Abstract
Background
In recent years, an idiosyncratic new class of bacterial enzymes, named BY-kinases, has been shown to catalyze protein-tyrosine phosphorylation. These enzymes share no structural and functional similarities with their eukaryotic counterparts and, to date, only few substrates of BY-kinases have been characterized. BY-kinases have been shown to participate in various physiological processes. Nevertheless, we are at a very early stage of defining their importance in the bacterial cell. In Escherichia coli, two BY-kinases, Wzc and Etk, have been characterized biochemically. Wzc has been shown to phosphorylate the UDP-glucose dehydrogenase Ugd in vitro. Not only is Ugd involved in the biosynthesis of extracellular polysaccharides, but also in the production of UDP-4-amino-4-deoxy-L-arabinose, a compound that renders E. coli resistant to cationic antimicrobial peptides.
Methodology/Principal Findings
Here, we studied the role of Ugd phosphorylation. We first confirmed in vivo the phosphorylation of Ugd by Wzc and we demonstrated that Ugd is also phosphorylated by Etk, the other BY-kinase identified in E. coli. Tyrosine 71 (Tyr71) was characterized as the Ugd site phosphorylated by both Wzc and Etk. The regulatory role of Tyr71 phosphorylation on Ugd activity was then assessed and Tyr71 mutation was found to prevent Ugd activation by phosphorylation. Further, Ugd phosphorylation by Wzc or Etk was shown to serve distinct physiological purposes. Phosphorylation of Ugd by Wzc was found to participate in the regulation of the amount of the exopolysaccharide colanic acid, whereas Etk-mediated Ugd phosphorylation appeared to participate in the resistance of E. coli to the antibiotic polymyxin.
Conclusions/Significance
Ugd phosphorylation seems to be at the junction between two distinct biosynthetic pathways, illustrating the regulatory potential of tyrosine phosphorylation in bacterial physiology.
Introduction
In bacteria, protein phosphorylation is catalyzed mainly by histidine-kinases which are key enzymes of the so-called “two component systems” [1], by proteins of the phosphotransferase system involved in sugar transport and phosphorylation as well as many regulatory functions and by Hanks-type Serine/Threonine Protein Kinases (STPKs) [2]. However, the presence of tyrosine-kinases has been proven in several bacterial species, and suggested in many more by homology-based gene annotation. These tyrosine-kinases share little structural similarities with their eukaryotic counterparts [3], [4] and most of them have been recently unified in a new enzyme family called BY-kinase [5]. Until now, BY-kinases have been found only in bacteria and they seem to constitute an idiosyncratic class of enzymes. They have been shown to be involved in several physiological processes such as DNA metabolism or heat shock response [6], [7]. In several bacteria, including both proteobacteria and firmicutes, they have also been established as co-polymerases involved in synthesis and export of extracellular polysaccharides [8], [9]. However, their accurate functions remain poorly understood due to slow progress in structural characterization and to the fact that only few phosphorylation substrates have been detected. BY-kinases are autophosphorylating enzymes and they are also able to phosphorylate endogenous proteins. It was only recently that sugar-dehydrogenases or -transferases involved in polysaccharide production [10]–[13], RNA polymerase sigma factors [7] and single-stranded DNA binding proteins [14] were identified as BY-kinase substrates. Nevertheless, recent phosphoproteomic studies indicate that BY-kinases could phosphorylate a significant number of other proteins [15], [16].
Escherichia coli produces two BY-kinases, Wzc and Etk. They are respectively encoded by genes located at approximately 46 min and 22 min on the E. coli chromosome in two gene-clusters both involved in the biosynthesis of extracellular polysaccharides [17], [18]. Accordingly, Wzc and Etk have been characterized as polysaccharide co-polymerases (PCP) belonging to multiprotein transmembrane machineries involved in synthesis and/or export of extracellular polysaccharides, and their autophosphorylation on several tyrosines has turned out to be a key feature in the production of these compounds [19]. In addition, it has been demonstrated that Wzc and Etk are involved in other processes. For instance, Wzc is able to phosphorylate and down regulate the activity of Int, the integrase of coliphage HK022 [20]. In the same way, Etk has been found to be involved in heat shock response by phosphorylating sigma and anti-sigma factors [7].
Protein Ugd of E. coli, a UDP-glucose dehydrogenase, is one of the first identified substrates of a BY-kinase [10]. Indeed, it has been shown that Ugd is phosphorylated in vitro by the BY-kinase Wzc. Other types of sugar-dehydrogenases, including the UDP-glucose dehydrogenase of Bacillus subtilis UgdBs [21] and the UDP-acetyl-mannosamine dehydrogenase CapO of Staphylococcus aureus [12], have recently been shown to be tyrosine-phosphorylated. Thus, it could be speculated that tyrosine phosphorylation of this class of enzymes is a common regulatory mechanism found in several bacteria. Ugd produces UDP-glucuronic acid (UDPGA) that is a precursor and an essential component in the biosynthesis of bacterial polysaccharides, notably of E. coli [19]. UDPGA is found in several capsular polysaccharides (K-antigens) and in colanic acid (M-antigen), an extracellular polysaccharide produced by many strains of E. coli. In addition, UDPGA participates in the production of a sugar derivative, UDP-4-amino-4-deoxy-L-arabinose (L-Ara4N) which is a crucial element in bacterial resistance to antibiotics such as polymyxin and cationic peptides of the innate immune system [22]–[25]. Thus, in the search of the role of tyrosine phosphorylation in bacteria, the question was raised whether Ugd phosphorylation could affect, on the one hand, the production of polysaccharides and, on the other hand, bacterial resistance to cationic peptides. In E. coli K-12, we had previously demonstrated that Wzc autophosphorylation influences the production of colanic acid, [26]. In addition, we had also shown that an etk knock-out mutant is much less resistant to polymyxin than a wild-type E. coli K-12 strain. [4], [27]. However, Ugd has not been described as being phosphorylated by Etk. Therefore, it seemed particularly interesting to investigate if Ugd is also a phosphorylation substrate for Etk and if Ugd phosphorylation by either Wzc or Etk affects colanic acid production or polymyxin resistance, respectively.
In this work, we provide in vivo evidence that, depending on the growth conditions, Ugd can be phosphorylated by either Wzc or Etk. We show that Ugd is phosphorylated on the same site by both BY-kinases, Wzc and Etk. We further show that Wzc-mediated phosphorylation of Ugd specifically affects the biosynthesis of colanic acid whereas resistance to the cationic antimicrobial peptide polymyxin is dependent upon Ugd phosphorylation by Etk. These data represent the first report of a bacterial protein phosphorylated by two distinct tyrosine-kinases. They contribute to define the role of tyrosine phosphorylation in bacteria and provide a basis for an emerging regulatory network in E.coli.
