Abstract
Laminin-5 (LN5) is a matrix component of epithelial tissue basement membranes and plays an important role in the initiation and maintenance of epithelial cell anchorage to the underlying connective tissue. Here we show that two distinct LN5 function-inhibitory antibodies, both of which bind the globular domain of the α3 subunit, inhibit proliferation of epithelial cells. These same antibodies also induce a decrease in mitogen-activated protein kinase activity. Inhibition of proliferation by the function-perturbing LN5 antibodies is reversed upon removal of the antibodies and can be overcome by providing the antibody-treated cells with exogenous LN5 and rat tail collagen. Because epithelial cells use the integrin receptor α3β1 to interact with both LN5 and rat tail collagen, we next investigated the possibility that integrin α3β1 is involved in mediating the proliferative impact of LN5. Proliferation of human epithelial cells is significantly inhibited by a function-perturbing α3 integrin antibody. In addition, antibody activation of β1 integrin restores the proliferation of epithelial cells treated with LN5 function-perturbing antibodies. These data indicate that a complex comprising LN5 and α3β1 integrin is multifunctional and contributes not only to epithelial cell adhesion but also to the regulation of cell growth via a signaling pathway involving mitogen-activated protein kinase. We discuss our study in light of recent evidence that LN5 expression is up-regulated at the leading tips of tumors, where it may play a role in tumor cell proliferation.
INTRODUCTION
Cell interaction with elements of the extracellular matrix impacts their adherence, motility, as well as protein and gene expression (for example, see Adams and Watt, 1993; Roskelly et al., 1995). In intact normal tissue epithelial cells bind to extracellular matrix molecules, which are organized into a complex multiprotein structure called the basement membrane. The major components of the latter include type IV collagen, proteoglycans, and laminins. One laminin isoform, laminin-5 (LN5),1 in particular plays an important role in establishing firm adherence of epithelial cells to the basement membrane, because it is necessary for the assembly and maintenance of stable anchorage devices between epithelial cells and matrix called hemidesmosomes (Baker et al., 1996; Green and Jones, 1996). However, recent reports also indicate that LN5 is expressed at the budding tips of invading tumor cells, i.e., at sites where cancer cells are undergoing cell division but where there are most likely no hemidesmosomes (Pyke et al., 1994, 1995). This provides an indication that LN5 has functions other than as an epithelial cell “glue.”
Two integrin receptors for LN5 have been identified. A variety of epithelial cells use the α3β1 integrin heterodimer to bind LN5 in vitro (Carter et al., 1991). However, for some cells this interaction appears to be transitory, and, both in vivo and in vitro, cell interaction with LN5 at some point switches to the α6β4 integrin (Xia et al., 1996). Indeed, the latter association is apparently essential for both hemidesmosome assembly as well as the maintenance of the structural integrity of this cell–matrix adhesion device (Jones et al., 1994; Baker et al., 1996; Borradori and Sonnenberg, 1996). Furthermore, LN5–α6β4 integrin complexes are believed to be conduits for signals from the external milieu of cells to the cytoplasm and potentially vice versa (Mainiero et al., 1995, 1997; Borradori and Sonnenberg, 1996; Giancotti, 1996; Shaw et al., 1997). In particular, the α6β4 integrin has recently been reported to be part of a cell signal cascade pathway that activates mitogen-activated protein (MAP) kinase and that regulates cell proliferation (Mainiero et al., 1997). In addition, α6β4 integrin, through interaction with phosphoinositide 3-OH kinase, promotes invasion of carcinoma cells (Shaw et al., 1997).
