA− strains HDM1701, HDM1702, and HDM1703 contained 4 μg/ml blasticidin (ICN Biomedicals, Eschwege, Germany). Buffers used were TBS (20 mM Tris-HCl, 150 mM NaCl, pH 7.5) and PBS (10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, pH 7.5). Escherichia coli strain XL1-Blue (Stratagene, Heidelberg, Germany) was used for all DNA work unless stated otherwise.
Cloning and Sequencing
The D. discoideum dymA gene was cloned using PCR techniques. The oligonucleotide pair used to amplify the dynamin-related sequences, 5′-GATTTCTTACCAGGAGGTTCAGGTAT-3′ and 5′-GCATCIGTACCTTCATCCAT-3′, corresponds to the conserved amino acid sequences DFLPRGSGI and MDEGTDA of human dynamin-1. For the isolation of the cDNA clone, a D. discoideum λ-gt11 cDNA library (Clontech, Heidelberg, Germany) was divided into ten fractions made from plates with ∼50,000–100,000 plaques. Each fraction was screened using PCR for the appearance of a 0.5-kilobase (kb) fragment. One of the positive fractions was subdivided into eight fractions made from plates with ∼15,000 plaques and rescreened. The final λ cDNA clone was isolated by filter screening of a positive fraction using the 500-base pair (bp) PCR fragment as the probe. Standard cloning and DNA hybridization methods were used (Sambrook et al., 1989). A genomic clone was isolated to obtain the entire coding sequence of dymA. D. discoideum genomic DNA was purified from Ax2 cells as described by Manstein et al., 1989. Southern blot analysis revealed that a 6- to 7-kb HindIII–EcoRI fragment harbored the complete DymA gene. Consequently a 6- to 7-kb HindIII–EcoRI partial genomic DNA library was created in pBluescript using E. coli strain XL2-Blue (Stratagene) as the host, and 1500 colonies were screened with the 500-bp PCR fragment described above. Sequencing confirmed that a 6.6- kb HindIII–EcoRI fragment contained the entire dymA gene, and the genomic clone was designated pBS-DYMA. Sequencing was performed using a Sequenase kit (Amersham Buchler, Braunschweig, Germany). Restriction enzymes and T4 DNA ligase were from Boehringer Mannheim (Mannheim, Germany).
Gene Replacement
Plasmid pBS-ΔDYMA was generated for the replacement of the dymA gene in D. discoideum (Figure 3). First, pBS-DYMA was cut with SpeI and religated to obtain pBS-DYMAΔSpe. The 134-bp BamHI–EcoRV DNA fragment of pBS-DYMAΔSpe was then replaced by the 1.4-kb blasticidin S resistance cassette from pBsr2 (Sutoh, 1993). The resulting construct (pBS-ΔDYMA) had the blasticidin S resistance cassette inserted 664 bp downstream of the dymA start codon and in the same orientation as the dymA gene.
Figure 3.
Disruption of dymA by gene replacement. (A) Known map of wild-type dymA locus. (B) Map of dymA− cells as derived from the Southern blot shown in C. The lighter lines with numbers underneath indicate the size of the restriction fragment (in kilobases) hybridizing with the probe when genomic DNA is digested with the enzymes shown on the right (X, XbaI; C, ClaI; N, NdeI; B, BamHI; E, EcoRV; S, SpeI; RI, EcoRI).
For transformation of wild-type Ax2 cells, 20 μg of pBS-ΔDYMA were digested with NdeI–SpeI to release the replacement fragment from the vector sequence. The DNA was dephosphorylated with shrimp alkaline phosphatase (Amersham Buchler), phenol/chloroform extracted, ethanol precipitated, and resuspended in 20 μl Tris-EDTA, pH 8.0. Alternatively, after digestion with NdeI–SpeI the replacement fragment was gel purified and resuspended in 20 μl Tris-EDTA, pH 8.0. Transformation of D. discoideum cells was carried out as described (De Hostos et al., 1991). Selection for transformants was applied 24 h after transformation in medium containing 4 μg/ml blasticidin S. Colonies appeared around day 5. Transformants were picked after 7–10 d and subcloned by spreading series of diluted cell suspensions onto SM agar plates containing Klebsiella aerogenes. Transformants were screened by PCR, and the replacement event was verified by Southern blot analysis.
Extrachromosomal Expression of Dynamin A
For complementation studies, the entire dymA gene was recovered from plasmid pBS-DYMA as a 6.6-kbp SalI–XbaI fragment. This fragment also contained 1.5 kbp of 5′-flanking region including the native promoter of dymA. The SalI–XbaI fragment was cloned into the Dictyostelium expression plasmid pDXA-3C (Manstein et al., 1995), cut SalI–XbaI thus releasing the actin 15 promoter. The resulting construct (pDX-DYMA) was transformed into dymA− cells, and transformants were selected in growth medium containing 10 μg/ml G418.
Expression of Green Fluorescent Protein (GFP)–Dynamin A
A vector for expression of a GFP–dynamin A fusion protein in D. discoideum under the control of the actin 15 promoter was constructed from the transformation vector pDEX H (Faix et al., 1992). The insert contained a continuous reading frame composed of, first, the coding sequence of S65T-GFP from A. victoria (Heim et al., 1995); second, an oligopeptide linker consisting of four glycine-serine-glycine repeats (Westphal et al., 1997); and third, the entire dymA coding sequence. The vector was introduced into the genome of Ax2 cells using electroporation, and transformants were selected in growth medium containing 10 μg/ml G418. The transformant clone HDM1718 was used for morphological studies.
Antibody Production and Western Blot Analysis
A fragment containing amino acids 1–380 was expressed as GST fusion protein and purified on a GST-agarose column. Polyclonal antisera against dynamin A were obtained from rabbits immunized with the GST-fusion protein. Antibodies were affinity purified using a dynamin fragment corresponding to amino acids 1–568 that was CNBr coupled to Sepharose. Eluted antibody was precipitated with 50% NH4SO4, resuspended in PBS, and dialyzed overnight at 4°C against PBS. For immunoblotting, D. discoideum proteins from wild-type and mutant strains were separated on a 10% SDS-PAGE gel and electroblotted onto nitrocellulose (Schleicher & Schüll, Dassel, Germany). The nitrocellulose blot was blocked in TBS containing 5% nonfat dry milk powder for 1 h, incubated with 1:1000 dilution of affinity-purified anti-dynamin antibody in the same buffer for either 1 h at room temperature or overnight at 4°C followed by detection with an HRP-conjugated secondary antibody (Bio-Rad, Munich, Germany). Detection by ECL was performed according to manufacturer’s instructions (Amersham Buchler).
Fluorescence Microscopy
Cells at a density of 1 × 106 cells/ml were placed on 12-mm coverslips and allowed to attach for 30 min. Fixation of cells was performed in methanol applying a temperature gradient from −85 to −35°C for 30 min (Neuhaus et al., 1998). The cells were rehydrated in PBS and blocked in PBS containing 3% BSA followed by incubation in primary antibody for 30 min at room temperature. Immunolocalization of dynamin A used antibody PAD1 at a dilution of 1:20 in PBS containing 3% BSA and 0.1% Triton X-100. For immunostaining of mitochondria, the monoclonal α-mitoporin-antibody 70-100-1 was used (Troll et al., 1992). Other primary antibodies used were monoclonal antibody 190-340-8 (Weiner et al., 1993) to stain the Golgi complex, monoclonal vacuolin antibody 221-1-1 to stain late endosomes (Rauchenberger et al., 1997), and a polyclonal GFP antibody (Clontech). Primary antibodies were diluted 1:5 before use or 1:200 in the case of the polyclonal GFP antibody. After washing with PBS and PBS/3% BSA, cells were incubated for 20 min in Cy3-labeled secondary antibody (Amersham Buchler) diluted 1:500. Nuclei were stained with 0.1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI) for 5 min. Coverslips were mounted on glass slides with Moviol (Heimer and Taylor, 1974). Double immunofluorescence was performed using Cy3-labeled secondary antibodies together with Alexa488-labeled secondary antibodies (Molecular Probes, Leiden, Germany). Images were recorded on a Zeiss Axiovert 135 inverted microscope equipped with a Quantix camera (Photometrics, Tucson, AZ), controlled by IP-Lab software. For confocal microscopy, a confocal microscope DM/IRB (Leica, Nussloch, Germany) was used, and optical sections were recorded at 0.4 μm per vertical step and 8 times averaging.
