Abstract
Oxidative stress can cause methionine oxidation that has been implicated in various proteins malfunctions, if not adequately reduced by the methionine sulfoxide reductase system. Recent evidence has found oxidized methionine residues in neurodegenerative conditions. Previously, we have described elevated levels of brain pathologies and an abnormal walking pattern in the methionine sulfoxide reductase A knockout (MsrA−/−) mouse. Here we show that MsrA−/− mice have compromised complex task learning capabilities relative to wild-type mice. Likewise, MsrA−/− mice exhibit lower locomotor activity and altered gait that exacerbated with age. Furthermore, MsrA−/− mice were less responsive to amphetamine treatment. Consequently, brain dopamine levels were determined. Surprisingly, relative to wild-type mice, MsrA−/− brains contained significantly higher levels of dopamine up to 12 months of age, while lower level of dopamine was observed at 16 months of age. Moreover, striatal regions of MsrA−/− mice showed an increase of dopamine release parallel to observed dopamine levels. Similarly, the expression pattern of tyrosine hydroxylase activating protein correlated with the age-dependent dopamine levels. Thus, it is suggested that dopamine regulation and signaling pathway are impaired in MsrA−/− mice, which may contribute to their abnormal bio-behavior. These observations may be relevant to age-related neurological diseases associated with oxidative stress.
Keywords: dopamine, oxidative stress, methionine oxidation, neurodegenerative diseases
Introduction
Accumulative posttranslational modification to proteins, mediated by the action of reactive oxygen species (ROS), is thought to be one of the major causes of aging and age-related diseases. ROS have been also implicated as possible risk factors in the development of neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD), which are associated with the deposition of oxidative-damaged proteins [1]. While most protein damage by ROS is irreversible, mechanisms have evolved to prevent or reverse specific protein modifications. Methionine sulfoxide (MetO) modifications to proteins can be reversed by the methionine sulfoxide reductase (Msr) system, which consists of MsrA (reduces S-MetO) and MsrB (reduces R-MetO), thioredoxin reductase, thioredoxin, and NADPH [2]. Evidence for the possible role of the Msr system in antioxidant defense is demonstrated by the hypersensitivity to oxidative stress in several organisms that results from MsrA ablation [2–5]. Loss of MsrA activity and elevated levels of protein-carbonyl were detected in brains diagnosed with Alzheimer’s disease (AD), relative to non-AD brains [6]. In addition, the hippocampal region of MsrA−/− mice exhibited enhanced neurodegeneration, hypersensitivity to H2O2, loss of astrocytes, elevated levels of beta-amyloid deposition, and tau phosphorylation compared to wild-type (WT) controls [7]. It is suggested that a deficiency in MsrA activity fosters an oxidative stress environment that is manifested by the accumulation of faulty proteins (via MetO), deposition of aggregated proteins, and premature brain cell death. The relation of compromised MsrA activity to either the development of AD or PD is probably dependent on the type of cell and proteins that encounter elevated oxidation of Met residues. Recently, examples of Met-oxidized proteins have been identified in brains diagnosed with AD and PD. For example, MetO-amyloid beta adducts were found in post-mortem senile plaques of AD patients [8] and the DJ-1 protein is irreversibly oxidized by carbonylation, as well as by MetO to methionine sulfone, in PD and AD [9]. Accordingly, these observations support the hypothesis that the occurrence of neurodegenerative diseases is linked to the accumulative oxidative damage to proteins (including MetO) that exacerbate with age and causes them to unfold, malfunction, and/or aggregate, thereby interfering with normal neuronal functions.
Given the hippocampal sensitivity to oxidative stress and markers of AD in the MsrA−/− mouse [7], we analyzed this mouse for a decline of complex task learning capability in the current study. The previously described “tip toe” walking pattern described for MsrA−/− mouse [4] prompted us to look into PD-associated bio-behavioral traits in this mouse strain. In PD, neurodegeneration (especially in the dopaminergic neurons of the substantia nigra) and altered dopamine (DA) levels have been linked to abnormal motor behavior, including walking distance and gait. Thus, locomotor activity and gait performance of the MsrA−/− mouse, compared to wild-type (WT) mouse, were monitored as function of age. The direct natural cause for these neuronal deaths in PD is yet to be revealed, but toxins can produce Parkinsonism via ROS and have been used to create animal models for PD. For example, both 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA, a neurotransmitter analogue toxic to dopaminergic neurons) reduces DA levels in the brain and results in PD-like motor syndromes. While 6-OHDA may cause direct elevation of ROS, MPTP is converted to MPP+, which by its inhibitory action on mitochondrial complex 1 may elevate ROS production. Therefore, behavioral characteristics of MPTP-treated mice served as a guide to demonstrating Parkinson’s-related abnormal locomotor behavior in MsrA−/−mice. Since DA is an important signaling molecule involved in motor regulation, we also determined the level of brain dopamine (post-mortem assay) and electrically stimulated brain DA secretion and reuptake in striatal neurons (in vitro, brain slice preparation). Additionally, the response of the mice to amphetamine was determined because 1) amphetamine blocks the DA transporter’s (DAT) ability to clear DA from the synaptic space, and 2) amphetamine is transported into the cell which contibutes to the DA efflux [10]. Together, the current observations show significant differences between MsrA−/−and WT mice with regard to bio-behavioral traits and brain DA levels, thereby providing a potential linkage between MetO, abnormal behavior, and age-related neurodegenerative diseases.