Results
Ugd is phosphorylated in vivo both by Wzc and Etk
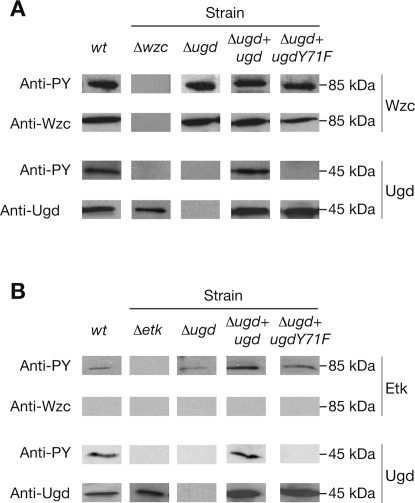
We have previously reported that Ugd is monophosphorylated in vitro by the catalytic domain of Wzc, Wzccyto [10] (Table S1). The Wzc protein is not synthesized in standard growth conditions and to characterize in vivo phosphorylation of Ugd by Wzc, we used a strain that encodes the transcriptional regulator RcsA [26]. RcsA is known to enhance the expression of wzc and ugd [28], [29] (Table S1) but not that of etk, which is not expressed in our culture conditions [30], [31]. After growth in Luria Bertani medium, a total protein extract was prepared, analyzed by SDS-PAGE and immunoblotted against either a monoclonal anti-Wzc antibody, or a monoclonal anti-Ugd antibody, or an anti-phosphotyrosine antibody (Fig. 1A). Immunorevelation with the Wzc- and Ugd-specific antibodies showed signals at around 81-kDa and 45-kDa, which are respectively consistent with the expression of the two proteins. The anti-phosphotyrosine antibody revealed an expected signal corresponding to autophosphorylated Wzc. Also, we observed a 45-kDa signal that could correspond to the phosphorylated form of Ugd. To strengthen these observations, we similarly analyzed a wzc knock-out mutant [26]. We did not detect any signal for Wzc or phosphotyrosine around 45-kDa, whereas Ugd was still detected by the Ugd-specific antibody (Fig. 1A). In addition, the ugd gene was inactivated (Table S1) and the Δugd strain obtained was analyzed as described above (Fig. 1A). We still observed the expression and phosphorylation of Wzc, but no 45-kDa signal was detected by either the Ugd-specific antibody or the anti-phosphotyrosine antibody. These data confirmed that Ugd was phosphorylated in vivo on tyrosine by Wzc.
Figure 1. Expression and in vivo phosphorylation of Ugd, UgdY71F and Wzc or Etk.
Western immunoblot of whole-cell lysates prepared when E. coli is grown under conditions allowing (A) colanic acid production or (B) polymyxin resistance were probed with either a Wzc-specific monoclonal antibody (Anti-Wzc), or a Ugd-specific monoclonal antibody (Anti-Ugd), or an anti-phosphotyrosine antibody (Anti-PY). E. coli wild-type strain (wt), wzc- or etk-deficient strain (Δwzc or Δetk), ugd-deficient strain (Δugd) and ugd-deficient strain complemented with wild-type ugd (Δugd+ugd) or mutated ugd (Δugd+ugdY71F).
E. coli cells encode another BY-kinase, namely Etk, that is homologous to Wzc. We wondered whether Etk was also able to phosphorylate Ugd. Like Wzc, Etk is not produced by E. coli K-12 under standard laboratory growth conditions. In addition, etk expression is not dependent on protein RcsA. It has previously been shown that etk, but not wzc, is expressed when E. coli K-12 grows in a culture medium at low pH and low concentration of magnesium and iron ions. Such conditions induce resistance to cationic antimicrobial peptides [27]. We verified that etk was expressed during growth of E. coli K-12 in such medium. No 81-kDa signal was observed when detection was carried out with the Wzc-specific antibody whereas a signal appeared at this position when we used the anti-phosphotyrosine antibody (Fig. 1B). This observation confirmed that wzc is not expressed and strongly suggested that autophosphorylated Etk is produced under these culture conditions. To validate this point, we analyzed an etk-deficient strain. Immunoblot analysis showed no 81-kDa signal and supported the assumption that the signal detected with the anti-phosphotyrosine antibody in the wild-type strain corresponded to Etk autophosphorylation. In addition, RT-PCR experiments were performed and we observed that the etk gene was effectively expressed under these growth conditions (data not shown). To assess if Etk can phosphorylate Ugd, we also analyzed total protein extracts of wild-type, ugd-deficient and etk-deficient strains of E. coli by immunorevelation with the Ugd-specific antibody and the anti-phosphotyrosine antibody (Fig. 1B). We observed that Ugd was produced and phosphorylated in the wild-type strain. On the contrary, no 45-kDa phosphorylation signal was detected with ugd and etk knock-out mutants while Ugd was still detected in the etk mutant. These observations confirmed that Ugd was also phosphorylated in vivo by Etk.
Ugd is activated by phosphorylation on Tyr71
To decipher the role of Ugd phosphorylation, it was necessary to identify the site of phosphorylation of E. coli Ugd. We had already observed that Ugd seemed to be monophosphorylated [10]. Mass spectrometry analysis (MALDI-TOF) failed to identify the Ugd phosphorylated tyrosine probably because of the low occupancy of bacterial phosphorylation sites [32]. Therefore, sequence alignments were performed with protein UgdBs, a UDP-sugar-dehydrogenase from Bacillus subtilis homologous to Ugd and also phosphorylated on tyrosine (Fig. S1A). Several tyrosines of Ugd (Tyr10, Tyr150, Tyr249, Tyr335 and Tyr380) that seemed conserved in UgdBs were mutated to phenylalanine. However, when each mutant protein was purified and phosphorylated in vitro by the catalytic domain of Wzc, Wzccyto, a radioactive signal corresponding to phosphorylated Ugd was still detected for each of them (Fig. S1B). While this work was in progress, a study of the B. subtilis phosphoproteome indicated that UgdBs was phosphorylated in vivo on tyrosine 70 [16]. According to sequence alignments, the closest tyrosine in the E. coli Ugd sequence is Tyrosine 71 (Tyr71) (Fig. S1A). We hypothesized that Tyr71 could be the phosphorylation site of Ugd and we constructed the Ugd mutant Tyr71Phe (UgdY71F).