Video Microscopy
For video microscopy, cells were transferred to covered chamber glasses (Nunc, Naperville, IN). Sequences were recorded in real time on videotape using a Sony SSC-370CE video camera with an Argus 20 controller (Hamamatsu Photonics, Herrsching, Germany). Time lapse series were acquired using the Photometrics Quantix camera and transferred to a Macintosh 9500 for analysis and storage.
Synchronous morphological differentiation was induced by starvation on MMC agar (20 mM 2-(N-morpholino)ethanesulfonic acid [MES], pH 6.8; 2 mM MgCl2; 0.2 mM CaCl2; 2% [wt/vol] Bacto agar [Difco, Detroit, MI]). Structures from various stages of development were visualized using an Olympus B061 microscope (Olympus, Lake Success, NY) and the Sony video camera. A Scion VG-5 frame grabber was used for transfer of images to a Macintosh 9500.
Transmission Electron Microscopy (TEM)
TEM for Figure 9 was performed as described by Novak and co-workers (1995). TEM for Figures 10 and 12 was carried out according to Neuhaus et al. (1998).
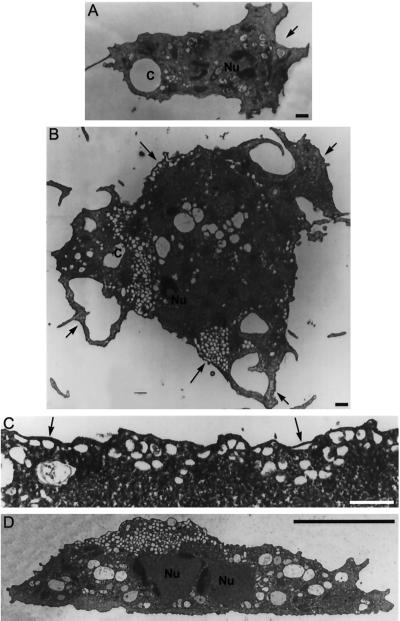
Figure 9.
Representative electron micrographs of wild-type and dymA− cells. Panels A, B, and C represent sections parallel to the surface of attachment. (D) This panel represents a perpendicular section. (A) Wild-type cells are smaller, with single nuclei (Nu) and one or two contractile vacuoles (c) with a few associated small vesicular or tubular profiles. Wild-type cells show a single main extension (arrow), reflecting movement in a particular direction. (B–D) Most dymA− cells are very large. However, due to limitations on illustration size, panels B and D show smaller dymA− cells that nevertheless illustrate the most important features of the dymA− phenotype. In contrast to wild-type cells, dymA− cells extend processes in several directions (short arrows) and display areas with numerous small vesicular profiles (long arrows) that may be sections through discrete vesicles or convoluted tubules. (C) Higher magnification of a region (arrows) showing many vesicular profiles under the cell membrane. Magnification in panels A and B is the same. Bar, 1 μm in panels A–C and 10 μm in panel D.
Figure 10.
Electron micrographs showing the distribution of coated structures (arrows) in dynamin A-depleted cells. (A) Area with numerous small vesicular profiles that may be sections through discrete vesicles or convoluted tubules. (B) Vesicles and coated vesicles are particularly enriched in the region near the MTOC (asterisk). Part of the cell nucleus (Nu) can be seen in the upper right corner of the panel. (C) Coated structures can be observed throughout the cell in association with tubular membrane structures. (D) Large, coated-pit–decorated vesicles are frequently found near the plasma membrane. Bar, 1 μm.
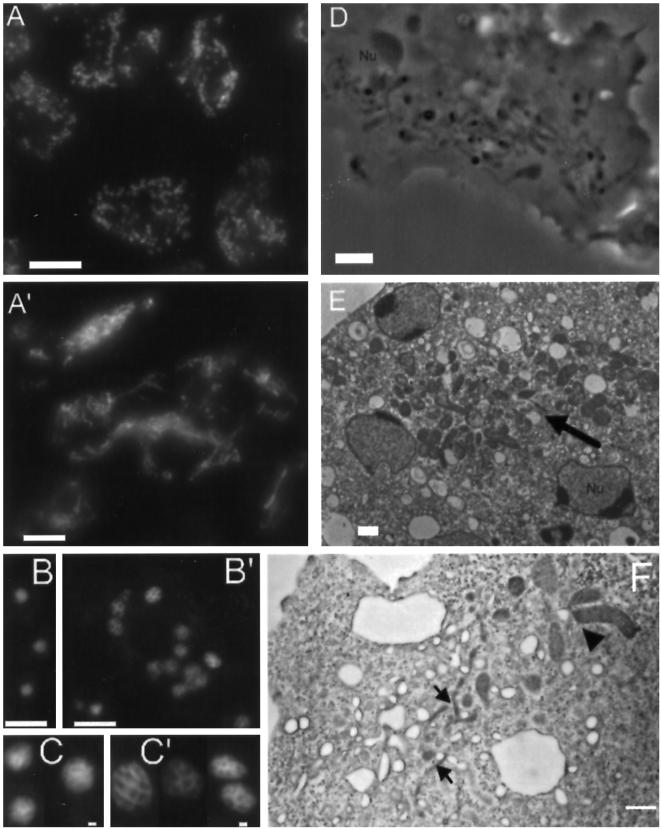
Figure 12.
Cells lacking a functional copy of dymA become multinucleated and display abnormal morphologies of nuclei and mitochondria. Panels A and A′ show cells stained with mAb 70-100-1 directed against a mitochondrial porin from D. discoideum. (A) Uniform, dispersed appearance of mitochondria in Ax2 cells. (A′) In dymA− cells dense clusters of mitochondria are frequently observed, and most mitochondria appear fused in a network of long threads. (B) Ax2 cells were stained with DAPI to visualize nuclei. (B′) DAPI staining of Dynamin A-depleted cells shows that these cells are multinucleated. Panels C and C′ show a gallery of DAPI-stained nuclei. In comparison to wild-type nuclei (C), dymA− nuclei (C′) display a lobed appearance and are more variable in size and shape. (D) The altered appearance of mitochondria in dymA− cells can also be observed in phase contrast images. (E) EM micrographs showing clustered mitochondria (arrow) in a dymA− cell. (F) EM micrographs showing a branched mitochondrial network consisting of thin filamentous structures (arrows) and large lobe-shaped structures (arrowhead) in dymA− cells. Bar, 10 μm in panels A, A′, B, and B′, and 1 μm in panels C, C′, D, E, and F.
Measurement of Pinocytosis and Phagocytosis
Fluid-phase endocytosis and exocytosis of surface-attached wild-type Ax2, rescue cells, and dymA− cells were quantitatively analyzed using a method adapted from Bacon et al. (1994). Cells were grown to confluency on 6-cm Petri dishes or in shaking culture at a density of 4 × 106 cells/ml. To determine fluid-phase uptake, cells were incubated with 1 mg/ml FITC-dextran in growth medium for various lengths of time. For measuring exocytosis, surface-attached cells were incubated with 1 mg/ml FITC-dextran for 2 h, washed twice, and transferred to fresh growth medium. To determine intracellular transit time of fluid phase, cells were incubated with 5 mg/ml FITC-dextran for 10 min, washed twice, and transferred to fresh medium. At the indicated times, cells were washed twice with 200 mM phosphate buffer, pH 6.3, and once with 50 mM phosphate buffer, pH 9.2. Cells were resuspended in 1 ml 50 mM phosphate buffer, pH 9.2, transferred to tubes, and lysed with 0.4% Triton X-100. After centrifugation the fluorescence of the supernatant was measured in an SLM 8000 spectrofluorimeter at excitation and emission wavelengths of 493 and 516 nm, respectively. The data shown were normalized with respect to total protein content of the cells, as determined with Bradford reagent (Bio-Rad).
Phagocytic ability was measured by a modification to the method of Temesvari and co-workers (1996). Briefly, 1-μm carboxylate-modified, fluorescent polystyrene beads (Molecular Probes) were incubated with cells grown in shaking suspension culture to 3 × 106/ml. The ratio of cells to beads was 1:100. Samples (1 ml) were mixed with 4 ml ice-cold MES buffer, pH 6.5, and placed on a 8-ml cushion of 20% PEG 8000 in MES buffer. Centrifugation at 2000 × g for 10 min separated ingested beads from uningested and attached beads. The uppermost layer and the cushion were removed by careful aspiration, and the cell pellet was washed in 5 mM glycine, 100 mM sucrose, pH 8.5. Cells were lysed in 100 μl of the same buffer containing 1% octylglucopyranosid. Samples were diluted to 1 ml, and fluorescence intensities were measured using excitation and emission wavelengths of 595 and 644 nm, respectively. Normalization of data to total protein content was as described above.