Materials and Methods
Operant learning procedures
Eight MsrA−/− mice and 7 WT mice were exposed to operant conditioning procedures in which the mice first acquired the capacity to press a lever to obtain small portions of condensed milk such that each lever press produced a reward (this procedure is termed fixed ratio 1 or FR1). The initial training, managed entirely by a computer program [11], occurred when the mice were 1 year old (mean ages of 12.9 months and 12.2 months for MsrA−/− and WT mice, respectively; not significantly different). Briefly, the computer-controlled lever-pressing training procedure used mouse operant chambers that were supplied by Med Associates (St. Albans, VT) and programmed to present condensed milk on concurrent FR1 and noncontingent variable time schedules of milk reinforcement in two consecutive-day, 2 hr sessions. These two sessions were followed by 4 consecutive-day FR1 training sessions 30 min in duration. The mice were returned to their home cages for a period of about 3.5 months with no training, but with daily restriction of food intake. Then the mice were retested for 7 consecutive daily sessions on FR1. The mice were then exposed to a computer-implemented shaping procedure aimed at gradually introducing increasingly greater task demands until they reached FR20 (i.e., 20 lever presses per reward). The FR-shaping procedure individually monitored each mouse’s behavior, and the FR requirement was increased only if the last two interresponse times of the preceding ratio were less than 1 s each. The training procedure was stopped after training session 32 when all WT mice reached the FR20 criterion. The dependent variable for describing the difference in learning was the number of sessions required by each mouse to reach the criterion. Mice not reaching the FR20 criterion were assigned a score of 32 sessions. Our FR shaping procedure was not a type of progressive ratio that is commonly used in experiments designed to reflect motivation to obtain a reward. In the conventional progressive ratio the trained animal is presented with a series of monotonically increasing ratios such that each completed ratio results in a reinforcer (reward) and the requirement for obtaining the reinforcer is incremented, eventually making the ratio of reinforcers to responses smaller and smaller until the animal “gives up”-the break point. Importantly, the criterion for reinforcement grows steadily without taking account of how well the animal is progressing. In the procedure we used, the animal was shaped to move on to increasing ratios; if it did not make two responses in moderately quick succession (two responses following one another each in less than 1 s), it could remain indefinitely at whatever ratio it was on and continue to be reinforced. We see our task as measuring the mouse’s sensitivity to the reinforcement contingency, which unimpaired animals learn quickly.
Locomotor activity
Locomotor activity and other behavioral attributes were assessed with four concurrently operative force-plate actometers [12]. The actometer and associated peripherals, as well as the methods of data collection and analysis, were used as described previously [12]. In the current studies, mouse locomotor activity was measured for 30 min. Six mice of each genotype (MsrA−/− or WT) and age (6 or 12-months old) were used for the analyses.
Gait dynamics
The DigiGait system (Mouse Specifics, Inc., MA) provides numerous spatial and temporal indices of gait dynamics and posture, including: stride length, stance width, stance duration, swing duration, braking duration, propulsion duration, stride frequency, and paw angle as described previously [10–12]. The mouse observation compartment is constructed of clear polycarbonate of fixed width with adjustable front and rear walls. The DigiGait apparatus performs ventral plane videography of rodents walking on a motorized transparent treadmill belt. A high-speed digital video camera continuously images the underside of the walking animals. DigiGait software generates “digital paw prints” to measure gait indices, representing the temporal record of paw placement relative to the treadmill belt. The gait signal of each limb comprises the stride duration, which includes the stance duration when the paw of a limb is in contact with the walking surface, plus the swing duration when the paw of the same limb is not in contact with the walking surface. Stance duration is subdivided into braking duration (increasing paw contact area over time) and propulsion duration (decreasing paw contact area over time). More than twenty gait indices are reported by the software, including the sciatic functional index (SFI), stance factor, and the step-sequence pattern. The incline of treadmill belt is fixed at horizontal. Imaging and analysis software automatically quantifies spatial and temporal indices of gait in walking or running animals. Additional details have been published previously [13–15]. The experimental design was similar to the locomotor activity measurements except that only one measurement session was conducted at each of the two age points. The experiments were conducted on the same mice (n=6 for each mouse genotype and age) a week after the actometer measurements. The belt speed was adjusted to the animals’ ability to walk without moving backwards. Belt speeds were kept constant for each age group.
Behavioral effects of amphetamine administration
In the amphetamine experiment, the doses studied were 5.0, 2.5, and 0.0 mg/kg (in this order, administered i.p. in a volume of 5 mL/kg) with 7 to 10 days separating treatments. Doses specified are in terms of the d-amphetamine sulfate salt (Sigma-Aldrich, St. Louis, MO). Amphetamine was administered 15 min before placement in the actometers. The primary behavioral measurement was distance traveled and secondary measurement was stereotypy.
Dopamine levels in post-mortem brains
Animals were sacrificed and their brains were dissected out and stored at −80°C until analysis. The DA analysis was performed on whole isolated brain. On the day of the assay, the brain was sonicated in 10 volumes (weight/volume), of 0.1 M perchloric acid containing 50 ng/mL dihydrobenzylamine as internal standard. After centrifugation at 15,000 X g for 15 minutes at 4°C, 20 μL of supernatant was injected onto a C18 reverse-phase HR-80 catecholamine column (ESA Inc., Bedford, MA, USA). The mobile phase consisted of 94% 50 mM sodium phosphate/0.2 mM EDTA/1.2 mM heptanesulfonic acid (pH 3.2) solution and 6% methanol. The flow rate was 1.5 mL/min. Peaks were detected by an ESA 8 Channel CoulArray system. Data was collected and processed using the CoulArray data analysis program.
Release and reuptake of dopamine in the striatum
Brain slices
Mice were deeply anesthetized by isoflurane inhalation and decapitated. The brains were immediately removed and placed in oxygen-saturated ice cold artificial cerebrospinal fluid (aCSF). From each brain, the cerebellum was excised and the cerebrum was glued on a Teflon block. Coronal slices of 300 μm thick were made using an NVSL vibratome (World Precision Instruments, Sarasota, FL). Brain slices containing the striatum, obtained at +0.3 to +1.5 mm from bregma, were stored in ice cold aCSF. A single slice was submerged under aCSF maintained at 34°C and continuously flowing (2 mL/min) through a superfusion chamber (Warner Instruments, Hamden, CT). Each brain slice was equilibrated for 30 minutes prior to obtaining measurements.
Electrochemistry
The procedure was based on method previously described by Bath et al. [16]. Carbon-fiber cylinder microelectrodes were made as previously described using T650 carbon fibers (Amoco, Greenville, SC) cut to a length of 25 μm. A triangular waveform, starting at −0.4 V, increasing to 1.0 V, and scanning back to −0.4 V (versus Ag/AgCl reference electrode), was applied to the carbon-fiber working electrode at a scan rate of 300 V/s and an update rate of 10 cyclic voltammograms/s. The carbon-fiber was inserted until the tip reached 100 μm below the surface of the brain slice in the dorsolateral caudate region of the striatum between the prongs of a bipolar stimulating electrode (FHC, Bowdoinham, ME), situated 200 μm apart. To evoke DA release, a single, biphasic stimulating current pulse (2 ms each phase, 350 μA in amplitude), was applied to the stimulating electrode. Successive cyclic voltammograms were collected every 20 s. The current arising from DA oxidation (at about 0.6 V) was measured and plotted versus time. To account for the naturally occurring heterogeneity of dopamine terminals in the striatum, DA release readings were obtained at four different randomly selected locations in the dorsolateral caudate and the resulting data were averaged and counted as a single reading. Electrodes were calibrated against DA standards of known concentrations in a flow cell before and after each brain slice experiment. The averages of these pre- and post-calibration measurements were used to calibrate the electrode.