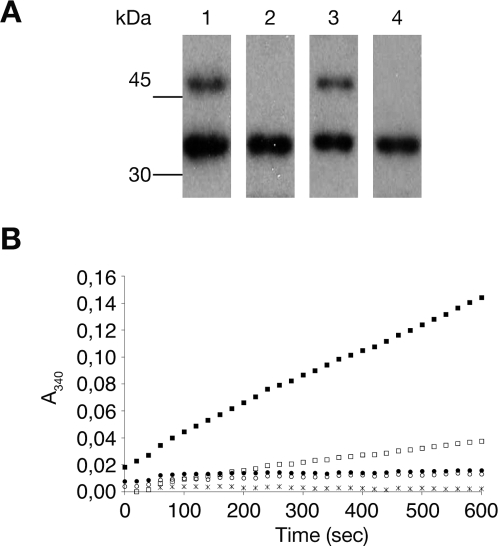
To check this hypothesis, the phosphorylation signal of Ugd was compared with that of UgdY71F. When incubated for 2 min with Wzccyto (kinase/substrate ratio of 1/100), we observed that Ugd was phosphorylated whereas no radioactive signal was detected for UgdY71F (Fig. 2A). These data suggested that Tyr71 is the phosphorylation site of Ugd. To check that the folding of the Ugd protein was not affected by the Y71F mutation, dynamic light scattering (DLS) measurements were performed. The hydrodynamic radius measured for Ugd and UgdY71F were the same (RS = 62+/−3 Å) confirming that the overall structure of Ugd was most likely not affected by the Tyr to Phe mutation. To confirm Tyr71 as the phosphorylation site responsible for activating the enzyme, the dehydrogenase activity of Ugd and UgdY71F was measured with or without prior incubation with Wzccyto and ATP (Fig. 2B). We have previously shown that Ugd phosphorylation increased its dehydrogenase activity [10]. As expected, Ugd activity was stimulated more than 10-fold upon phosphorylation. By contrast, UgdY71F activity remained unaffected by incubation with Wzccyto. In addition, UgdY71F was less active than Ugd which is in agreement with our previous observations showing that Ugd purified from E. coli is partially phosphorylated, and that its extensive dephosphorylation by the phosphotyrosine phosphatase Wzb reduces its activity [10]. These data indicated that UgdY71F was no longer activated by phosphorylation and we concluded that Tyr71 was the regulatory phosphorylation site of Ugd.
Figure 2. Activation of the UDP-glucose dehydrogenase activity of Ugd by phosphorylation on Tyr71.
(A) Autoradiography of SDS-PAGE on which reaction mixtures containing [γ-32P]ATP and either Ugd and Wzccyto (lane 1) or UgdY71F and Wzccyto (lane 2) or Ugd and Etkcyto (lane 3) or UgdY71F and Etkcyto (lane 4) were analyzed. (B) UDP-glucose dehydrogenase activity was monitored at 340 nm for 10 min by measuring NADH formation: Ugd (□), Ugd previously phosphorylated by Wzccyto (▪), UgdY71F (○) and UgdY71F previously incubated with Wzccyto (•). As a control, a reaction mixture without Ugd was used (*). Standard deviations are not indicated because of low variations.
Ugd phosphorylation by Wzc on Tyr71 influences the production of colanic acid
The cps operon that includes wzc, and the ugd gene that is located elsewhere on the genome, are required for the synthesis of colanic acid, the extracellular polysaccharide of E. coli K-12. Expression of both cps operon and ugd are dependent on the Rcs system [29] and, as previously shown, the strain producing the transcriptional regulator RcsA is able to synthesize colanic acid [26]. In this strain, we showed that Ugd is phosphorylated in vivo by Wzc (Fig. 1A). We therefore used this strain (referred to herein as the wild-type) to assess the importance of Ugd phosphorylation on colanic acid synthesis. First, we confirmed that Ugd Tyr71 was not phosphorylated in vivo by Wzc. For this, we complemented the ugd-deficient strain (Δugd strain) with an episomal copy of either the ugd gene (Δugd+ugd strain) or the ugd Y71F allele (Δugd+ugdY71F strain) (Table S1). Total protein extracts were prepared from these two strains and the protein synthesis and phosphorylation for both Wzc and Ugd were analyzed by antibodies as described above (Fig. 1A). We observed that proteins Wzc, Ugd, and UgdY71F, were produced. In contrast, Ugd was phosphorylated only in the strain Δugd+ugd. Indeed, no 45-kDa phosphorylation signal was detected for the strain producing UgdY71F. These data confirmed that in vivo phosphorylation of Ugd by Wzc occurs at Tyr71.
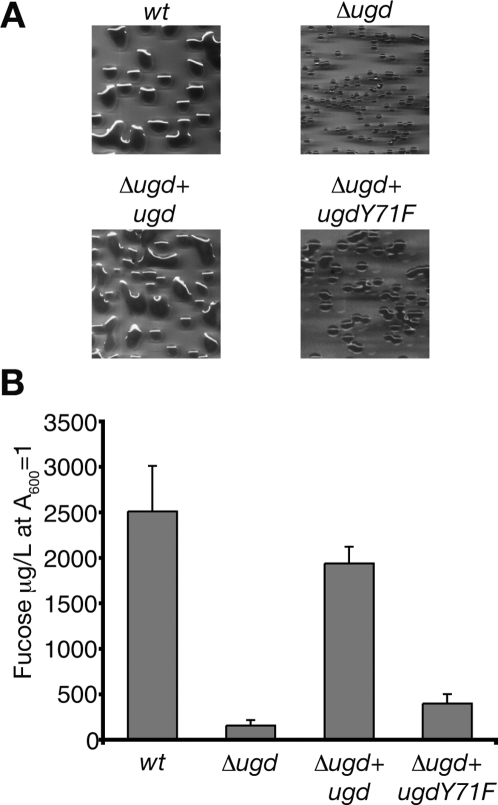
E. coli K-12 producing colanic acid exhibits a mucoid phenotype, and on plates, colonies have a fatty and shiny appearance. In contrast, smaller and duller colonies are observed when colanic acid is not produced. Therefore, we compared the colony morphology of strains producing Ugd, wild-type form or mutated on Tyr71 (Fig. 3A). We first observed that colony morphologies of the wild-type and Δugd+ugd strains were almost indistinguishable and they had characteristics of a mucoid phenotype due to the production of colanic acid (Fig. 3A). By contrast, colonies formed by the Δugd strain differed considerably and were not mucoid. When looking at the colony morphology of Δugd+ugdY71F strain, we also observed a non-mucoid phenotype similar to the Δugd strain (Fig. 3A). These data supported the hypothesis that the Wzc-mediated phosphorylation of Ugd on Tyr71 controls colanic acid production. To strengthen this observation, we prepared and quantified colanic acid produced by each strain used (Fig. 3B). Quantification was performed by measuring the amount of fucose, which is exclusively found in this polysaccharide [17]. As expected, the wild-type and Δugd+ugd strains produced comparable amounts of colanic acid, whereas the amount of colanic acid determined for the Δugd strain was up to 10-fold lower. Concerning the Δugd+ugdY71F strain, a 5-fold reduction was measured compared to Δugd+ugd strain. Colanic acid still produced by the Δugd+ugdY71F strain was likely due to the basal activity of UgdY71F (Fig. 2B). Accordingly, colony morphology of Δugd+ugdY71F strain began to be mucoid when growth was allowed for more than 48 hours (data not shown). These data nevertheless confirm the two distinct phenotypes: mucoid for the wild-type and Δugd+ugd strains, and non-mucoid for the Δugd and Δugd+ugdY71F strains. They also confirm that phosphorylation of UgdTyr71 by Wzc affects colanic acid production.
Figure 3. Influence of Ugd Tyr71 phosphorylation on colanic acid production.
(A) Colony morphology of E. coli wt and mutants. Photographs were taken after growth on LB Agar plates for 24 h at 37°C. (B) Production of colanic acid by wild-type E. coli and mutants. The amount of colanic acid was determined in each strain by measuring fucose, and was expressed as µg/L of culture of A600 = 1. Standard deviations from four independent experiments are indicated with error bars.