A number of antibodies against LN5 have been shown to inhibit either cell adhesion and/or hemidesmosome assembly (Carter et al., 1991; Rousselle et al., 1991; Baker et al., 1996). One such antibody, CM6, recognizes the globular or G domain of the α3 chain of rat LN5 (rtLN5) (Baker et al., 1996). Remarkably, we show here that the division of 804G rat epithelial cells treated with the antibody is compromised, despite their attaching to and spreading on substrate. We have also investigated the effects of three different human-specific function-inhibiting LN5 monoclonal antibodies on the breast epithelial cell line MCF-10A. MCF-10A cells, like 804G cells, secrete an LN5-rich matrix and assemble hemidesmosomes (Stahl et al., 1997). All three monoclonal antibodies inhibit MCF-10A proliferation. We will also present results indicating that LN5 impacts the proliferation of epithelial cells via its association with the integrin receptor α3β1. Furthermore, LN5 and α3β1 integrin appear to be part of a distinct cell signaling pathway that regulates MAP kinase activity.
MATERIALS AND METHODS
Cell Culture
804G cells were maintained as previously detailed (Riddelle et al., 1991). MCF-10A cells were obtained from American Type Culture Collection (Rockville, MD) and were maintained in a 1:1 mix of Dulbecco’s modified Eagle medium and Ham’s F-12 medium supplemented with 5% equine serum, 0.01 mg/ml insulin, 20 ng/ml epidermal growth factor (EGF), 100 ng/ml cholera toxin, and 500 ng/ml hydrocortisone. OVCA429 cells were generously provided by Dr. Sharon Stack (Northwestern University Medical School) and were maintained according to Moser et al. (1996).
Antibodies
Antibody CM6, which inhibits rtLN5 function, and the control antibody 5C5, which does not inhibit rtLN5 function, were described by Baker et al. (1996). The human LN5 (hLN5) function-inhibitory antibody P3H9-2 was purchased from Chemicon (Temecula, CA). Antibody BM165 against hLN5 was a kind gift from Dr. Robert Burgeson (Harvard University, Cambridge, MA). Mouse monoclonal antibody RG13 was prepared using MCF-10A LN5-rich matrix as immunogen according to Langhofer et al. (1993). The TS2/16.2.1 mouse hybridoma line was obtained from the American Type Culture Collection, and hybridoma supernatant containing TS2/16.2.1 antibody was collected from subconfluent dishes of actively growing cells (van de Wiel-van Kemenade et al., 1992). Fibronectin (FN) antibody (clone II), an antibody against α2 integrin (P1E6), antibody P1B5 against α3 integrin, and antibody 3E1 against β4 integrin were obtained from Life Technologies (Gaithersburg, MD). The fluorescein-conjugated anti-bromodeoxyuridine (BrdU) monoclonal antibody was purchased from Boehringer Mannheim (Indianapolis, IN). Antibodies specific to BM28, a cell cycle antigen, were affinity purified as described previously and visualized in cells by indirect immunofluorescence using a fluorescein-conjugated anti-rabbit IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA) (Todorov et al., 1994). An affinity-purified polyclonal antibody (anti-ACTIVE MAP kinase [MAPK] p42/p44), specific for phosphorylated p42/p44, was purchased from Promega (Madison, WI), whereas anti-p42/p44 antibody, which recognizes p42/p44 regardless of its activation state, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse immunoglobulin G (IgG) was purchased from Jackson ImmunoResearch (West Grove, PA).
Matrix Molecules and Preparation of Recombinant G Domain of the LN5 α3 Subunit
LN1, FN, and rat tail collagen (RTC) type I were purchased from Collaborative Research (Bedford, MA). These were coated onto cell supports according to the instructions of the supplier. rtLN5 and hLN5 were prepared from 804G or MCF-10A cell conditioned medium, respectively (Baker et al., 1996; Stahl et al., 1997). In brief, cell medium was fractionated by cation exchange chromatography. Fractions enriched in LN5 were further processed by anion exchange chromatography, and a final purification was achieved using hydroxyapatite chromatography. This procedure will be detailed elsewhere (Fitchmun, Jones, and Falk-Marziller, unpublished procedure).