Functional Analysis of the Contractile Vacuolar System
To assess the function of the contractile vacuolar system the method described by Kuwayama and colleagues (1996) was used. Amebae were harvested by centrifugation, washed free of medium, and starved for 1 h in phosphate buffer. Cells were incubated in the presence of 300 mM glucose, water, or phosphate buffer as different osmotic environments in plastic tissue culture dishes. Cells were then monitored visually by video microscopy as described above. For quantitative analysis of viability after treatment a certain amount of cells were plated on bacteria lawns and the number of colonies counted after 4 d.
Functional Analysis of the Mitochondrial System
To assess the function of the mitochondrial system, the activity of the mitochondrial marker succinate dehydrogenase was measured in whole-cell lysates as described by Morré and co-workers (1987). As a control, the activity of the cytoplasmic marker alkaline phosphatase was determined. Cells were washed in MES buffer, resuspended at 3 × 107 cells/ml, and lysed by forced passage through a 5-μm pore filter (Schleicher & Schüll). To measure succinate dehydrogenase activity, 10–50 μl of whole-cell lysate were mixed with a buffer containing 40 mM sodium phosphate (pH 7.4), 250 μg/ml tetranitroblue tetrazoliumchloride (Sigma), 10 mM sodium succinate to a final volume of 500 μl and incubated at room temperature. After 30–60 min the reaction was stopped by the addition of 500 μl 4% SDS, and absorbance was measured at 570 nm.
Alkaline phosphatase activity was measured by mixing 10–50 μl of whole cell lysate with a solution consisting of 50 mM Tris-HCl (pH 9.0), 10 mM MgCl2, and 2.6 mg/ml p-nitrophenyl phosphatase (Sigma) to a final volume of 1 ml. After incubation for 30 min at room temperature, absorbance was measured at 404 nm. The data obtained in the succinate dehydrogenase assay were normalized for protein content or alkaline phosphatase activity.
RESULTS
We used PCR techniques to identify dymA, the gene encoding dynamin A. Both cDNA and gDNA libraries were screened to obtain a full-length dymA clone. Comparison of cDNA and gDNA revealed a single 83-bp intron near the 5′- end of the gene.
The nucleotide sequence of dymA revealed an open reading frame of 2559 bp encoding the 853-amino acid polypeptide chain of dynamin A (Figure 1A). With a calculated molecular mass of 96.1 kDa, dynamin A is similar in size to other members of the dynamin family (Obar et al., 1990, van der Bliek and Meyerowitz, 1991, Guan et al., 1993). Dynamin A contains the dynamin consensus motif LPRGSGIVTR (residues 51–60) and sequences corresponding to the tripartite GTP-binding motif (Bourne et al., 1991). The high homology of dynamin A to other members of the dynamin family is mainly confined to the N terminus. For the first 360 amino acids the sequence identity is 57% to rat DLP1, 56% to human dynamin-1, 54% to D. melanogaster shibire protein, and 55% to yeast Dnm1p (Figure 1B). The GGARI motif (residues 360–364) marks the carboxy-terminal end of the region of high similarity between dynamin A and the shibire-like dynamins. As previously described for all dynamin-like GTPases, the C terminus shows a higher level of sequence divergence. In the central part of dynamin A (residues 365–500), the highest degree of sequence similarity is observed with DLP1 (49%), Dnm1p (43.0%), and Vps1p (38.3%).
Figure 1.
Sequence analysis of D. discoideum dynamin A. (A) Predicted amino acid sequence of dynamin A. Dynamin A has a calculated molecular mass of 96.1 kDa and an isoelectric point of 7.0. The tripartite GTP-binding motif is underlined. (B) Sequence alignment of the GTP-binding domains of D. discoideum dynamin A (top), human dynamin-1 (middle), and yeast Dnm1p (bottom). An insert in the Dnm1p sequence corresponding to residues 73–114 was omitted from the alignment. The position of the insert is marked by an X in the alignment. Gaps are presented as —. (C) Sequence alignment of the putative GTPase effector domains of dynamin A, human dynamin-1, and the shibire gene product. (D) Diagram of dynamin A structural domains. The GTPase domain is shown in solid black, and the tripartite GTP-binding motif is indicated as white bars. LZ, leucine zipper; GED, GTPase effector domain; QNS, glutamine-asparagine-serine-rich domain. The basic, proline-rich part of the QNS-rich region, corresponding to residues 573 to 624, is shown in dark gray.
The region of dynamin A formed by residues 500–730 shows no similarity to the primary sequence of other members of the dynamin family. Secondary structure analysis shows that this region is highly hydrophilic and has a high surface probability. This part of dynamin A is almost devoid of charged residues, but is rich in polar amino acids (67%) and contains a high proportion of glutamine (25%), asparagine (23%), and serine (14%) residues that appear in long stretches of up to 13 amino acids (Figure 1A). The central part of the QNS-rich region, corresponding to residues 573–624, comes closest to the proline-rich domain observed with other dynamin family members. This region is basic in composition (pI = 10.0), and 12 of 51 residues are prolines.
The C-terminal domain of dynamin A (residues 730–853) shares 51 and 43% sequence identity with the corresponding regions of DPL1 and Dnm1p. This part of the protein is predicted to be α-helical and marked by an abundance of charged residues (40%), with the number of basic residues (20) almost equaling the number of acidic residues (22). The C-terminal end of classical dynamins is formed by a basic, proline- and arginine-rich domain (PRD) that exhibits no significant sequence similarity to that of dynamin A. However, residues 660–742 of dynamin-1 share 43% sequence identity with residues 730–853 of dynamin A (Figure 1C). This 13-kDa domain of dynamin-1 corresponds to a GTPase effector domain (GED) and is required for efficient GTPase activity (Muhlberg et al., 1997).
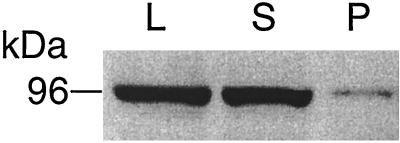
After lysis of wild-type cells and cells overproducing the protein, dynamin A was predominantly (∼90%) found in the soluble fraction (Figure 2). Further subcellular fractionation of the membrane-associated protein by sucrose gradient centrifugation showed the protein to be enriched in the microsomal fraction (data not shown). The polyclonal antibody PAD1, specific for the N-terminal 380 amino acids of dynamin A, was used for immunoblot analysis. This antibody reacts predominantly with a 96-kDa antigen in whole-cell lysates of Ax2 (see below). The size of the detected antigen agrees well with that of the purified protein (Wienke et al., 1997) and the molecular mass of dynamin A deduced from its amino acid sequence.
Figure 2.
Cellular distribution of dynamin A. Dynamin A is abundant in cytosol. Immunoblots stained with anti-dynamin A antibody PAD1 showing D. discoideum whole-cell lysate (L), supernatant after 15 min centrifugation at 15,000 × g (S), and pellet (P).
Generation of dymA− Cells by Gene Replacement
The dymA locus was disrupted by transforming wild-type Ax2 cells with linearized replacement fragments from vector pBS-ΔDYMA, using resistance against blasticidin S as a selectable marker. After 5–8 d of selection, typically 100–150 colonies were obtained. Thirty to 40 transformants were cloned and screened for the disruption of the dymA gene by PCR (data not shown). In three independent experiments, screening indicated that 5–15% of the transformants were potentially dymA−. The frequency of the desired recombination event was higher (30–40%) when the replacement fragment was gel purified before transformation.
Positive transformants from the PCR screen were analyzed by Southern blotting to confirm the disruption of the dymA gene locus and to determine its molecular organization in the mutant strains. A 0.6-kb fragment from the 5′-end of the dymA cDNA was used as a probe. The expected hybridization pattern for this probe with wild-type DNA is depicted in Figure 3A. Homologous recombination with the replacement construct is expected to cause a band shift for the XbaI–EcoRI digest from 7.5 to 3.3 kb and for the ClaI digest from 2.6 to 3.8 kb in the dymA− cells (Figure 3B). The Southern blot in Figure 3C is consistent with the predicted change in the hybridization pattern. Further Southern blot analysis with the blasticidin resistance cassette as a probe confirmed that only one copy of the replacement fragment had been integrated in the dymA gene and no other gene loci had been affected (data not shown). Cell lines HDM1701, HDM1702, and HDM1703, each with a disrupted dymA gene locus, were obtained from three different transformations and will be referred to, in the following text, as dymA− cells.