Ugd phosphorylation by Etk on Tyr71 influences polymyxin resistance
In E. coli K-12, Ugd catalyzes the first step of the biosynthesis of L-Ara4N, which confers bacterial resistance to cationic antimicrobial peptides and antibiotics such as polymyxin [33]. It has been previously reported that E. coli resistance to polymyxin depends also on the expression of etk [4], [27]. Here, we showed that Ugd was phosphorylated in vivo by Etk when E. coli was grown under culture conditions allowing resistance to polymyxin [34] (Fig. 1B). Therefore, it was tempting to assume that Etk could regulate polymyxin resistance by phosphorylating Ugd. We therefore tested whether Etk-catalyzed in vivo phosphorylation of Ugd would also occur on Tyr71. For this, the Δugd+ugd and the Δugd+ugdY71F strains were grown in the conditions allowing polymyxin resistance and total protein extracts were prepared and analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 1B, detection with the Ugd-specific antibody indicated that Ugd was produced in both Δugd+ugd and Δugd+ugdY71F strains. However, when immunoblotted with the anti-phosphotyrosine antibody, a phosphorylation signal was detected only when the wild-type form of Ugd was produced. This observation confirmed that Ugd was specifically phosphorylated in vivo on Tyr71 by Etk and that Ugd Tyr71 phosphorylation could consequently influence polymyxin resistance.
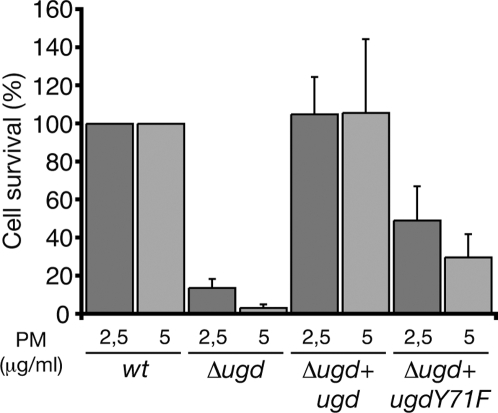
To check this, each strain was assayed for polymyxin resistance by measuring the percentage of surviving cells (Fig. 4). E. coli strains were grown in the culture medium allowing resistance to polymyxin, incubated for 1 hour in the presence of varying concentrations of polymyxin and plated onto agar-LB plates. Survival rates are expressed as the percentage of the number of colonies formed with a strain grown in the presence of polymyxin with respect to the number of colonies obtained with the same strain grown in the absence of the antibiotic. Optimal resistance of the wild-type strain was 39% or 25% survival in the presence of 2.5 or 5 µg/ml polymyxin, respectively. As expected, the Δugd strain exhibited 7% to 1% survival, which represents only 17% to 3% of the wild-type strain resistance (Fig. 4). The Δugd+ugd strain survival values were not significantly different from those of the wild-type strain. By contrast, the strain expressing UgdY71F showed a survival level decreased over 2 and 3-fold depending on the polymyxin concentration (Fig. 4). We therefore conclude that Ugd phosphorylation by Etk on Tyr71 participates in polymyxin resistance.
Figure 4. Influence of Ugd Tyr71 phosphorylation on polymyxin resistance.
Polymyxin resistance of E. coli mutants are expressed relatively to the survival of wild type taken as 100%. Resistance was measured in the presence of a polymyxin concentration of either 2.5 or 5 µg/ml. Standard deviations from six independent experiments are indicated with error bars.
Discussion
In this work, we showed that Ugd of E. coli is phosphorylated on tyrosine 71 by the BY-kinases Wzc and Etk. This finding constitutes the first example of a bacterial protein phosphorylated on tyrosine by two distinct BY-kinases (Fig. 5). In E. coli, so far only 3 proteins have been characterized as being phosphorylated by Etk or Wzc [7], [20]. However, no less than 7 other proteins have recently been found to be tyrosine phosphorylated in E. coli cells grown under standard laboratory conditions [15]. These proteins carry out functions as diverse as tRNA synthesis, transport of amino acids, protein translation and stress response. Since, Wzc and Etk are the only tyrosine kinases characterized in E. coli, it is possible that they phosphorylate these proteins. Alternatively, presently unidentified tyrosine kinases could be present in E. coli. To support the first hypothesis, it has been demonstrated that the colanic acid operon (cps), which includes 19 genes, contains a stem-loop transcriptional attenuator which is located immediately after the third gene, namely wzc [35]. wzc might therefore be expressed independently of the other cps genes and be involved in the phosphorylation of other proteins so as to regulate other cellular functions. There are also arguments for the second hypothesis, namely that other tyrosine kinases could be encoded by the genome of E. coli. Several proteins of unknown function harbor the Walker A and B motifs [36] that constitute the active sites of Wzc and Etk, and they might function as tyrosine kinases. Furthermore, Zheng and coworkers have recently reported that the protein of unknown function YihE indeed exhibits a eukaryotic-like kinase fold despite sharing no sequence homology with eukaryotic kinases [37]. This finding supports the existence of still unknown bacterial kinases that would harbor a new type of phosphorelay mechanism. Be that as it may, the role of BY-kinases in phosphorylating other proteins seems underestimated and new regulatory networks based on tyrosine phosphorylation are likely to exist not only in E. coli but also in a large number of proteobacteria and firmicutes in which BY-kinases have been identified [5].
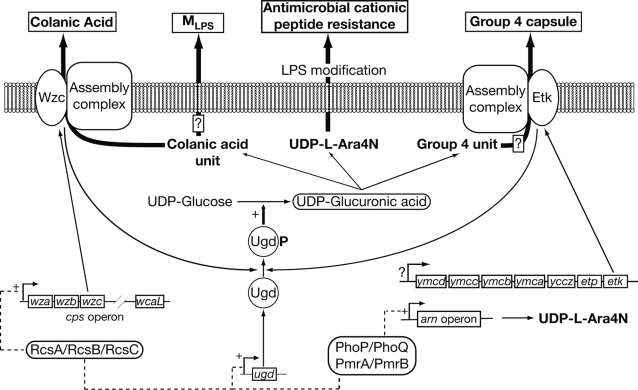
Figure 5. Schematic model for Ugd phosphorylation by Wzc or Etk as control element for extracellular polysaccharides production and resistance to antimicrobial cationic peptide.
The two-component systems PhoP/PhoQ and PmrA/PmrB, or the RcsA/RcsB/RcsC system alone allow expression of genes involved in L-Ara4N production (arn and ugd) or colanic acid biosynthesis (cps operon and ugd), respectively. In both situations, phosphorylation of Ugd by Wzc and/or Etk influences UDP-glucuronic acid production and consequently the two phenotypes. Since, Ugd is involved in MLPS and Group 4 capsule synthesis, it is assumed that Ugd phosphorylation could also influence their production (boxed question mark symbols). A question mark symbol is also used to indicate that the mechanisms governing the expression of the ymc operon are still unknown.