Genomic and western blot analyses
Genomic analysis
The mRNAs from three brains of each mouse genotype at 6 months of age were purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). The purified mRNA from each brain was used for genomic screening analyses probing with Affymetrix Mouse Genome 430 2.0 Array (using Affymetrix GeneChip System at the University of Kansas Genomic Facility). Only the tyrosine hydroxylase (TH) and TH-activating protein (Ywhaz) genes were evaluated for the current study.
Western blot analysis
Protein-expression levels of TH and TH-activating protein (Ywhaz) were detected by extracting protein from a pool of 5 mouse brains. For each sample, 30 micrograms of soluble protein were subjected to gel-electrophoresis, followed by western blot analysis using primary antibodies against Ywhaz (Abcam) and TH (Millipore).
Statistical analysis
Data presented are the mean ± standard deviation of the mean. Statistical analysis was carried out using paired or unpaired student’s t-test to determine if the observed changes between the two mouse strains had a significant effect (p<0.05). For locomotor activity, the distance traveled was analyzed with a repeated measures 2-way ANOVA with genotype as the between groups factor and 5-min time block as the repeated measures factor.
Results
Behavioral effects of MsrA targeted deletion
Assessment of operant learning
Eight MsrA−/− mice and 7 WT mice were exposed to operant conditioning procedures. The mice first acquired the capacity to press a lever to obtain small portions of condensed milk such that each lever press produced a reward (FR1). During initial training, the two types of mice were not significantly different, but numerically the MsrA−/− mice lagged behind the WT mice. The mice were returned to their home cages for a period of about four months with no training in this interval. Then the mice were retested for seven consecutive daily sessions on FR1. All mice showed evidence of relearning the task and attained similar lever press rates on the seventh day. The mice were then exposed to a second computer-implemented shaping procedure aimed at gradually introducing increasingly greater task demand until the requirement for reward was FR20 (i.e., 20 lever presses per reward). This increase in demand revealed striking differences between the MsrA−/− and WT mice. The WT mice learned the FR20 task significantly faster than the MsrA−/− mice (Fig. 1). Over the 32 training sessions, the mean number of training sessions to reach FR20 was 10.9 for the WT mice and 30.6 for the MsrA−/− mice, t13=6.14, p<0.001 (t13 refers to a “between-groups” t-test with 13 degrees of freedom). It is important to note that the MsrA−/− mice continued to lever press for reward during the FR shaping procedure (i.e., they did not “extinguish”). Therefore, the deficit of the MsrA−/− mice was not failure to perform a previously acquired habit, but, instead, was a failure to learn a new habit. These results clearly support the hypothesis that the MsrA−/− mice have functional behavioral/cognitive impairments at about 16 months of age compared to WT control mice, which may be related to the enhanced hippocampal neurodegeneration observed in the 12-month-old MsrA−/− mice [7].
Figure 1. Operant task in both mouse strains.
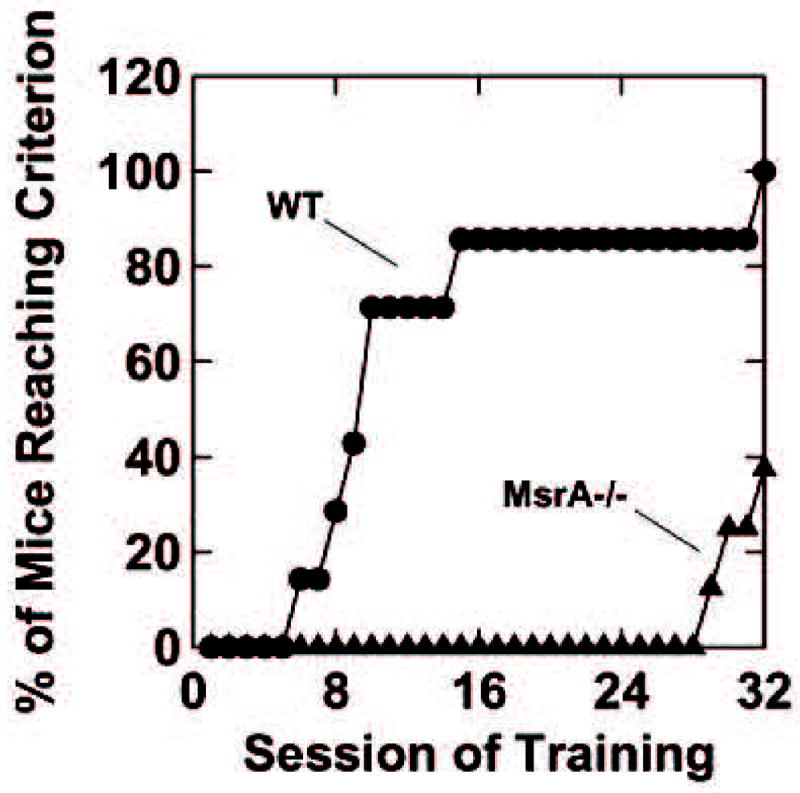
At the age of ~16 months, the control mice quickly learned to transition from FR1 to FR20 schedule of milk reward (WT), but the MsrA−/− mice lagged significantly behind the WT mice in learning to perform under these conditions (despite the fact that they were not different when the task demand was FR1). WT, n=7; MsrA−/−, n=8. Over the 32 training sessions shown in Fig. 1, the mean number of training sessions to reach FR20 was 10.9 for the WT mice and 30.6 for the MsrA−/− mice, t13=6.14, p<0.001(t13 refers to a “between-groups” t-test with 13 degrees of freedom).
Locomotor activity
At 12 months of age, the MsrA−/− were less active by up to ~50% relative to control mice (Fig. 2). However, no obvious difference in locomotor activity between the two mouse strains was detected at 6 months of age (data not shown). Decreased spontaneous motor activity was also observed in mice that were shown to have signs of PD or AD [17, 18].
Figure 2. Spontaneous locomotor activity of MsrA−/−- and WT control mice measured in a force-plate actometer apparatus.

Five wild-type (WT) and 5 MsrA−/− mice (12 months old) were individually placed in a dark force-plate actometer apparatus and their behavior was recorded for 30 min. The effect of genotype was significant: F (1, 8) =12.1, p=0.008 as was the effect of time block: F (5, 40) = 43.3, p<0.001. The interaction effect: F (5, 40) =1.75, p=0.145, was not significant.