As expected from the disruption of the dymA gene locus, the 96-kDa antigen was not detectable in whole-cell lysates of dymA− cells by immunoblot analysis (Figure 4, lane 2).
Figure 4.
Immunoblot analysis of dymA− cells. Whole-cell protein (50 μg) from Ax2 cells (lane 1), dymA− cells (lane 2), and dymA− cells rescued by transformation with a plasmid bearing the wild-type dymA gene (lane 3) were separated on a 12% SDS-PAGE gel, blotted, and probed with polyclonal antibody PAD1 directed against the 380 N-terminal residues of dynamin A.
Complementation of the dymA− Cells
To confirm that phenotypic changes in the dymA− cells are explicitly attributable to the loss of the dymA gene product, the mutant strain was rescued by reintroduction of functional copies of dymA. The multicopy plasmid pDX-DYMA, harboring the entire dymA gene under the control of its native promoter, was used to transform dymA− cells. Complementation of dymA− cells led to the production of the 96-kDa antigen at levels 10- to 50-fold higher than those observed for Ax2 cells (Figure 4, lane 3) and rescued all phenotypic changes observed with dynamin A-depleted cells.
Growth and Appearance of dymA−Cells
The dymA gene product is not essential for D. discoideum growth. However, dymA− cells grow under all conditions tested approximately twofold slower than the parental Ax2 cells. In axenic media, Ax2 cells grow with doubling times <12 h. Exponentially growing cultures of dymA− cells double every 24 h under the same conditions. In suspension culture, Ax2 cells reach cell densities of 1 × 107 cells/ml and above while dymA− cells grow to a maximum cell density of 2 × 106 cells/ml.
Unusually large cells and the frequent occurrence of cytoplasmic bridges connecting neighboring cells are prominent features of populations of surface-attached dymA− cells (Figure 5A). Staining with DAPI showed that most dymA− cells are multinucleated with 40% of the cells containing three or more nuclei and some upwards of 20 nuclei. This is in comparison to wild-type cells where 98% of the cells have one or two nuclei with <2% having three or more nuclei. The frequent occurrence of large multinucleated cells and cytoplasmic bridges is reminiscent of myosin II-defective cells, which are unable to carry out normal cytokinesis and whose ability to divide requires attachment to a surface (De Lozanne and Spudich, 1987; Neujahr et al., 1997).
Figure 5.
Cell division in dymA− cells. (A) Populations of dynamin A-deficient cells contain many unusually large cells, some of which are connected via cytoplasmic bridges. After transfer from suspension culture to a Petri dish or a similar substratum dymA−, cellsfrequently divide by amitotic traction-mediated cytofission. (B) Time-lapse video microscopy demonstrated that dymA− cells are able to pass through all the phases of cell division at wild-type speed. The defect only occurs at the final stage of cytokinesis, at which the incipient daughter cells are merely connected by a cytoplasmic bridge. Images were taken at 20-s intervals. (C) In wild-type cells this connection was usually short lived, while the long cytoplasmic bridges of dymA− cells persisted for several minutes to several hours and reached more than 100 μm in length. Bar, 10 μm.
However, unlike myosin II-depleted cells, dymA− cells are able to grow in suspension culture. When dymA− cells are transferred from shaken culture to Petri dishes, the multinucleated cells start within minutes to undergo amitotic cell divisions by tearing themselves into smaller fragments by traction-mediated cytoplasmic fission (Figure 5A). Amitotic cell divisions are also observed at an increased frequency shortly after the transfer of dymA− cells to nutrient-free salt solutions. However, when cells are grown axenically or in association with bacteria, cytokinesis is in most instances (<95%) synchronized with mitosis. We used time-lapse video microscopy to follow cytokinesis in surface-attached and synchronized dymA− and Ax2 cells. The timing and the sequence of shape changes during cytokinesis were similar for both strains. Approximately 2–3 min were required to pass through cleavage furrow constriction until only a thin filamentous connection persisted (Figure 5B). This connection is very short lived in wild-type cells. In all recorded events the connection broke within 2 min and reached a maximal length of 20 μm. In contrast, long cytoplasmic bridges are a distinct phenotypic feature of surface-attached dymA− cells (Figure 5C). They persist for several minutes to several hours and reach up to 300 μm in length. The incipient daughter cells can undergo additional rounds of cytokinesis without disruption of the cytoplasmic bridges, leading to networks of interconnected cells. As long as the cytoplasmic bridges persist, the almost completely separated daughter cells can fuse again, thus giving rise to large multinucleated cells. A similar defect in cytokinesis was previously reported for calmodulin-depleted D. discoideum cells (Liu et al., 1992).
The average dymA− cell appears to lack polarity but is highly dynamic (Figure 6). Cells were frequently observed to cyclically extend and retract lamellopodia around the entire cell periphery. Pseudopodial extensions and long filopodia were also commonly observed; however, their distribution on the cell surface appears random. When we measured the speed of cells undergoing random walks on a glass surface, we found that dymA− cells are very motile and move at rates similar or greater than those observed for wild-type cells. Cell movement was measured under two different nutrient conditions. First, in axenic medium, dymA− cells moved at a rate of 4.4 μm/min (SD ± 2.7; n = 98) and Ax2 cells at 4.7 μm/min (SD ± 2.9; n = 86). Second, movement was tested after transfer to a buffered salt solution. The motility of D. discoideum cells varies with the developmental stage and reaches a maximum at the onset of aggregation about 8 h after transfer to starvation conditions (Varnum et al., 1985). Thus, transfer to starvation conditions induced an increase in the velocity of Ax2 cells to 5.8 μm/min (SD ± 3.2; n = 74) compared with vegetative cells. The maximum velocity of dymA− cells was 6.8 μm/min (SD ± 3.2; n = 74) during early development. Simultaneously, the multinucleated dymA− cells undergo several rounds of traction-mediated cytoplasmic fission, which inhibits the directed movement of the cells. The chemotactic response of dymA−cells was normal (data not shown).
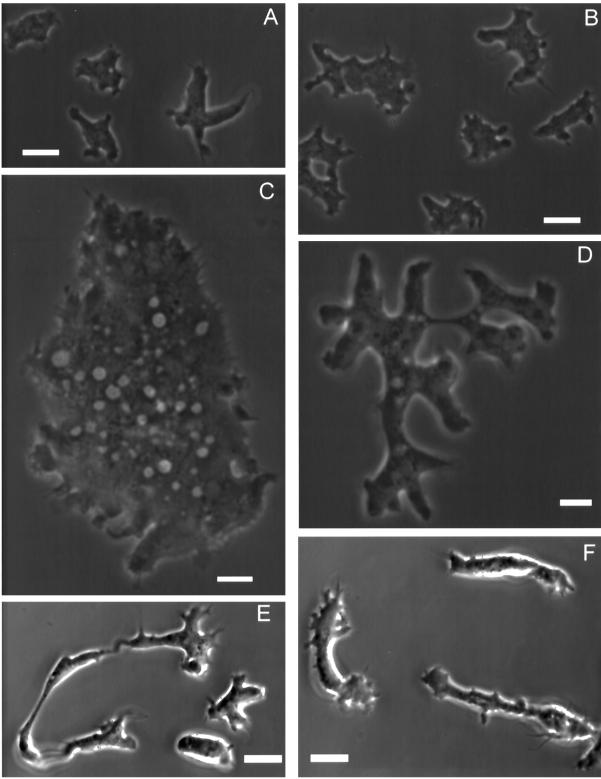
Figure 6.
Cells lacking dynamin A are large and irregularly shaped and show multiple pseudopodia. (A) Phase-contrast micrograph of untransformed Ax2 cells in axenic culture. (B) Phase-contrast micrograph of Ax2 cells 4 h after transfer in a nutrient-free, buffered salt solution. (C and D) Dynamin A-depleted cells show a striking difference in size and morphology and appear apolar in axenic culture. (E and F) When placed under starvation conditions for 4 to 5 h, dymA− cells take on a very elongated shape, form many cell surface projections, and show increased motile activity. Bar, 10 μm.