We established that Wzc-mediated phosphorylation of Ugd influences the production of colanic acid (Fig. 5). It has already been demonstrated that the phosphorylation-dephosphorylation of BY-kinases is involved in the biosynthesis of extracellular polysaccharides in various bacteria and more precisely in the assembly or the export of the nascent polysaccharide [26], [38]–[42]. The function of BY-kinases has been mainly studied by performing mutations in their catalytic site. Therefore, one might assume that, in addition to an effect due to the phosphorylation-dephosphorylation process of a BY-kinase itself, an effect due to the altered phosphorylation of a potential substrate might also influence the biosynthesis of the polysaccharide. Ugd, that is involved in the biosynthesis of the repeat unit of colanic acid, is in line with this idea (Fig. 5) [17]. Therefore, colanic acid biosynthesis is controlled by phosphorylation at two levels: the assembly and export of the polyssacharide (Wzc phosphorylation) and the synthesis of the colanic acid repeat unit (Ugd phosphorylation). This analysis could be extended to other bacteria. For instance, Minic and co-workers have described the phosphorylation of the UDP-glycosyl-transferase EpsE, which is involved in the production of the expolysaccharide of Streptococcus thermophilus, by the BY-kinase EpsD [13].
In the particular case of E. coli K-12, the phosphorylation of Ugd could also have side effects on the production of other polysaccharidic compounds. A recent study has demonstrated that variation of polysaccharide chain length depends on the UDP-glucose (UDPG) concentration available in the bacterial cell [43]. In this work, we did not observe such a variation of the colanic acid polymer length (data not shown) even though the UDPG/UDPGA ratio might vary in accordance with the Ugd phosphorylation level. Also, UDPGA is involved in the biosynthesis of certain Group 4 capsules (G4C) produced by some pathogenic strains of E.coli [44]. G4C are well-established virulence factors and require Etk to be secreted [18], [30]. Accordingly, Etk-mediated phosphorylation of Ugd could influence the production of G4C (Fig. 5). Similarly, Meredith and co-workers have reported that colanic acid repeats could modify the lipopolysaccharide (LPS) of E. coli K-12, forming thus a novel LPS glycoform henceforth called MLPS [45]. The biosynthesis of MLPS could also be affected by phosphorylation of Ugd (Fig. 5). Therefore, tyrosine phosphorylation of Ugd, and more generally of enzymes involved in polysaccharide biosynthesis, could provide keys to understand the biosynthesis of polysaccharide which might be far more complex than presently believed.
We also demonstrated that Etk-mediated phosphorylation of Ugd is connected with E. coli resistance to polymyxin (Fig. 5). The etk gene is located in the ymc operon which is thought not to be expressed in E. coli K-12 strain grown in LB medium, but only in pathogenic E. coli strains [18]. Indeed, an insertion sequence IS1 is found in the promoter of the ymc operon in E. coli K-12 but not in pathogenic E. coli strains. Therefore, our results raise the question of the regulation of etk expression in E. coli K-12. We have previously shown that an etk-deficient strain of E. coli is unable to resist to polymyxin [27]. This finding has recently been confirmed by a report indicating that the kinase activity of Etk is required per se for polymyxin resistance [4]. In addition, an etk knock-out mutant of an E. coli K-12 strain has been reported to be altered in its heat shock response [7]. These observations demonstrate that etk is expressed in E. coli K-12 in spite of the IS1 sequence, at least under some particular growth conditions. No data have been reported concerning the expression of the ymc operon (Fig. 5) but, as etk is the last gene of this operon, one might suggest that a cryptic promoter could be involved in etk specific expression. More likely, it could be speculated that the IS1 insertion could have a positive transcriptional effect under particular conditions. For example, genomic transposition within the regulatory locus bglR constitutes the major class of activating mutations that enable transcription of the bgl operon, which is silent in wild-type E. coli strains under laboratory conditions [46].
Colanic acid synthesis or polymyxin resistance depend each on two distinct sets of proteins, that include respectively Wzc or Etk, and that are synthesized under specific conditions (Fig. 5). Beside the influence of Ugd phosphorylation, one cannot preclude that other events would affect those biological processes. To illustrate this, it has been shown that Wzc expression does not complement polymyxin resistance of an etk-deficient strain [27]. Similarly, Wzc proteins from the E. coli K12 and K30 strains are also not interchangeable because of specific interactions between each Wzc proteins and their cognate capsule assembly complex [47]. Therefore, we assume that Wzc and Etk themselves are not likely only crucial in Ugd phosphorylation, but also in establishing interactions with other proteins involved in colanic acid synthesis and polymyxin resistance. Another possibility is that Etk and Wzc would likely phosphorylate other proteins [15]. Therefore, one cannot exclude that Wzc and/or Etk would specifically phosphorylate other endogenous proteins involved in colanic acid production or polymyxin resistance. For instance, WcaJ protein is involved in colanic acid synthesis in E. coli K12 [17] and its homolog in S. thermophilus, EpsE, is phosphorylated on tyrosine by the BY-kinase EpsD [13]. Therefore, Wzc-mediated phosphorylation of WcaJ would also influence colanic acid synthesis.
One can speculate that certain environments would induce simultaneous both expression of colanic acid and polymyxin resistance. At this moment, some factors could also participate in determining whether colanic acid or polymyxin resistance is expressed. The presence of these factors could depend on activation of the Rcs, PmrA/PmrB and PhoP/PhoQ two-components sytems, that govern the expression of the cps and arn operons (Fig. 5), but that are also known to regulate other numerous cellular activities [48], [49]. For instance, it has already been brought up that BY-kinases, namely Wzc and Etk, could act as membrane receptors, capable of sensing input signals, thereby affecting their kinase activity and controlling signal transduction [5], [50]. In line with our hypothesis, some factors produced by either the Rcs, or PmrA/PmrB or PhoP/PhoQ systems could influence specifically Etk or Wzc kinase activity or their ability to function in colanic acid synthesis and polymyxin resistance.
Complicated as it may, both high-throughput phosphoproteomic studies [50] and structural characterization of Wzc and Etk [4] will be helpful to generate valuable data to understand further their biological role in E. coli. More generally, our data represent a step toward deciphering the regulatory role of tyrosine phosphorylation in bacteria and illustrate that bacterial protein phosphorylation networks could be more complex than initially expected.
Methods
Bacterial strains
Strains and plasmids used in this study are listed in Table S1. E. coli JM83, wild type or mutated, was used to perform experiments on colanic acid production. E. coli W3110, wild type or mutated, was used to perform polymyxin resistance assay. E. coli XL1-Blue strain was used to propagate plasmids in cloning experiments. Bacteria were grown in LB medium at 37°C or in the medium inducing polymyxin resistance (see below). Antibiotics were added at the following concentrations: 50 µg/ml ampicillin, 25 µg/ml kanamycin, 15 µg/ml tetracyclin.
Gene disruption, mutagenesis and cloning
Gene replacement of ugd in E. coli W3110 or JM83 strain was performed by one-step inactivation as previously described [51]. Here, ugd was replaced with the kanamycin resistance cassette (KanR) (Table S1). Two-way mutagenic PCR was performed to substitute Ugd tyrosines to phenylalanines. For in vivo analysis of colanic acid production and polymyxin resistance, ugd and ugdY71F were expressed after cloning either in the pUC-rcsA plasmid opened with SacI and Acc65I, or in the pUC plasmid opened with Acc65I and BamHI, respectively. The 840-bp etk fragment encoding the cytoplasmic domain of Etk (amino acids 447–726) was cloned into the pQE30 vector previously opened with BamHI and HindIII. Constructs were checked by DNA sequencing. Primers used in this study are described in Table S2.