Gait dynamics
Gait disturbances including shortened stride length [19, 20] and abnormal stride frequency [21] are symptomatic of PD. A ventral plane videography apparatus using a clear treadmill [15, 22] was chosen to analyze the gait indices of mice at a constant speed (Fig. 3), compared to traditional “paw painting” techniques that do not control speed. The MsrA−/− displayed significant differences in stride length, frequency and duration relative to WT control mice (Fig. 3). Specifically, we observed a reduced stride length and stride duration as well as an increased stride frequency in MsrA−/− mice. The decreased parameters suggest a lack of motor control, and the increase frequency occurs to maintain the animal’s speed as governed by the constant rate of the belt. Moreover, this novel technology has been previously used to analyze the gait dynamics of MPTP-injected mice [15], a common PD mouse model (shown in Table 1 for comparison). The gait data were pooled from all four limbs for analysis, as was done in previous analyses [15]. The values in the graph (Fig. 3) show the differences by genotype and age relative to the 6-month-old control mice (WT young mice normalized to 100%). Actual numbers of the averaged values are given in Table 1. All stride indices were significantly different between the two types of mice, and these differences increased with age (aged mice are approximately 16 months of age). Also, Table 1 shows the percent change relative to the corresponding genotype. Reduced stride length is indicative of basal ganglia dysfunction [23]. Indeed, the current results for MsrA−/−mice are comparable in magnitude to the data reported by Amende et al. [15] for MPTP-treated C57BL/6 mice (Table 1). There is no evidence that MPTP administration and MsrA ablation affect the same biochemical pathway. However, the gait abnormalities found in the PD mouse model using MPTP administration and the MsrA−/− mouse may be indicative of some similarities in brain function/s of both mouse types, which may affect gait performance.
Figure 3. Gait dynamics of MsrA−/− and WT control mice.

The horizontal axis represents the type of mouse and the dependent variables from the DigiGait Imaging System. The graphic shows differences in stride dynamics of MsrA−/− mice at two age points, 6 months (young, slashes) and 16 months (aged, gray solid) versus young and aged WT controls (white and black, respectively). Values are given as a percentage relative the WT young value to show progression of abnormalities with age. The DigiGait-treadmill speed: young mice: 26 cm/s; aged mice: 15 cm/s, MPTP-injected mice: 34 cm/s [14]. Significance was determined by t-test and indicated between strains by * (p < 0.05) or ** (p < 0.001); and between age groups in each strain by † (p < 0.05) or †† (p < 0.005). Each strain, n = 6.
Table 1. Stride dynamics of MsrA−/− versus wild-type (WT) and MPTP mice.
The mean value from actual numbers determined by DigiGait Analysis in Figure 3 expressed as percentile of MsrA−/− or MPTP value [14] of respective WT or saline control, respectively.
| Strain | Stride length
(cm) |
Stride frequency
(steps/second) |
Stride duration
(ms) |
|---|---|---|---|
| WT (young) | 7.24±0.18 | 3.67±0.10 | 257±7 |
| MsrA −/− (young) | 6.63±0.18 | 4.06±0.13 | 252±7 |
| WT (aged) | 5.50±0.13 | 2.80±0.07 | 367±9 |
| MsrA −/− (aged) | 2.90±0.21 | 5.90±0.39 | 194±1 |
| MsrA −/− vs. WT (young) | 91.5% | 110.7% | 91.6% |
| MsrA −/− vs. WT (aged) | 52.8% | 209.8% | 52.9% |
| MPTP vs. Saline | 93% | 108% | 93.7% |
Behavioral effects of amphetamine administration
Force-plate actometers were used to measure the locomotor-activating effects of amphetamine in MsrA−/− mice and WT controls. Numerical data for distance traveled are shown in Fig. 4 and tracings of movement trajectories are shown in Fig. 5. Analysis of variance indicated a significant difference between the two types of mice (F(1,9)=7.10, p=0.026). The dose effect was also significant (F(2,18)=36.9, p<0.001), as was the dose-by-type interaction effect (F(2,18)=8.25, p=0.003)). A significant three-way interaction (dose X time block X type of mouse) was also obtained, (F(10,90)=15.4, p<0.001). The statistical interaction effects were the result of the marked divergence of the behavior in the two types of mice at the 5.0 mg/kg dose, with the WT mice increasing their activity and the MsrA−/− mice decreasing in activity as session time passed. Because it is known that higher doses of amphetamine can induce in rodents focused stereotypies that reduce amount of locomotion [24], it is important to examine the behavior for indications of the potential occurrence of focused stereotypy in response to amphetamine. Data shown in Fig. 5 (examples for 4 animals per mouse strain) can be used to address this issue. Consistent with Figure 3 at the 5.0 mg/kg dose of amphetamine, the WT mice exhibited a high degree of locomotor stimulation which was accompanied by partial spatial confinement in mice number 3 and number 5, whereas the MsrA−/− mice showed declining stimulation with time but no spatial confinement of the kind seen for the WT mice. There was no focused sterotypies observed at 0.0 dose of amphetamine for either mouse strain. Intensified spatial confinement was observed for the WT mice when treated with amphetamine 2.5 mg/kg, which was the second dose treatment. The MsrA−/− mice also showed evidence of spatial confinement after the 2.5 mg/kg dose, but less than the WT mice. The somewhat greater spatial confinement effect on the second dose (even though it was lower than the first dose) is consistent with the idea that both types of mice were capable of expressing behavioral sensitization. At the 5.0 mg/kg dose the data suggest a deficient ability of the MsrA−/− to sustain motor response to amphetamine that may be related to an abnormality in the mouse’s dopamine neurotransmitter system, which is the predominate modality through which amphetamine produces its increase in locomotor activity.
Figure 4. Response to amphetamine administered to both mouse strains.

Distance traveled by MsrA−/− mice (filled circles, n=5) and controls (triangles, n=6) in response to the indicated doses of d-amphetamine sulfate (Amp) given ip. The drug was administered 15 min before the 30-min recording session in a force-plate actometer. Mice were about 16 months old. Analysis of variance indicated a significant difference between the two types of mice (F(1,9)=7.10, p=0.026). The dose effect was also significant (F(2,18)=36.9, p<0.001), as was the dose-by-type interaction effect (F(2,18)=8.25, p=0.003)). A significant three-way ANOVA interaction (dose X time block X type of mouse) was also obtained, (F(10,90)=15.4, p<0.001).