Development
The developmental program of D. discoideum is initiated upon starvation. Cells start emitting cAMP pulses and aggregate into mounds consisting of ∼105 cells. These mounds undergo a series of morphological changes that culminate in the formation of a fruiting body (Raper, 1935). The transcriptional regulation of dymA expression during development was assessed by probing Northern blots of RNA isolated at a series of developmental stages. A ∼3-kb dymA transcript was detected in vegetative cells and, at similar levels, at all later developmental stages (data not shown).
When dymA− cells were placed in starvation conditions on nonnutrient agar plates, the cells took 10 h to start streaming in comparison to 7 h observed for wild-type cells. Except for this difference in timing, early development of dymA− cells appeared similar to that of the wild-type cells. The delay in the onset of streaming of dymA− cells may, in part, be explained by the requirement to undergo several rounds of cytokinesis by traction mediated cytofission before efficient development can be initiated. Development of dymA− cells is completed by 30–36 h, while Ax2 cells completed development within 24–26 h. The final developmental morphology of dymA− cells results in shorter fruiting bodies consisting of a tapered stalk with enlarged basal disk and spore mass at the apical tip and a second cluster of spores laterally attached to the center of the stalk (Figure 7B). Only 10–20% of the spores formed by dymA− cells were resistant to heat treatment and germinated after incubation at 42°C for 30 min, in contrast to ∼90% for wild-type cells (data not shown).
Figure 7.
Development of fruiting bodies in wild-type Ax2 and dymA− cells. In comparison to the Ax2 cells (A) the final developmental morphology of dymA− cells results in shorter fruiting bodies consisting of a tapered stalk with enlarged basal disk and spore mass at the apical tip and a second cluster of spores laterally attached to the center of the stalk (B). Bar, 1 mm.
When D. discoideum cells are plated at low density in association with bacteria on nutrient agar plates, the bacteria form a confluent lawn and the D. discoideum cells form clonal plaques within the lawn as the bacteria are digested. Consistent with their reduced rate of growth under axenic conditions, the rate at which dymA− cells advanced on a bacterial lawn was approximately twofold slower than that of wild-type cells. In areas behind the advancing edge, where the dymA− cells had depleted the bacteria, the cells starved and gave rise to fruiting bodies that resembled those observed on nonnutrient agar plates.
Endocytosis and Osmoregulation in dymA− Cells
The role of dynamin A in the endosomal-lysosomal system of D. discoideum was tested by measuring the uptake of the fluid phase marker fluorescein-labeled dextran. In comparison to cells containing a functional copy of dymA, the extent to which the marker was accumulated was approximately twofold reduced in dymA− cells (Figure 8A). The initial rate of uptake for the first 40 min was 33% slower for dymA− than the parental wild-type or rescue cells. Thus, the lack of a dynamin A has a clear inhibitory effect on the initial rate of internalization and the long-term accumulation of the fluid phase marker. Similar results were obtained for cells grown on a substratum or in suspension.
Figure 8.
The role of dynamin A in pinocytosis and phagocytosis. For quantification of fluid-phase marker uptake, cells were incubated with 1 mg/ml FITC-dextran, and intracellular fluorescence was measured at the time points indicated. The amount of FITC-dextran internalized was plotted as percent of total uptake of Ax2 cells after 160 min. (A) In comparison to wild-type Ax2 cells (closed circles) and a rescued cell line (open circles), the extent to which the marker was accumulated was approximately twofold reduced in dymA− cells (open triangles). The initial rate of uptake for the first 40 min was 33% slower for dymA− than the parental wild-type or rescue cells. (B) For analysis of exocytosis, cells were incubated with 1 mg/ml FITC-dextran for 2 h, washed, and incubated with fresh medium for the time points indicated. Intracellular fluorescence was measured and plotted as percent of FITC-dextran remaining in the cells. Similar rates of exocytosis were determined for dymA− cells and cells producing dynamin A. (C) Intracellular transit time of fluid phase was measured by incubating the cells in 5 mg/ml FITC-dextran for 10 min and transfer to fresh media for the time indicated. Intracellular fluorescence was measured and plotted as percent of FITC-dextran remaining in the cells. In dymA− cells transit time was prolonged more than twofold. (D) Phagocytotic ability was assayed by following the uptake of 1 μm polystyrene microspheres (100 beads/cell) in axenic medium. In dymA− cells phagocytosis is increased by 50% compared with control cells. All the assay data are normalized to protein content and represent averages from at least three independent experiments.
The release of fluid phase marker over time was measured to show that the reduced rate of FITC-dextran uptake by the dymA− cells can be attributed to a deficiency in internalization and not recycling to the media by a faster rate of exocytosis. Cells were loaded for 2 h with FITC-dextran, washed free of the marker, and resuspended in fresh medium. The amount of fluid-phase marker that remained in the cells over time was measured. Similar rates of exocytosis were determined for dymA− cells and cells producing dynamin A (Figure 8B).
Examination of intracellular transit time by exposing the cells to a 10-min pulse of FITC-dextran revealed that the retention of the marker is more than twofold prolonged in dymA− compared with wild-type and rescue cells. The half-life for internalized FITC-dextran was 60 min in control cells and 150 min in dymA− cells (Figure 8C).
Phagocytosis is an inducible actin-dependent process. It is initiated by adhesion of a particle to any region on the surface of a D. discoideum cell. To investigate whether dynamin A plays a role in phagocytosis, dymA− and control cells were incubated with fluorescent polystyrene beads of 1 μm diameter for various lengths of time. In contrast to the pinocytosis assays, particle uptake experiments were performed with cells grown in shaking suspension culture. Compared with Ax2 and rescue cells, the dymA− mutants exhibited a 50% increase of total particle uptake (Figure 8D).
The best characterized function of dynamin-1 is its role in receptor-mediated endocytosis via clathrin-coated pits (Herskovits et al., 1993; van der Bliek et al., 1993; Takei et al., 1995). Therefore, we were particularly interested in comparing the phenotype of dymA− cells and clathrin heavy chain-deficient D. discoideum cells (CHC− cells). Coated vesicles are particularly enriched near the microtubule-organizing center (MTOC) of wild-type cells and were found to be completely absent in CHC− cells (O’Halloran and Anderson, 1992). In dymA− cells, coated vesicles were observed throughout the cytoplasm and in large numbers (>20) near the MTOC. Coated pits were frequently observed in association with larger vesicles but not at the plasma membrane. One of the most prominent defects of CHC− cells is the absence of large vacuoles that form part of the contractile vacuole system (Ruscetti et al., 1994). Examination of the morphology and function of this osmoregulatory organelle in dymA− cells showed the contractile vacuole system to function normally. Observation of dymA− cells by phase microscopy revealed the cyclic filling and emptying of large vacuoles. Exposure to hypo- and hyperosmotic conditions had no adverse effect on the viability of dymA− cells. Localization of two contractile vacuole markers, calmodulin and the 100-kDa subunit of the V-H+-ATPase (Fok et al., 1993; Zhu et al., 1993), by immunofluorescence revealed no difference in the appearance and organization of the contractile vacuole system of dymA− cells and that of the parental cell line (data not shown).
Disruption of dymA Affects Organelle Morphology
The clear differences in cell morphology between Ax2 cells and dymA− cells led us to examine the ultrastructure of dymA− cells at light and electron microscopic levels. Transmission electron micrographs showed a striking accumulation of membrane tubules in proximity to nuclei and the cell membrane in dymA− cells (Figures 9 and 10). These spongiome-like structures could represent sections through convoluted vesicular or tubular profiles. Additionally, coated vesicles (90–150 nm) or pits could be readily detected in electron microscopic (EM) micrographs of dymA− cells, and the number of coated structures appeared higher than in wild-type cells. Coated vesicles were observed in association with spongiome-like structures and were particularly enriched near the MTOC (Figure 10, A and B). Coated pits were frequently found on vesicular or tubular structures near the plasma membrane but not at the plasma membrane itself (Figure 10, C and D).