RNA manipulation
Total RNA was prepared from E.coli cells collected in the postexponential phase of growth. RNA was purified with the High Pure RNA isolation kit (Roche). Contaminating DNA was removed by additional treatment for 20 min at 37°C with 10 U DNase I (Roche). RT-PCR amplification was carried out with the SuperScript II reverse Transcriptase (Invitrogen) following the manufacturer's recommendations. A control sample without reverse transcriptase was included to confirm the absence of contaminating DNA.
Protein purification, kinase assay and UDP-glucose dehydrogenase assay
Wzccyto, Etkcyto, Ugd or UgdY71F were expressed in E. coli XL1-Blue cells. The purification procedure and in vitro kinase assays were performed as previously described [10]. For kinase assays, 1 µg of protein Ugd was incubated for 30″ to 15 min at 37 °C with varying amount of Wzccyto ranging from 0.002 µg to 1 µg. The reaction mixtures were then analyzed by SDS-PAGE. After electrophoresis, gels were soaked in 20% TCA for 10 min at 90°C, stained with Coomassie blue and dried. Radioactive proteins were visualized by autoradiography using direct exposure films. Ugd dehydrogenase activity measurement were performed in a thermostated cuvette at 37 °C, on a PowerWave 340 BIO-TEK spectrophotometer as described [52].
Dynamic light scattering (DLS) measurements
DLS measurements were performed at 18 °C in a buffer containing 50 mM NaH2PO4 pH 7.8, 300 mM NaCl, 10% glycerol and 150 mM imidazol with a Zetasizer Nano series Malvern instrument. A light path of 3 mm was used. Protein concentration was of 14 mg/mL and 8 mg/mL for Ugd and UgdY71F, respectively.
Immunoblot analysis
Bacterial cell extracts were analyzed by Western blotting after SDS-PAGE. Proteins Wzc and Ugd were detected using specific monoclonal antibodies prepared in our laboratory according to the procedure described [53] and a goat-anti-mouse secondary antibody HRP conjugate (Biorad). Phosphorylation was detected using PY20 monoclonal anti-phosphotyrosine-HRP conjugate antibody (Sigma).
Colanic acid purification
The method used was based on the procedure previously described [54]. 50 ml cell culture were heated for 15 min at 100 °C to denature EPS-degrading enzymes. After cooling, they were centrifuged at 13,200 ×g at 4 °C for 30 min. 40 ml of the supernatant fraction were then precipitated by addition of three volumes of ethanol. The mixture was maintained at 4°C overnight and centrifuged in the same conditions as above. The resulting pellet was dissolved in 5 ml of distilled water, dialyzed for 48 h against distilled water (Membrane MWCO 3,500 Da) and dried. Residual polypeptides were removed by precipitation with 5 ml of 10% (v/v) trichloroacetic acid and centrifuged again at 13,200 ×g at 4 °C for 30 min. The supernatant was dialyzed again for 5 days against distilled water and dried. The resulting preparation was re-suspended in 1 ml of distilled water and stored until quantification.
Quantification of colanic acid
Quantification of colanic acid was carried out according to [55] by measuring the amount of non-dialyzable methylpentose (6-deoxy-hexose), namely fucose which is a specific component of this exopolysaccharide. 10 to 100 µl of the colanic acid preparation were diluted to 1 ml with distilled water, and mixed with 4.5 ml of H2SO4/H2O (6∶1 v/v). The mixture was prepared at room temperature, then heated at 100 °C for 20 min, and finally cooled down to room temperature. For each sample, absorbance at 396 nm and 427 nm was measured either directly (control sample (A-co)) or after addition of 100 µl of cysteine hydrochloride (cysteine sample (A-cy)). Indeed, biological extracts often contain compounds which under heating with H2SO4 yield brown products absorbing between 396 and 427 nm. The absorption due to this unspecific reaction was subtracted from the total absorption of the sample: A396-co and A427-co were respectively subtracted from A396-cy and A427-cy to obtain DA396 and DA427. Values of (DA396–DA427) were directly correlated to methylpentose concentration by using a standard curve obtained with a fucose concentration ranging from 5 to 100 mg/ml.
Polymyxin resistance assay
Tested strains were grown for 12 h in LB medium at 37°C, diluted 100 fold and grown again overnight in N-minimal medium at pH 7.7, in the presence of 0.2% glucose and 10 mM Mg2+. Cultures were then harvested and washed three times with a N-minimal medium at pH 5.8 (mild acid conditions), in the presence of 0.2% glucose and 10 µM Mg2+ (low magnesium conditions) and 1 µM Fe3+ (Resistance-Inducing medium) [56]. Cells were diluted 1∶100 into the same medium and incubated for 4 h at 37 °C. 100,000 bacteria were thus recovered and incubated for one additional hour in the presence of a varying concentration of polymyxin B from 0.5 to 5 µg /ml at 37°C. Finally, 50 ml of the solution were plated onto LB agar plates and the number of colonies was counted after overnight aerobic incubation at 37°C. The percentage of survival (%) was calculated as follows: number of colonies of polymyxin-treated culture / number of colonies of control culture ×100.
Supporting Information
Analysis of the Ugd amino acids sequence to characterize the phosphorylated tyrosine. (A) Comparison of both the amino-acid sequences and the predicted secondary structure of Ugd and YwqF. β, α, and η indicate β-sheet, α-helices and 3.10 helices, respectively. Secondary structure elements of Ugd and YwqF have been predicted using Streptococcus pyogenes UDP-glucose dehydrogenases (PDB code 1DL1) and Pseudomonas aeruginosa GDP-mannose dehydrogenase (PDB code 1MV8) as templates respectively (Gouet et al., 2003; Rost and Liu, 2003) Conserved tyrosines are indicated in cyan. Tyr70 of YwqF and Tyr71 of Ugd are highlighted in green. (B) Autoradiography of SDS-PAGE on which reaction mixtures containing [γ-32P]ATP and either Ugd and Wzccyto (lane 1), or UgdY10F and Wzccyto (lane 2), or UgdY150F and Wzccyto (lane 3), or UgdY249F and Wzccyto (lane 4), or UgdY335F and Wzccyto (lane 5), or UgdY380F and Wzccyto (lane 6) were analyzed. References 1.Gouet, P., Robert, X., and Courcelle, E. (2003) ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res 31: 3320-3323. 2.Rost, B., and Liu, J. (2003) The PredictProtein server. Nucleic Acids Res 31: 3300-3304.
(21.88 MB TIF)
Bacterial strains and plasmids used in this study
(0.04 MB DOC)
Primers used in this study
(0.04 MB DOC)
Acknowledgments
Thanks are due to Patrice Gouet, Michel Becchi and Brice Obadia respectively for DLS measurements, mass spectrometry analysis and expert technical assistance. Josef Deutscher and Sébastien Guiral are gratefully acknowledged for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the CNRS, the University of Lyon and the Agence National de la Recherche (ANR-07-JCJC0125-01). IM was supported by a grant from the Danish Natural Research Council (FNU).