Figure 5. Movement trajectories for the data shown in Figure 4.

Each square represents 6 min of recording. The top row for each treatment occurred first in time. These mice were approximately 16 months old.
Assessment of the molecular changes in MsrA−/− mouse brain
Dopamine levels in post-mortem brains
Changes in brain DA levels are associated with abnormal motor behavior. Thus, we measured DA level in total brain extracts of 6-, 12-, and 16-month-old mice using reverse-phase HPLC-column linked to an electrochemical detector. Surprisingly, brains of the MsrA−/− mice exhibited 47% and 63% more dopamine compared to their counterpart WT brains at 6- and 12-month-old brains, respectively (Fig. 6). However, at older age (16 months), there was a ~50% decline in DA level in MsrA−/− compared to WT mice. It is important to note that the DA level in WT brain increased at 16 months of age to the DA level observed in MsrA−/− at 12 months. Consequently, it was important to evaluate the possibility of enhanced DA synthesis and release as shown by monitoring the expression levels of tyrosine hydroxylase (TH), TH-activating protein (TH has been reported to require binding of 14-3-3 proteins (Ywhaz) for optimal activation by phosphorylation [25], and DA release/reuptake in striatum of brain slices in vitro, as shown below.
Figure 6. Concentration of brain dopamine in MsrA−/− and WT control mice at various ages.
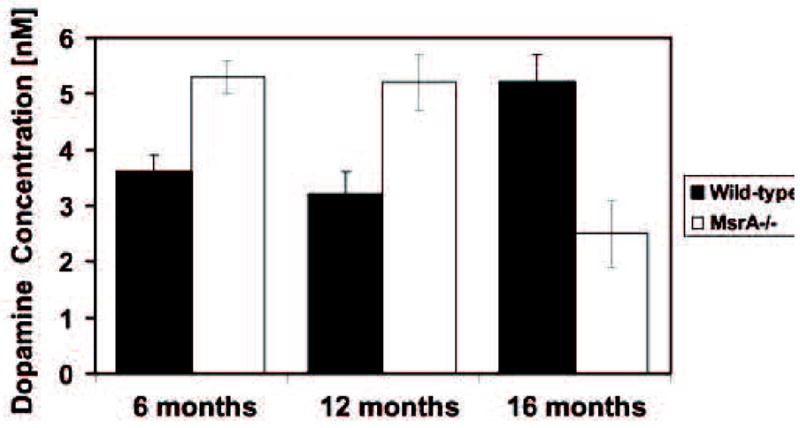
Neurotransmitter level was assayed by HPLC with electrochemical detection. Dopamine levels in the MsrA−/− brains were significantly higher than in the WT mice at ages of 6 and 12 months and lower than WT at 16 months of age, respectively, as determined by t-test, P<0.01. The higher dopamine level in WT at age of 16 months relative to ages of 6 and 12 months was also significant, as determined by t-test, p<0.01. Each strain, n=6.
Release and reuptake of dopamine in the striatum of brain slices
To gain insight into presynaptic dopaminergic malfunctions that may occur in MsrA−/−mice, we measured electrically evoked DA release and reuptake in striatal brain slices using fast-scan cyclic voltammetry (FSCV) at carbon-fiber microelectrodes. Electrodes were placed in the dorsolateral caudate because dopaminergic innervation in this brain region comes almost exclusively from the nigrostriatal pathway [26]. As shown in a representative concentration-time plot (Fig. 7), a sharp increase in DA concentration is measured at the electrode upon application of a single stimulus pulse. This increase is followed by a more gradual decrease as DA is taken up by DAT [27]. Cyclic voltammograms (upper right in Fig. 7) obtained at the maxima confirm the detection of DA. Amplitudes of stimulated DA release obtained from brain slices of MsrA−/− mice are dramatically increased (272% of WT on average, aggregated data are shown in Table 2). Plots of stimulated DA release at carbon-fiber microelectrodes in brain slices may be distorted by diffusional contributions and DA reuptake by DAT [16]. To account for these contributions, concentrations of DA released per stimulus pulse ([DA]p) were estimated by modeling the concentration–time curves (Fig. 7). The curved line superimposed over the points of the sample DA release plot represents best-fit curve derived using curve-fitting software [28]. On average, [DA]p was significantly increased (333% of WT, p < 0.05, n = 3 mice per genotype, 12 month-old mice) in brain slices harvested from MsrA−/− mice (Fig. 8). [DA]p progressively decreased with increasing time in culture of the MsrA−/− brain-slices, while remaining flat in the first 90 min for the WT brain slices and increasing by 200% at 180 min time point (Fig. 8).
Figure 7. Dopamine release in striatal brain slices of MsrA−/− and WT control mice.
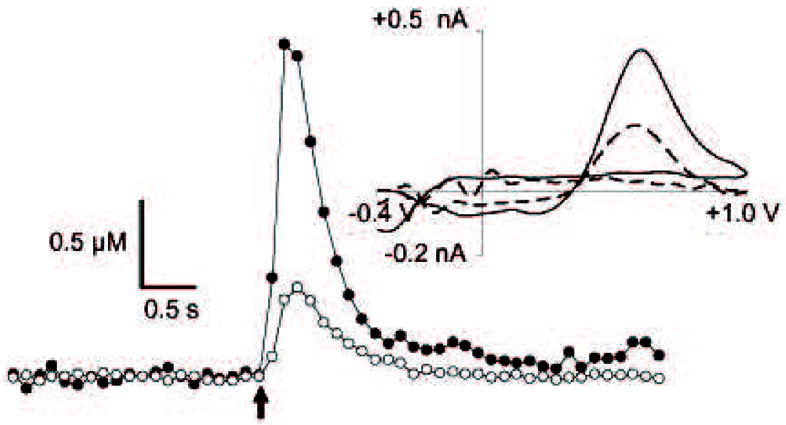
A representative stimulated DA release plot generated from measurements obtained from striatal brain slices (from 12-month-old post-mortem brains). Fast scan cyclic voltammetry was used to collect DA release measurements. The cyclic voltammograms plots (shown above and to the right) confirm the presence of DA. Legend: MsrA−/−, closed symbols on release plot, solid line on cyclic voltammogram; WT, open symbols on release plot, dashed line on cyclic voltammograms.
Table 2. Peak dopamine release from brain slices harvested from MsrA−/− and WT control mice.