The localization of proteins related to the endo-lysosomal system and the Golgi complex was investigated by immunofluorescence, to identify the nature of the spongiome-like compartment (Figure 11). No differences were observed for endosomal compartments that can be detected with an antibody that recognizes the actin-binding protein coronin (De Hostos et al., 1991). However, when anti-vacuolin antibody 221-1-1 was used for staining late endosomal compartments (Rauchenberger et al., 1997), the immunofluorescence pattern in dymA− cells was clearly altered. Two to four late endosomes of similar size were observed per nucleus in Ax2 cells. In contrast, the number of stained vesicles per nucleus is increased to 10 or more in dymA− cells. The vacuolin-positive compartment of dymA− cells is more variable in size and seems to form a tubular network compared with the discrete ring-like structures observed in Ax2 cells (Figure 11, A and A′). Cellular localization of GFP–dynamin A by immunofluorescence microscopy produced a similar, but more punctate, labeling of membranous structures, and double immunofluorescence showed an almost complete colocalization of vacuolin and dynamin A (Figure 11B). The organization of the Golgi apparatus was visualized using antibody 190-340-8 (Weiner et al., 1993). Perinuclear structures with short tubular extensions were observed in Ax2 (Figure 11C). The perinuclear Golgi network appeared somewhat enlarged in the dymA− cells, and there was a higher background of diffuse staining throughout the cells. The increase in diffuse background staining was also observed in mononucleate dymA− cells of comparable size to the wild-type cells. In larger, multinucleated dymA− cells the prominent juxtanuclear labeling of all nuclei indicated that each was associated with a Golgi complex (data not shown).
Figure 11.
Immunolocalization of dynamin A, postlysosomal compartments, the Golgi-complex, and mitochondria by confocal laser scanning microscopy. (A and A′) Anti-vacuolin antibody 221-1-1 was used to stain a postlysosomal compartment that acquires endocytic markers shortly before exocytosis. Single confocal sections of anti-vacuolin antibody-labeled cells revealed a substantial increase in the number of vesicular structures per nucleus in dymA− cells (A′) compared with wild-type cells (A). In contrast to the uniform size of immunostained late endosomal compartments in wild-type cells, the accumulated structures in dymA− cells display a wider variety in size and shape and frequently appear reticular. (B) Confocal sections showing the colocalization (yellow) of dynamin A (Alexa488, green) and vacuolin (Cy3, red) in Ax2 cells expressing GFP–dynamin A. The large vacuolin-positive compartment shows prominent, punctate dynamin A staining (see also right cell in panel D). (C) Confocal sections showing the localization of GFP–dynamin A (Alexa488, green) and the Golgi apparatus as visualized with antibody 190-340-8 (Cy3, red). (D) Localization of GFP–dynamin A (Alexa488, green) in comparison to the distribution of mitochondria (Cy3, red). Mitochondria were labeled with mAb 70-100-1, directed against a mitochondrial porin from D. discoideum. Bar, 10 μm. Magnification in panel A is identical to A′.
The effects of dymA disruption were not restricted to the endo-lysosomal system. Normally mitochondria are dispersed uniformly throughout the cytoplasm of D. discoideum cells. In dymA− cells, mitochondria form continuous reticular structures of variable diameter that consist primarily of tubules and are branched at multiple points (Figure 12). The striking difference in mitochondrial morphology was best visualized by indirect immunofluorescence (Figure 12, A and A′) using an anti-porin antibody (Troll et al., 1992). Clusters of mitochondria and mitochondria forming continuous reticular structures could also be readily observed in living dymA− cells by phase contrast microscopy or after appropriate fixation by electron microscopy (Figure 12, D–F). In addition to these morphological changes, loss of dynamin A expression appears to affect mitochondrial function. The activity of the respiratory chain-marker enzyme, succinate dehydrogenase, was approximately twofold reduced (47 ± 8%) in homogenates of dymA− cells when compared with wild-type cells.
The EM micrographs also indicated differences in the size and shape of cell nuclei between dymA− cells and the parental Ax2 cells. Cells were stained with the DNA-specific dye DAPI to further investigate these differences in nuclear morphology (Figure 12, B, B′, C, and C′). On average, the nuclear diameters determined for dymA− cells (3.4 ± 0.8 μm; n = 376) and Ax2 cells (3.3 ± 0.5 μm; n = 212) were similar. However, ∼2% of dymA− nuclei measured 6–8 μm in diameter while none of the Ax2 nuclei was larger than 6 μm, and only one was larger than 5 μm. In addition to the occurrence of large nuclei in populations of dynamin A-depleted cells, DAPI-stained dymA− nuclei can easily be distinguished from wild-type nuclei by their lobed appearance, indicative of a difference in chromatin organization, and aberrant shape.
DISCUSSION
Dynamin A represents a new member of the family of dynamin-related proteins. The results presented here establish that this D. discoideum protein shares hallmark features, such as the tripartite GTP-binding motif, the consensus pattern LPRGSGIVTR, and a high degree of sequence identity with the N-terminal half of other dynamin-like proteins (Figure 1). The C-terminal half of dynamin A contains sequence motifs that could potentially serve roles similar to those of the PRD and GED of dynamin-1. However, the organization of these regions in the polypeptide chain of dynamin A differs from that of dynamin-1. Dynamin A contains a QNS-rich domain, and similar asparagine and glutamine repeats have been found in several other D. discoideum proteins, e.g., protein tyrosine phosphatase PTP3 (Gamper et al., 1996) and the catalytic subunit of cAMP-dependent protein kinase PKAC (Burki et al., 1991; Mann and Firtel, 1991). The significance of these repeats is not well understood, but it has been speculated that glutamine repeats may form polar zippers that make protein oligomerize or able to interact with other proteins containing glutamine repeats (Stott et al., 1995). Huntington’s disease and six other neurodegenerative diseases have been linked to abnormally expanded stretches of polyglutamine in the affected proteins.
Phylogenetic analysis places dynamin A in the same branch as the yeast proteins, Vps1p and Dnm1p, and the mammalian proteins, DVLP and DLP1. In primary structure and domain organization, dynamin A is more closely related to DLP1 and Dnm1p than dynamin-1. So far, little is known about the biochemical and structural features of this group of dynamin-like proteins. The members of this group of dynamin-like proteins appear to support vesicle trafficking processes at cytoplasmic sites distinct from the plasma membrane. Vps1p is associated with the Golgi and is required for proper sorting of proteins to the yeast vacuole (Vater et al., 1992; Wilsbach and Payne, 1993; Nothwehr et al., 1995). Dnm1p is mostly cytosolic and believed to function primarily in the maintenance of yeast mitochondrial network morphology (Shaw et al., 1997). Human DVLP is predominantly found in the perinuclear region, but its precise role and membrane localization are unknown (Shin et al., 1997). Rat DLP1 is predominately found in the soluble cytosolic fraction, but a substantial amount associates with endoplasmic reticulum tubules and vesicles that move unidirectionally from the cell periphery toward the perinuclear region; it may function in the transport of peripheral endoplasmic reticulum elements to the Golgi compartment (Yoon et al., 1998).
To gain insight into the cellular function of dynamin A, we used gene replacement techniques to create D. discoideum cells that no longer produce the protein. The resulting dymA− cells were viable under all conditions tested, indicating that the dymA gene product is not essential for D. discoideum growth. However, dynamin A-deficient cells displayed pleiotropic phenotypic changes in comparison to the parental Ax2 wild-type cells.
In agreement with a role of dynamin A in vesicle sorting and trafficking, the ability to internalize fluid phase markers by pinocytosis was reduced in dymA− cells. Both the initial rate of uptake and the extent to which the marker was accumulated were affected. At the same time efflux of the fluid phase marker was normal, indicating dynamin A participates in endosomal trafficking, but not in recycling of internalized fluid. The prolonged transit time for fluid phase in the dymA− cells indicates that the intracellular processing of fluid phase cargo is altered. Immunofluorescence analysis was used to further dissect the defect in endocytosis. Coronin, a cytoplasmic actin-associated protein and a marker of endocytic compartments, is strongly enriched in a cytoskeletal coat that transiently surrounds both particle- and fluid-containing vacuoles in D. discoideum (Maniak et al., 1995; Hacker et al., 1997). Within the first minute after internalization, both coronin and actin are gradually released from the phagosome or macropinosome. After dissociation of the cytoskeletal coat, acidification and digestion of the vesicle contents occur. The rapid acidification to pH 5 is followed by neutralization and reassociation with a cytoskeletal coat. The ingested cargo is thus progressively shuttled to a large, neutral compartment that acquires fluid-phase markers 60–80 min after endocytosis, just before exocytosis (Rauchenberger et al., 1997). While the cellular localization of coronin and the frequency of coronin-associated vacuolar structures appeared unchanged, the vacuolin-decorated, postlysosomal compartment was greatly enriched in dynamin A-depleted cells and may, at least in part, account for the accumulation of large vacuoles (>300 nm) and tubular structure in these cells (Figures 9 and 10). The colocalization of dynamin A and vacuolin supports the idea that dynamin A might be required for the breakdown of the vacuolin-decorated postlysosomal vesicles.