References
- 1.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 2.Bakal CJ, Davies JE. No longer an exclusive club: eukaryotic signalling domains in bacteria. Trends Cell Biol. 2000;10:32–38. doi: 10.1016/s0962-8924(99)01681-5. [DOI] [PubMed] [Google Scholar]
- 3.Olivares-Illana V, Meyer P, Bechet E, Gueguen-Chaignon V, Lazereg-Riquier S, et al. Structural basis for the regulation mechanism of the tyrosine kinase CapB from Staphylococcus aureus. PloS Biol. 2008 doi: 10.1371/journal.pbio.0060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DC, Zheng J, She YM, Jia Z. Structure of Escherichia coli tyrosine kinase Etk reveals a novel activation mechanism. Embo J. 2008 doi: 10.1038/emboj.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grangeasse C, Cozzone AJ, Deutscher J, Mijakovic I. Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem Sci. 2007;32:86–94. doi: 10.1016/j.tibs.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Petranovic D, Michelsen O, Zahradka K, Silva C, Petranovic M, et al. Bacillus subtilis strain deficient for the protein-tyrosine kinase PtkA exhibits impaired DNA replication. Mol Microbiol. 2007;63:1797–1805. doi: 10.1111/j.1365-2958.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- 7.Klein G, Dartigalongue C, Raina S. Phosphorylation-mediated regulation of heat shock response in Escherichia coli. Mol Microbiol. 2003;48:269–285. doi: 10.1046/j.1365-2958.2003.03449.x. [DOI] [PubMed] [Google Scholar]
- 8.Tocilj A, Munger C, Proteau A, Morona R, Purins L, et al. Bacterial polysaccharide co-polymerases share a common framework for control of polymer length. Nat Struct Mol Biol. 2008;15:130–138. doi: 10.1038/nsmb.1374. [DOI] [PubMed] [Google Scholar]
- 9.Whitfield C, Larue K. Stop and go: regulation of chain length in the biosynthesis of bacterial polysaccharides. Nat Struct Mol Biol. 2008;15:121–123. doi: 10.1038/nsmb0208-121. [DOI] [PubMed] [Google Scholar]
- 10.Grangeasse C, Obadia B, Mijakovic I, Deutscher J, Cozzone AJ, et al. Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J Biol Chem. 2003;278:39323–39329. doi: 10.1074/jbc.M305134200. [DOI] [PubMed] [Google Scholar]
- 11.Mijakovic I, Poncet S, Boel G, Maze A, Gillet S, et al. Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases. Embo J. 2003;22:4709–4718. doi: 10.1093/emboj/cdg458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soulat D, Grangeasse C, Vaganay E, Cozzone AJ, Duclos B. UDP-acetyl-mannosamine dehydrogenase is an endogenous protein substrate of Staphylococcus aureus protein-tyrosine kinase activity. J Mol Microbiol Biotechnol. 2007;13:45–54. doi: 10.1159/000103596. [DOI] [PubMed] [Google Scholar]
- 13.Minic Z, Marie C, Delorme C, Faurie JM, Mercier G, et al. Control of EpsE, the phosphoglycosyltransferase initiating exopolysaccharide synthesis in Streptococcus thermophilus, by EpsD tyrosine kinase. J Bacteriol. 2007;189:1351–1357. doi: 10.1128/JB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mijakovic I, Petranovic D, Macek B, Cepo T, Mann M, et al. Bacterial single-stranded DNA-binding proteins are phosphorylated on tyrosine. Nucleic Acids Res. 2006;34:1588–1596. doi: 10.1093/nar/gkj514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macek B, Gnad F, Soufi B, Kumar C, Olsen JV, et al. Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol Cell Proteomics. 2008;7:299–307. doi: 10.1074/mcp.M700311-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Macek B, Mijakovic I, Olsen JV, Gnad F, Kumar C, et al. The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol Cell Proteomics. 2007;4:697–707. doi: 10.1074/mcp.M600464-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson G, Andrianopoulos K, Hobbs M, Reeves PR. Organization of the Escherichia coli K-12 gene cluster responsible for production of the extracellular polysaccharide colanic acid. J Bacteriol. 1996;178:4885–4893. doi: 10.1128/jb.178.16.4885-4893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peleg A, Shifrin Y, Ilan O, Nadler-Yona C, Nov S, et al. Identification of an Escherichia coli operon required for formation of the O-antigen capsule. J Bacteriol. 2005;187:5259–5266. doi: 10.1128/JB.187.15.5259-5266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 20.Kolot M, Gorovits R, Silberstein N, Fichtman B, Yagil E. Phosphorylation of the integrase protein of coliphage HK022. Virology. 2008;375:383–390. doi: 10.1016/j.virol.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Mijakovic I, Petranovic D, Deutscher J. How tyrosine phosphorylation affects the UDP-glucose dehydrogenase activity of Bacillus subtilis YwqF. J Mol Microbiol Biotechnol. 2004;8:19–25. doi: 10.1159/000082077. [DOI] [PubMed] [Google Scholar]
- 22.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, et al. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 24.Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem. 2001;276:43122–43131. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- 25.Breazeale SD, Ribeiro AA, Raetz CR. Origin of lipid A species modified with 4-amino-4-Deoxy-L-Arabinose in polymyxin resistant mutants of Escherichia coli:An aminotransferase (ArnB) that generates UDP-4-amino-4-deoxy-L-arabinose. J Biol Chem. 2003;278:24731–24739. doi: 10.1074/jbc.M304043200. [DOI] [PubMed] [Google Scholar]
- 26.Obadia B, Lacour S, Doublet P, Baubichon-Cortay H, Cozzone AJ, et al. Influence of Tyrosine-Kinase Wzc Activity on Colanic Acid Production in Escherichia coli K12 Cells. J Mol Biol. 2007;367:42–53. doi: 10.1016/j.jmb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 27.Lacour S, Doublet P, Obadia B, Cozzone AJ, Grangeasse C. A novel role for protein-tyrosine kinase Etk from Escherichia coli K-12 related to polymyxin resistance. Res Microbiol. 2006;157:637–641. doi: 10.1016/j.resmic.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Gottesman S, Trisler P, Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985;162:1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouslim C, Groisman EA. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol Microbiol. 2003;47:335–344. doi: 10.1046/j.1365-2958.2003.03318.x. [DOI] [PubMed] [Google Scholar]
- 30.Ilan O, Bloch Y, Frankel G, Ullrich H, Geider K, et al. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. Embo J. 1999;18:3241–3248. doi: 10.1093/emboj/18.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent C, Duclos B, Grangeasse C, Vaganay E, Riberty M, et al. Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in gram-negative bacteria. J Mol Biol. 2000;304:311–321. doi: 10.1006/jmbi.2000.4217. [DOI] [PubMed] [Google Scholar]
- 32.Macek B, Mijakovic I, Olsen J, Gnad F, Kumar C, et al. Deciphering the prokaryotic Ser/Thre/Tyr phosphoproteome: the case of Bacillus subtilis. 2006; Seattle, WA, USA.