Peak dopamine release ([DA]max) was measured by fast scan cyclic voltammetry from four different locations in each quadrant (dorsolateral, dorsomedial, ventrolateral, ventromedial) of coronal brain slices. Values represent the average of each set of measurements in each quadrant of each slice analyzed ± SEM. Significance as determined by t-test: p < 0.05 (MsrA−/− versus WT mice).
| Genotype | [DA]max |
|---|---|
| MsrA−/− | 2.07 ± 0.49 μM |
| WT | 0.76 ± 0.07 μM |
Figure 8. Peak dopamine release presented from the WT and MsrA−/− slices.

Successive measurements were collected from striatal brain slices of MsrA−/−, and WT mice 90 minutes apart. Bar graph values represent the average of release measurements collected from four different locations within the ventral lateral caudate/putamen. DA release from MsrA−/− brain slices diminished over time, whereas it did not diminish in WT slices. Error bars represent ±SEM. Significance determined by t-test: * p < 0.05, ** p < 0.001.
From the analysis of the stimulated release curves we also determined Vmax, a kinetic parameter describing the maximum rate of DA reuptake by DAT. The average values determined from MsrA−/− and WT mice were consistent with previously published values of Vmax (4μM/s) in mice [29]. No significant difference in Vmax between MsrA−/− mice and aged-matched WT littermates was found n= 3 mice per genotype, data not shown). The KM for reuptake is the concentration of extracellular DA at which DAT functions at 1/2Vmax. The KM serves as a measure of DAT efficiency, whereas, Vmax is directly proportional to DAT concentration. Values of KM in the absence of reuptake inhibitors have been determined in synaptosomal preparations to be approximately 0.2 μM [30]. Dopamine reuptake follows Michaelis–Menten kinetics [30–32]. Therefore, the rate of DA reuptake in a given plot of stimulated DA release is dependent upon DA concentration and is independent of the time point in the curve at which the concentration is measured. Thus, to qualitatively assess DA reuptake efficiency, we overlaid the averaged MsrA−/− and WT DA reuptake curves, time shifted so that the initial concentrations were approximately equal, from brain slice data obtained. This approach to observing relative DAT efficiency has been employed previously [30]. The MsrA−/−curves are not statistically different from their respective WT curves in the concentration range of KM (0.2 μM; p > 0.05, Mann–Whitney, n = 3, each genotype, 12-month-old MsrA−/− vs. age-matched WT; graphic not shown). This suggests that the KM, and therefore the efficiency of DA reuptake by DAT, is similar in MsrA−/− and WT mice.
Analyses of TH and TH-activating protein
Increased DA levels observed may be attributed to elevated DA synthesis. We performed brain genomic screening analyses and found that the MsrA−/− brain had about 4-fold induction in Ywhaz (TH-activating protein) mRNA content over the WT brain gene at age of 6 months, respectively (p<0.001). The up-regulation of the Ywhaz gene in MsrA−/−brain was confirmed on the protein level following western blot analysis of MsrA−/− and WT brain extracts at ages of 6 and 12 months (Fig. 9A). Additionally, the protein level of Ywhaz was reduced at 16 months versus 12 months of age in the MsrA−/− brain and elevated in WT brain at 16 months of age compared to 12 months of age, respectively (Fig. 9A). Thus, there is a good correlation between the expression of Ywhaz gene and brain DA levels across the monitored strains and age. This correlation implies that Ywhaz may play an important role in the regulating brain DA synthesis as a function of age and performance of the Msr system. Differences in dopaminergic neuron quantities can alter the TH-activating protein content. However, the relative expression level of TH in both mouse strains was similar across all monitored ages (mRNA data not shown; and Fig. 9B for the protein expression level). The unchanged TH level suggests no significant age-dependent neurodegeneration in MsrA−/− dopaminergic neurons compared to WT, respectively.
Figure 9. Protein-expression levels of TH and Ywhaz in WT and MsrA−/− brains.

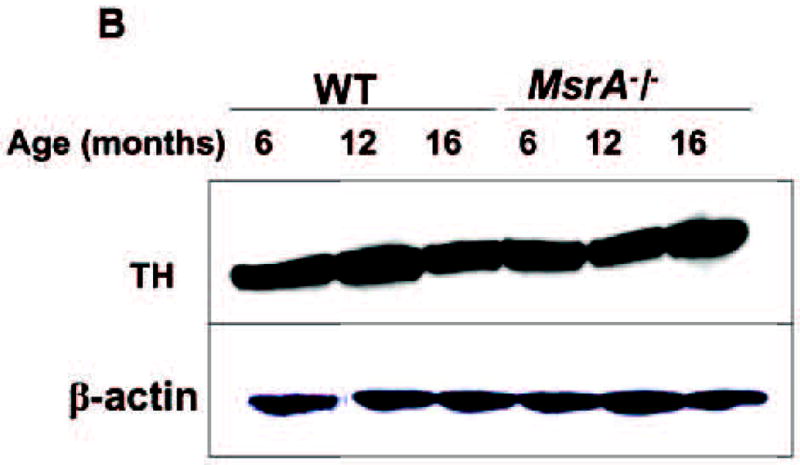
Pool of 5 mouse brain extracts were subjected to gel-electrophoresis, followed by western blot analyses. Thirty microgram of soluble brain proteins were loaded in each lane. A) Expression levels of Ywhaz (TH-activating protein) in both mouse strains as function of age (6, 12, and 16 months). B) Expression levels of TH as function of age (6, 12, and 16 months). Respectively, β-actin levels for each lane are shown in Panel A and panel B.
Discussion
In our previous studies we have found that MsrA−/− mice display elevated brain pathologies and a “tip-toe” walking pattern. Here we show evidence that the MsrA−/−mouse exhibits abnormal cognitive and locomotor behaviors reminiscent of age-related neurodegenerative diseases. In addition, the mice lacking MsrA display atypical dopamine regulation. The initial increased MsrA−/− dopamine levels are a finding that, to our knowledge, has only been reported in studies not related to oxidative stress. The MsrA−/− mouse was shown to be more vulnerable to oxidative stress and showed enhanced neurodegeneration in the hippocampus [5, 7]. Thus, it is possible that the general observed hypersensitivity of the MsrA−/− to oxidative stress may affect the function of specific proteins that are involved in DA physiology.