The role of mammalian dynamin-1 in clathrin-mediated endocytosis is well established (Herskovits et al., 1993; van der Bliek et al., 1993; Damke et al., 1994). It was shown that fluid-phase uptake is affected in a HeLa cell line expressing a temperature-sensitive dynamin mutant. However, after shift to the nonpermissive temperature, these HeLa cells rapidly and completely compensate for the loss of clathrin-dependent endocytosis by inducing an alternate endocytic pathway (Damke et al., 1995; Lamaze and Schmid, 1995). In contrast, pinocytosis is constitutively impaired in D. discoideum cells lacking either dynamin A or the clathrin heavy chain (O’Halloran and Anderson, 1992; Ruscetti et al., 1994). Apart from this reduced activity in pinocytosis, the dymA− and CHC− mutants display a spectrum of common and separate defects. CHC− and dymA− cells share a defect in cytokinesis. However, while CHC− cells are unable to grow in suspension cultures and fail to divide due to an inability to form a functional contractile ring during cell division (Niswonger and O’Halloran, 1997a), dymA− cells grow in suspension culture and pass through all stages of cytokinesis up to the formation of the midbody. Only the final step, severing of the thin cytoplasmic bridge connecting the incipient daughter cells, is inhibited in dymA− cells. A distinct defect of the CHC− cells is the lack of a contractile vacuole and the resulting impairment in osmoregulation. In contrast, the contractile vacuole system of dymA− cells was found to be fully functional, and its morphological organization was similar to that of wild-type cells. The developmental program is affected both in CHC− and dymA− cells. It was demonstrated that clathrin increases the efficiency of early development and is essential for differentiation of mature spore cells (Niswonger and O’Halloran, 1997b). Dynamin A-deficient cells show a comparable defect in the early stages of development; however, progress from the formation of a pseudoplasmodium to the culmination of the fruiting body was normal. Fruiting bodies formed by dynamin A-deficient cells display significant morphological alterations, but they are still capable of producing viable spores, although spore viability is reduced to 10–20% of wild-type levels. Finally, while CHC− cells display defects in sorting and secretion of lysosomal enzymes (Ruscetti et al., 1994), this pathway does not seem to be affected by disruption of dymA. Lysosomal α-mannosidase is processed normally and apparently not missorted in dymA− cells, and secretion of mature forms of the enzyme also appears to be normal (data not shown).
The strong impairment in endocytosis of fluid phase markers in CHC− mutants suggested initially that fluid entry proceeds in D. discoideum mainly through clathrin-mediated endocytosis (O’Halloran and Anderson, 1992). However, a number of recent studies indicate that, in D. discoideum, fluid-phase uptake proceeds predominantly by macropinocytosis and that the reduction in pinocytosis capacity of axenically grown CHC− cells is caused by cross-talk between the actin- and clathrin-dependent routes of fluid entry during endocytosis (Adessi et al., 1995; Hacker et al., 1997). Similarly, the observed reduction in the uptake of fluid-phase markers by dymA− cells may be an indirect effect of dynamin A depletion. This interpretation is best supported by the observed distribution of coated structures in dymA− cells and is in agreement with a general role of dynamins in the budding of vesicles from membranous structures and vesicle scission (Hinshaw and Schmid, 1995; Takei et al., 1995). The absence of coated pits from the plasma membrane and the prominent and frequent occurrence of coated pit-decorated vesicles (Figure 10D) support the hypothesis that, in D. discoideum, fluid-phase uptake is an actin-dependent process and that dynamins play a role in the breakdown of macropinosomes and endosomes rather than the direct entry of extracellular fluid (Aubry et al., 1997).
Pleiotropic effects of shibire and dynamin-1 mutations are well known (Poodry et al., 1973; Chen et al., 1991; van der Bliek et al., 1993). Depletion of the closely related yeast Dnm1p, which, like dynamin A and DLP1, is mostly cytosolic, results in a twofold delay in the transport of endocytic material to the vacuole (Gammie et al., 1995). However, recent studies provide strong evidence that Dnm1p localizes to the tip of mitochondria and functions primarily in the maintenance of yeast mitochondrial network morphology (Shaw et al., 1997). Functions unrelated to vesicle trafficking have also been reported for the dynamin-like yeast proteins, Vps1p/Spo15p and Mgm1p. Vps1p/Spo15p is required for the timely separation of spindle-pole bodies in meiosis (Yeh et al., 1991), while Mgm1p is needed for the maintenance of the mitochondrial genome (Jones and Fangman, 1992; Guan et al., 1993), respectively. Our findings imply that dynamin A is likely to function in vesicle-trafficking processes related to the endo-lysosomal system of D. discoideum. The observed alterations in mitochondrial and nuclear morphology on dynamin A depletion are probably indirect consequences of a more general defect in membrane transport processes. This view is supported by the morphological alterations of the postlysosomal compartment in dymA− cells (Figure 9), a strong increase in the number of coated structures decorating intracellular membrane compartments in dymA− cells (Figure 10), and the finding that GFP-labeled dynamin A colocalizes mostly with vacuolin-decorated membranes and does not colocalize with either nuclei or mitochondria (Figure 11).
Further investigations will be greatly facilitated by the fact that D. discoideum is readily accessible by biochemical, cell biological, and molecular genetic approaches. This will help to clarify the involvement of dynamin A in cytokinesis and define the exact role of the protein in vesicle targeting and transport processes.
Accession Numbers
Dynamin A sequences reported in this article have been submitted to the GenBank/EMBL data bank with the accession numbers Q94464 for the peptide sequence and X99669 for the nucleotide sequence.
ACKNOWLEDGMENTS
We thank R. Albrecht, R. Batra, C. Heizer, E. Hirst, D. Hunt, R. Neujahr, M.D. Peterson, J. Sabry, S. Sinda, and I. Weber for help and advice; and J.A. Cardelli and co-workers for help with the secretion assays. We are especially grateful to G. Gerisch, H. Horstmann, M. Maniak, M. Sheetz, T. Soldati, and M. Titus for their many ideas and discussions. G. Gerisch, M. Maniak and A. Noegel provided antibodies 70-100-1, 221-1-1, and 190-340-8. We thank K.C. Holmes and W. Almers for support and encouragement. The work was supported by the Max Planck Society (Germany). M.L.W.K. was supported by a long-term fellowship from the European Molecular Biology Organization.