- 33.Breazeale SD, Ribeiro AA, McClerren AL, Raetz CR. A formyltransferase required for polymyxin resistance in Escherichia coli and the modification of lipid A with 4-Amino-4-deoxy-L-arabinose. Identification and function oF UDP-4-deoxy-4-formamido-L-arabinose. J Biol Chem. 2005;280:14154–14167. doi: 10.1074/jbc.M414265200. [DOI] [PubMed] [Google Scholar]
- 34.Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol. 2006;4:705–709. doi: 10.1038/nrmicro1478. [DOI] [PubMed] [Google Scholar]
- 35.Bailey MJ, Hughes C, Koronakis V. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol Microbiol. 1997;26:845–851. doi: 10.1046/j.1365-2958.1997.6432014.x. [DOI] [PubMed] [Google Scholar]
- 36.Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- 37.Zheng J, He C, Singh VK, Martin NL, Jia Z. Crystal structure of a novel prokaryotic Ser/Thr kinase and its implication in the Cpx stress response pathway. Mol Microbiol. 2007;63:1360–1371. doi: 10.1111/j.1365-2958.2007.05611.x. [DOI] [PubMed] [Google Scholar]
- 38.Wugeditsch T, Paiment A, Hocking J, Drummelsmith J, Forrester C, et al. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J Biol Chem. 2001;276:2361–2371. doi: 10.1074/jbc.M009092200. [DOI] [PubMed] [Google Scholar]
- 39.Niemeyer D, Becker A. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J Bacteriol. 2001;183:5163–5170. doi: 10.1128/JB.183.17.5163-5170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morona JK, Morona R, Miller DC, Paton JC. Mutational Analysis of the Carboxy-Terminal (YGX)(4) Repeat Domain of CpsD, an Autophosphorylating Tyrosine Kinase Required for Capsule Biosynthesis in Streptococcus pneumoniae. J Bacteriol. 2003;185:3009–3019. doi: 10.1128/JB.185.10.3009-3019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bender MH, Cartee RT, Yother J. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J Bacteriol. 2003;185:6057–6066. doi: 10.1128/JB.185.20.6057-6066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakar D, Gutnick DL. Involvement of a protein tyrosine kinase in production of the polymeric bioemulsifier emulsan from the oil-degrading strain Acinetobacter lwoffii RAG-1. J Bacteriol. 2003;185:1001–1009. doi: 10.1128/JB.185.3.1001-1009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ventura CL, Cartee RT, Forsee WT, Yother J. Control of capsular polysaccharide chain length by UDP-sugar substrate concentrations in Streptococcus pneumoniae. Mol Microbiol. 2006;61:723–733. doi: 10.1111/j.1365-2958.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- 44.Goldhar J, Peri R, Zilberberg R, Lahav M. Enterotoxigenic Escherichia coli (ETEC) isolated in the Tel-Aviv (Isreal) area. Med Microbiol Immunol. 1980;169:53–61. doi: 10.1007/BF02123712. [DOI] [PubMed] [Google Scholar]
- 45.Meredith TC, Mamat U, Kaczynski Z, Lindner B, Holst O, et al. Modification of lipopolysaccharide with colanic acid (M-antigen) repeats in Escherichia coli. J Biol Chem. 2007;282:7790–7798. doi: 10.1074/jbc.M611034200. [DOI] [PubMed] [Google Scholar]
- 46.Moorthy S, Mahadevan S. Differential spectrum of mutations that activate the Escherichia coli bgl operon in an rpoS genetic background. J Bacteriol. 2002;184:4033–4038. doi: 10.1128/JB.184.14.4033-4038.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid AN, Whitfield C. functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J Bacteriol. 2005;187:5470–5481. doi: 10.1128/JB.187.15.5470-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 49.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jers C, Soufi B, Grangeasse C, Deutscher J, Mijakovic I. Phosphoproteomics in bacteria-towards a systemic understanding of bacterial phosphorylation networks. Expert Rev Proteomics. 2008;5 doi: 10.1586/14789450.5.4.619. in press. [DOI] [PubMed] [Google Scholar]
- 51.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagni M, Lazarevic V, Soldo B, Karamata D. Assay for UDPglucose 6-dehydrogenase in phosphate-starved cells: gene tuaD of Bacillus subtilis 168 encodes the UDPglucose 6-dehydrogenase involved in teichuronic acid synthesis. Microbiology. 1999;145 (Pt 5):1049–1053. doi: 10.1099/13500872-145-5-1049. [DOI] [PubMed] [Google Scholar]
- 53.Doublet P, Grangeasse C, Obadia B, Vaganay E, Cozzone AJ. Structural organization of the protein-tyrosine autokinase Wzc within Escherichia coli cells. J Biol Chem. 2002;277:37339–37348. doi: 10.1074/jbc.M204465200. [DOI] [PubMed] [Google Scholar]
- 54.Bergmaier D, Lacroix C, Guadalupe Macedo M, Champagne CP. New method for exopolysaccharide determination in culture broth using stirred ultrafiltration cells. Appl Microbiol Biotechnol. 2001;57:401–406. doi: 10.1007/s002530100764. [DOI] [PubMed] [Google Scholar]
- 55.Dische Z, Shettles LB. A new spectrophotometric test for the detection of methylpentose. J Biol Chem. 1951;192:579–582. [PubMed] [Google Scholar]
- 56.Groisman EA, Kayser J, Soncini FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040–7045. doi: 10.1128/jb.179.22.7040-7045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of the Ugd amino acids sequence to characterize the phosphorylated tyrosine. (A) Comparison of both the amino-acid sequences and the predicted secondary structure of Ugd and YwqF. β, α, and η indicate β-sheet, α-helices and 3.10 helices, respectively. Secondary structure elements of Ugd and YwqF have been predicted using Streptococcus pyogenes UDP-glucose dehydrogenases (PDB code 1DL1) and Pseudomonas aeruginosa GDP-mannose dehydrogenase (PDB code 1MV8) as templates respectively (Gouet et al., 2003; Rost and Liu, 2003) Conserved tyrosines are indicated in cyan. Tyr70 of YwqF and Tyr71 of Ugd are highlighted in green. (B) Autoradiography of SDS-PAGE on which reaction mixtures containing [γ-32P]ATP and either Ugd and Wzccyto (lane 1), or UgdY10F and Wzccyto (lane 2), or UgdY150F and Wzccyto (lane 3), or UgdY249F and Wzccyto (lane 4), or UgdY335F and Wzccyto (lane 5), or UgdY380F and Wzccyto (lane 6) were analyzed. References 1.Gouet, P., Robert, X., and Courcelle, E. (2003) ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res 31: 3320-3323. 2.Rost, B., and Liu, J. (2003) The PredictProtein server. Nucleic Acids Res 31: 3300-3304.
(21.88 MB TIF)
Bacterial strains and plasmids used in this study
(0.04 MB DOC)
Primers used in this study
(0.04 MB DOC)