Lower locomotor activity present as early as 12 months of age in MsrA−/− relative to WT mice (Fig. 2), and measurable gait disturbances detected at 6 months and worsening with age in MsrA−/− (Fig. 3), suggests that the motor deficiencies in the MsrA−/− are exacerbated with age; relative to WT declining locomotor activity. The data presented in Fig. 3 and Table 1 suggests a magnitude of gait abnormality in the MsrA−/− mice that is comparable to what is seen in MPTP treated mice. The gait abnormalities found in the PD mouse model using MPTP administration, which has also been demonstrated in MsrA−/− mouse, may be a consequence of a common response to direct or indirect effects prompted by the ablation of MsrA and MPP+ action in the brain. Studies of gait in mouse models will help establish parallels with human pathologies, and these parallels may be useful in identifying therapeutic approaches to human neurological diseases. Alteration in DA metabolism is strongly associated with abnormal locomotor activity. The drug amphetamine blocks the re-uptake of already released DA, by blocking DAT function, and thus was used to treat the mice to elevate their locomotor activity.
Surprisingly, the presented data showed that amphetamine-treated MsrA−/− mice have a compromised response to this treatment (Figs. 4 and 5) at 5.0 mg/kg. From the results obtained on DA release and re-uptake from the striatum, using fast scan cyclic voltammetry (Figs. 7 and 8, Table 2), it appears that the DAT in MsrA−/− mice functions properly, while a dramatic increase in DA release is observed. Thereby, it is unlikely that the observed high levels of MsrA−/− brain DA at ages of 6 and 12 months (Fig. 6) are the outcome of insufficient reuptake of DA. Rather, these findings imply that the measured high level of MsrA−/− brain DA (Fig. 6) is related to an enhancement in DA synthesis. Supportive evidence to this hypothesis arises from the fact that the upregulation or downregulation of the activating enzyme of TH (TH-activating protein, Ywhaz) correlate with the levels of brain-DA in both mouse strains (Fig. 9A and Fig. 6). At an age of 16 months, MsrA−/− mice showed reduction in DA level compared to the previous ages (50%), while the WT reached the DA levels of the MsrA−/− at ages of 6 and 12 months (Fig. 6). One possible explanation for this observation is that, relative to WT, the MsrA−/−brain is functioning under oxidative stress conditions from an early age and it is responding by elevating DA levels (possibly as a compensatory response). However, at an older age this response mechanism to oxidative stress may fail in the MsrA−/− brain. In contrast, MsrA expression and activity in WT brains decline only at older ages [33, 34]. The increase of dopamine content at 16 months old wild-type mice may reflect, in light of the DA-data presented for the MsrA KO mice, an enhanced oxidative stress at older age before dopaminergic neurodegeneration occurs. Thus, the same phenomenon may occur in WT, but it is delayed until MsrA levels are decreased in normal aging.
The unchanged levels of TH in MsrA−/− relative to WT brain (Fig. 9B) reduce the possibility of enhanced dopaminergic neurodegeneration in MsrA−/− brain up to 16 months of age. Similarly, lack of an obvious in vivo dopaminergic neurodegeneration was shown in major genetic mouse models for PD; for example, in the DJ-1 knockout mouse [35] and in various types of mouse models overexpressing α-synuclein [36]. The different promoters and mouse transgenes of α-synuclein lead to a wide variety of phenotypes accompanied by nonexistent, late onset, or non-specific neurodegeneration [36]. Thus, it is proposed enhanced level of dopaminergic neurodegeneration is probably an outcome of multi-component events manifested at late stages of cell death; whereas MetO participates in earlier stages of neurodegeneration. Nevertheless, the type of brain cell having a compromised MsrA system may determine the level of neurodegeneration. For example, it seems that certain cells of the hippocampus are more sensitive to neurodegeneration [7] compared to dopaminergic neurons (Fig. 9B).
The inability of high DA levels in the MsrA−/− mice to increase their locomotor and gait performances (in the absence of DAT abnormality) supports the hypothesis that malfunction of downstream signaling events (due to MetO) may be the rate-limiting factors in attenuating the MsrA−/− response to DA. Moreover, the increased bio-behavior abnormities observed in the aged MsrA−/− mice may be due to the additional age-dependent general decline in antioxidant defense. For example, it is speculated that the DA receptors in MsrA−/− mice may be more affected by MetO with age, thereby causing either lower efficiency in DA receptors binding to DA, downregulation of DA-receptors expression, or both.
The MsrA−/− mice exhibited a compromised learning capability when challenged with increased task demands. As shown in Fig. 1, the WT mice learned the FR20 task significantly faster than the MsrA−/− mice. Given the older age of these mice (16 months), this observed difference in learning is possibly the result of having malfunctioning proteins involved in the learning processes as oxidative damage increases with age. Reduced operant capability is indicative of problems residing within the cortico-striatal-thalamo-cortical loops. Thus, it is possible that irregularities in dopamine physiology may contribute to the altered learning capacity in the MsrA−/− mice, in addition to the already demonstrated pathologies in the MsrA−/− hippocampus [7].
The fast scan cyclic voltammetry results (Figs. 7 and 8, Table 2) clearly suggest that there is a rapid exaggerated release of DA in MsrA−/− brain upon stimulation. It is well established that DA plays a pivotal role in motor behavior activity and that the rate of DA release from dopaminergic neurons contributes significantly to its potential action on targeted receptors. Dopaminergic neurons have the capacity to release DA not only from their axon terminals, but also from their somatodendritic compartment. The endoplasmic reticulum and its associated tubulovesicles have been proposed as possible storage sites for somatodendritic DA. These structures indeed appear to contain DA [37–39], and similar structures have been shown to express proteins of the SNAP-receptor (SNARE) complex, otherwise known to be essential for exocytosis [33]. Consequently, one possible explanation for the observed increased DA release in MsrA−/− brain is an upregulation of the expression levels of the proteins participating in DA release mechanisms.
In summary, elevated protein oxidation at older age may cause irreversible MetO leading to protein malfunction and/or abnormal accumulation. Thus, irregularities in various physiological processes may occur, including abnormalities in neurological functions manifested by attenuated locomotor behavior and reduced learning capabilities. The molecular basis for such events may involve altered DA synthesis and release (this study) and post-synaptic physiology (future studies), which are associated with age-dependent reduction in the proper function of the Msr system. Accordingly, it is proposed to use the MsrA−/− mouse as a model to further studies on the relationships between MetO, neuronal metabolism, behavioral functions, and aging.