REFERENCES
- Adessi C, Chapel A, Vincon M, Rabilloud T, Klein G, Satre M, Garin J. Identification of major proteins associated with Dictyostelium discoideum endocytic vesicles. J Cell Sci. 1995;108:3331–3337. doi: 10.1242/jcs.108.10.3331. [DOI] [PubMed] [Google Scholar]
- Aubry L, Klein G, Satre M. Cytoskeletal dependence and modulation of endocytosis in Dictyostelium discoideum amoebae. In: Maeda Y, Inouye K, Takeuchi I, editors. Dictyostelium a Model System for Cell and Developmental Biology. Tokyo: Universal Academy Press; 1997. pp. 65–72. [Google Scholar]
- Bacon RA, Cohen CJ, Lewin DA, Mellman I. Dictyostelium discoideum mutants with temperature-sensitive defects in endocytosis. J Cell Biol. 1994;127:387–399. doi: 10.1083/jcb.127.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli JA. Evidence for a recycling role of Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki E, Anjard C, Scholder JC, Reymond CD. Isolation of 2 genes encoding putative protein-kinases regulated during Dictyostelium-discoideum development. Gene. 1991;102:57–65. doi: 10.1016/0378-1119(91)90538-m. [DOI] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991;10:4097–4104. doi: 10.1002/j.1460-2075.1991.tb04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy-chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Faix J, Gerisch G, Noegel AA. Overexpression of the csA cell adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium. J Cell Sci. 1992;102:203–214. doi: 10.1242/jcs.102.2.203. [DOI] [PubMed] [Google Scholar]
- Fok AK, Clarke M, Ma L, Allen RD. Vacuolar H(+)-ATPase of Dictyostelium discoideum. A monoclonal antibody study. J Cell Sci. 1993;106:1103–1113. doi: 10.1242/jcs.106.4.1103. [DOI] [PubMed] [Google Scholar]
- Gammie AE, Kurihara LJ, Vallee RB, Rose MD. DNM1, a dynamin-related gene, participates in endosomal trafficking in yeast. J Cell Biol. 1995;130:553–566. doi: 10.1083/jcb.130.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper M, Howard PK, Hunter T, Firtel RA. Multiple roles of the novel protein tyrosine phosphatase PTP3 during Dictyostelium growth and development. Mol Cell Biol. 1996;16:2431–2444. doi: 10.1128/mcb.16.5.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Verma DP. Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. EMBO J. 1996;15:695–704. [PMC free article] [PubMed] [Google Scholar]
- Guan K, Farh L, Marshall TK, Deschenes RJ. Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr Genet. 1993;24:141–148. doi: 10.1007/BF00324678. [DOI] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Heimer GV, Taylor CE. Improved mountant for immunofluorescence preparations. J Clin Pathol. 1974;27:254–256. doi: 10.1136/jcp.27.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE, Schmid SL. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Jones BA, Fangman WL. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes & Dev. 1992;6:380–389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983a;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol. 1983b;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama H, Ecke M, Gerisch G, Van Haastert PJ. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science. 1996;271:207–209. doi: 10.1126/science.271.5246.207. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Liu T, Williams JG, Clarke M. Inducible expression of calmodulin antisense RNA in Dictyostelium cells inhibits the completion of cytokinesis. Mol Biol Cell. 1992;3:1403–1413. doi: 10.1091/mbc.3.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell. 1995;83:915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Mann SK, Firtel RA. A developmentally regulated, putative serine/threonine protein kinase is essential for development in Dictyostelium. Mech Dev. 1991;35:89–101. doi: 10.1016/0925-4773(91)90060-j. [DOI] [PubMed] [Google Scholar]
- Manstein DJ, Schuster H-P, Morandini P, Hunt DM. Cloning vectors for the production of proteins in Dictyostelium discoideum. Gene. 1995;162:129–134. doi: 10.1016/0378-1119(95)00351-6. [DOI] [PubMed] [Google Scholar]
- Manstein DJ, Titus MA, De Lozanne A, Spudich JA. Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 1989;8:923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré DJ, Brightman AO, Sandelius AS. In: In: Biological Membranes: A Practical Approach. Findlay JBC, Evans WH, editors. Oxford, England: IRL Press; 1987. pp. 37–50. [Google Scholar]
- Muhlberg AB, Warnock DE, Schmid SL. Domain structure and intramolecular regulation of dynamin GTPase. EMBO J. 1997;16:6676–6683. doi: 10.1093/emboj/16.22.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus EM, Horstmann H, Almers W, Maniak M, Soldati T. Ethane-freezing/methanol-fixation of cell monolayers—a procedure for improved preservation of structure and antigenicity for light and electron microscopies. J Struct Biol. 1998;121:326–342. doi: 10.1006/jsbi.1998.3971. [DOI] [PubMed] [Google Scholar]
- Neujahr R, Heizer C, Gerisch G. Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: redistribution of the nuclei, re-arrangement of the actin system and formation of the cleavage furrow. J Cell Sci. 1997;110:123–137. doi: 10.1242/jcs.110.2.123. [DOI] [PubMed] [Google Scholar]
- Niswonger ML, O’Halloran TJ. A novel role for clathrin in cytokinesis. Proc Natl Acad Sci USA. 1997a;94:8575–8578. doi: 10.1073/pnas.94.16.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswonger ML, O’Halloran TJ. Clathrin heavy chain is required for spore cell but not stalk cell differentiation in Dictyostelium discoideum. Development. 1997b;124:443–451. doi: 10.1242/dev.124.2.443. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KD, Peterson MD, Reedy MC, Titus MA. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J Cell Biol. 1995;131:1205–1221. doi: 10.1083/jcb.131.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990;347:256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- O’Halloran TJ, Anderson RG. Characterization of the clathrin heavy chain from Dictyostelium discoideum. DNA Cell Biol. 1992;11:321–330. doi: 10.1089/dna.1992.11.321. [DOI] [PubMed] [Google Scholar]
- Park JM, Cho JH, Kang SG, Jang HJ, Pih KT, Piao HL, Cho MJ, Hwang I. A dynamin-like protein in Arabidopsis thaliana is involved in biogenesis of thylakoid membranes. EMBO J. 1998;17:859–867. doi: 10.1093/emboj/17.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poodry CA, Hall L, Suzuki DT. Developmental properties of shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973;32:373–386. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- Raper KB. Dictyostelium discoideum, a new species of slime mold from decaying forest leaves. J Agric Res. 1935;58:135–147. [Google Scholar]
- Rauchenberger R, Hacker U, Murphy J, Niewohner J, Maniak M. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr Biol. 1997;7:215–218. doi: 10.1016/s0960-9822(97)70093-9. [DOI] [PubMed] [Google Scholar]
- Ruscetti T, Cardelli JA, Niswonger ML, O’Halloran TJ. Clathrin heavy chain functions in sorting and secretion of lysosomal enzymes in Dictyostelium discoideum. J Cell Biol. 1994;126:343–352. doi: 10.1083/jcb.126.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shaw JM, Otsuga D, Keegan B, Hermann G, Bleazard W. The dynamin-like GTPase Dnm1p is required for maintenance of yeast mitochondrial network morphology. Mol Biol Cell. 1997;8:2470a. (Abstract). [Google Scholar]
- Shin HW, Shinotsuka C, Torii S, Murakami K, Nakayama K. Identification and subcellular localization of a novel mammalian dynamin-related protein homologous to yeast Vps1p and Dnm1p. J Biochem. 1997;122:525–530. doi: 10.1093/oxfordjournals.jbchem.a021784. [DOI] [PubMed] [Google Scholar]
- Stott K, Blackburn JM, Butler PJG, Perutz M. Incorporation of glutamine repeats makes protein oligomerize: implications for neurodegenerative diseases. Proc Natl Acad Sci USA. 1995;92:6509–6513. doi: 10.1073/pnas.92.14.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. A transformation vector for Dictyostelium discoideum with a new selectable marker bsr. Plasmid. 1993;30:150–154. doi: 10.1006/plas.1993.1042. [DOI] [PubMed] [Google Scholar]
- Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-γ-S in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Temesvari LA, Bush JM, Peterson MD, Novak KD, Titus MA, Cardelli JA. Examination of the endosomal and lysosomal pathways in Dictyostelium discoideum myosin I mutants. J Cell Sci. 1996;109:663–673. doi: 10.1242/jcs.109.3.663. [DOI] [PubMed] [Google Scholar]
- Troll H, Malchow D, Muller-Taubenberger A, Humbel B, Lottspeich F, Ecke M, Gerisch G, Schmid A, Benz R. Purification, functional characterization, and cDNA sequencing of mitochondrial porin from Dictyostelium discoideum. J Biol Chem. 1992;267:21072–21079. [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum B, Edwards KB, Soll DR. Dictyostelium amebae alter motility differently in response to increasing versus decreasing temporal gradients of cAMP. J Cell Biol. 1985;101:1–5. doi: 10.1083/jcb.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OH, Murphy J, Griffiths G, Schleicher M, Noegel A. The actin-binding protein comitin (p24) is a component of the Golgi apparatus. J Cell Biol. 1993;123:23–34. doi: 10.1083/jcb.123.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Jungbluth A, Heidecker M, Muhlbauer B, Heizer C, Schwartz JM, Marriott G, Gerisch G. Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion protein. Curr Biol. 1997;7:176–183. doi: 10.1016/s0960-9822(97)70088-5. [DOI] [PubMed] [Google Scholar]
- Wienke DC, Batra R, Knetsch MLW, Manstein DJ. Overexpression, purification and characterization of a Dictyostelium dynamin-homologue. Mol Biol Cell. 1997;8:2471a. (Abstract). [Google Scholar]
- Wilsbach K, Payne GS. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 1993;12:3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Driscoll R, Coltrera M, Olins A, Bloom K. A dynamin-like protein encoded by the yeast-like sporulation gene SPO15. Nature. 1991;349:713–715. doi: 10.1038/349713a0. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Pitts KR, Dahan S, McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J Cell Biol. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Liu T, Clarke M. Calmodulin and the contractile vacuole complex in mitotic cells of Dictyostelium discoideum. J Cell Sci. 1993;104:1119–1127. doi: 10.1242/jcs.104.4.1119. [DOI] [PubMed] [Google Scholar]