Acknowledgments
This study was supported by the J.R. and Inez Jay Award and Higuchi Biosciences Center at University of Kansas, KIDDRC center grant number (HD02528), and part by the National Institute of Aging, AG027363.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabner BJ, El-Agnaf OM, German MJ, Fullwood NJ, Allsop D. Protein aggregation, metals and oxidative stress in neurodegenerative diseases. Biochem Soc Trans. 2005;33:1082–1086. doi: 10.1042/BST20051082. [DOI] [PubMed] [Google Scholar]
- 2.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochimica Et Biophysica Acta-Proteins and Proteomics. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, Brot N, Weissbach H. Escherichia-Coli Peptide Methionine Sulfoxide Reductase Gene -Regulation of Expression and Role in Protecting against Oxidative Damage. Journal of Bacteriology. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide methionine sulfoxide reductase functions as an antioxidant in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskovitz J, Bar-Noy S, Williams WM, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer’s disease brain. Journal of Neurochemistry. 1999;73:1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 7.Pal R, Oien DB, Ersen FY, Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Experimental Brain Research. 2007;180:765–774. doi: 10.1007/s00221-007-0903-6. [DOI] [PubMed] [Google Scholar]
- 8.Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. Journal of Biological Chemistry. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 11.McKerchar TL, Zarcone TJ, Fowler SC. Differential acquisition of lever pressing in inbred and outbred mice: Comparison of one-lever and two-lever procedures and correlation with differences in locomotor activity. Journal of the Experimental Analysis of Behavior. 2005;84:339–356. doi: 10.1901/jeab.2005.95-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. Journal of Neuroscience Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- 13.Hampton TG, Stasko MR, Kale A, Amende I, Costa ACS. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiology & Behavior. 2004;82:381–389. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle & Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amende I, Kale A, McCue S, Glazier S, Morgan James P, Hampton Thomas G. Gait dynamics in mouse models of Parkinson’s disease and Huntington’s disease. J Neuroengineering Rehabil FIELD Full Journal Title:Journal of neuroengineering and rehabilitation. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Analytical Chemistry. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 17.Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. Journal of Neuroscience. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KW, Lee SH, Kim H, Song JS, Yang SD, Paik SG, Han PL. Progressive cognitive impairment and anxiety induction in the absence of plaque deposition in C57BL/6 inbred mice expressing transgenic amyloid precursor protein. Journal of Neuroscience Research. 2004;76:572–580. doi: 10.1002/jnr.20127. [DOI] [PubMed] [Google Scholar]
- 19.Salarian A, Russmann H, Vingerhoets FJG, Dehollain C, Blanc Y, Burkhard PR, Aminian K. Gait assessment in Parkinson’s disease: Toward an ambulatory system for long-term monitoring. Ieee Transactions on Biomedical Engineering. 2004;51:1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 20.Weller C, Oneill CJA, Charlett A, Bowes SG, Purkiss A, Nicholson PW, Dobbs RJ, Dobbs SM. Defining Small Differences in Efficacy between Anti-Parkinsonian Agents Using Gait Analysis - a Comparison of 2 Controlled Release Formulations of Levodopa Decarboxylase Inhibitor. British Journal of Clinical Pharmacology. 1993;35:379–385. doi: 10.1111/j.1365-2125.1993.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartolic A, Pirtosek Z, Rozman J, Ribaric S. Postural stability of Parkinson’s disease patients is improved by decreasing rigidity. European Journal of Neurology. 2005;12:156–159. doi: 10.1111/j.1468-1331.2004.00942.x. [DOI] [PubMed] [Google Scholar]
- 22.Kale A, Amende N, Meyer GP, Crabbe JC, Hampton TG. Ethanol’s effects on gait dynamics in mice investigated by ventral plane videography. Alcoholism-Clinical and Experimental Research. 2004;28:1839–1848. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- 23.Fernagut PO, Diguet E, Labattu B, Tison F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. Journal of Neuroscience Methods. 2002;113:123–130. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- 24.Lyon M, Robbins T. The action of central nervous stimulant drugs: A general theory concerning amphetamine effects. In: W E, L V, editors. Current Developments in Psychopharmacology. New York: Spectrum; 1975. pp. 79–163. [Google Scholar]
- 25.Kleppe R, Toska K, Haavik J. Interaction of phosphorylated tyrosine hydroxylase with 14-3-3 proteins: evidence for a phosphoserine 40-dependent association. J Neurochem. 2001;77:1097–1107. doi: 10.1046/j.1471-4159.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- 26.Gerfen CR, Herkenham M, Thibault J. The Neostriatal Mosaic.2. Patch-Directed and Matrix-Directed Mesostriatal Dopaminergic and Nondopaminergic Systems. Journal of Neuroscience. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. Journal of Neuroscience. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. Journal of Neuroscience Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- 29.Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphetamine. Journal of Neurochemistry. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of Dopamine Uptake in the Basolateral Amygdaloid Nucleus, Caudate-Putamen, and Nucleus-Accumbens of the Rat. Journal of Neurochemistry. 1995;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM. Regulation of Transient Dopamine Concentration Gradients in the Microenvironment Surrounding Nerve-Terminals in the Rat Striatum. Neuroscience. 1992;51:55–64. doi: 10.1016/0306-4522(92)90470-m. [DOI] [PubMed] [Google Scholar]
- 32.Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-Time Characterization of Dopamine Overflow and Uptake in the Rat Striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- 33.Petropoulos I, Mary J, Perichon M, Friguet B. Rat peptide methionine sulphoxide reductase: cloning of the cDNA, and down-regulation of gene expression and enzyme activity during aging. Biochem J. 2001;355:819–825. doi: 10.1042/bj3550819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadtman ER, Moskovitz J, Berlett BS, Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem. 2002;234–235:3–9. [PubMed] [Google Scholar]
- 35.Yamaguchi H, Shen J. Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-deficient mice. Mol Neurodegener. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesselet MF. In vivo alpha-synuclein overexpression in rodents: A useful model of Parkinson’s disease? Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linder JC, Klemfuss H, Groves PM. Acute Ultrastructural and Behavioral-Effects of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (Mptp) in Mice. Neuroscience Letters. 1987;82:221–226. doi: 10.1016/0304-3940(87)90134-0. [DOI] [PubMed] [Google Scholar]
- 38.Groves PM, Linder JC. Dendro-Dendritic Synapses in Substantia-Nigra -Descriptions Based on Analysis of Serial Sections. Experimental Brain Research. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- 39.Hattori T, McGeer PL, McGeer EG. Dendro axonic neurotransmission. II. Morphological sites for the synthesis, binding and release of neurotransmitters in dopaminergic dendrites in the substantia nigra and cholinergic dendrites in the neostriatum. Brain Res FIELD Full Journal Title:Brain Research. 1979;170:71–83. doi: 10.1016/0006-8993(79)90941-7. [DOI] [PubMed] [Google Scholar]