To produce the C-terminal G domain of the hLN5 α3 subunit, a fragment encoding amino acid residues 747-1560 of the α3 subunit was subcloned into the HindIII and XhoI sites of the pET32b vector (Novagen, Madison, WI) and transfected into DE3α cells. A His fusion protein was induced, and the cells expressing the fusion protein were extracted in SDS buffer, as described above. The fusion protein of 110 kDa was identified using a His-HRP probe (SuperSignal HisProbe Western blotting kit; Pierce, Rockford, IL) and on an SDS-PAGE gel after protein staining in Coomassie Brilliant Blue (Sigma, St. Louis, MO).
Cell Proliferation and Cell Cycle Assays
Approximately 2 × 104 804G or MCF-10A cells were plated into wells of a 24-well plate in the presence of antibody on various substrate supports in complete, serum-containing medium. Immunofluorescence assays were used to confirm that antibodies are still present after 48 h (our unpublished results). At specific time points, cells were trypsinized and then counted. For each experimental condition, three wells were assayed. For the BrdU assays, cells were plated onto glass chamber slides under various experimental conditions, and 18 h later 10 μM BrdU (Sigma) was added directly to the cell culture medium. After 1 h the cells were extracted in −20°C methanol and allowed to air dry. DNA was subsequently denatured by incubating the extracted cells in 2N HCl at 37°C for 1 h. After washing in PBS, the cell preparations were overlaid with a fluorescein-conjugated monoclonal anti-BrdU antibody (Boehringer Mannheim) and incubated for 1 h at 37°C.
For visualization of BM28 antigen, cells were extracted as described by Todorov et al. (1995). In brief, cells grown on coverslips were washed in PBS containing 2 mM MgCl2 and extracted in 0.5% Triton X-100, 20 mM Tris-HCl (pH 7.4), 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.5 mM PMSF for 5 min at 20°C. After washing in PBS the cells were fixed and extracted in methanol (−20°C) followed by acetone (−20°C) and processed for immunofluorescence using rabbit anti-BM28 antibodies. DNA was visualized by staining with 0.1 μg/ml 4,6-diamidino-2-phenylindole (DAPI). Fixed and stained cells were viewed using a Zeiss (Thornwood, NY) Photomicroscope III fitted with epifluorescence optics.
Cell Adhesion Assays
Approximately 2 × 105 MCF-10A cells or OVCA429 cells were plated onto FN- or RTC-coated wells of a non-tissue culture–treated 96-well plate (Sarsedt, Newton, NC), respectively. In some instances MCF-10A cells were plated in the presence of a 1:250 dilution of an FN antibody, whereas the OVCA429 cells were plated into medium containing a 1:50 dilution of the anti-α2 integrin antibody PIE6. After 30 min at 37°C the cells were washed extensively in Dulbecco’s PBS, fixed for 15 min in 3.7% formaldehyde in PBS, and then incubated at room temperature with 0.5% crystal violet for 10 min. The dye was then solubilized with 1% SDS (100 μl/well), and absorbance at 570 nm measured on a Vmax plate reader (Molecular Devices, Menlo Park, CA).
MAP Kinase Assays
Cells were maintained for 48 h in complete medium. The cells were then washed twice in PBS and scraped off their substrate into Laemmli-type gel sample buffer containing 2% SDS. The cell extracts were sonicated briefly and heated at 95°C for 3 min before gel electrophoresis (Laemmli, 1970). The MEK1 inhibitor that selectively inhibits the MAPK cascade, PD98059, was purchased from New England Biolabs (Beverly, MA). A stock solution of inhibitor at a concentration of 50 mM in DMSO was prepared. The inhibitor was added directly to complete medium to a final concentration of 50 μM. The same volume of DMSO without inhibitor was added to cell cultures as a control. After 48 h the cells were washed and harvested in gel sample buffer as above.
SDS-PAGE, Western Immunoblots, and Scanning Densitometry
SDS-PAGE and immunoblotting were carried out as described previously with the exception that blots were developed using a chemiluminescence kit (Pierce) (Zackroff et al., 1984; Klatte et al., 1989). Immunoblots were scanned and quantitated using Molecular Analyst (Bio-Rad, Richmond, CA).
RESULTS
Antibody CM6 against the α3 LN Subunit Inhibits Proliferation of 804G Cells
The mouse monoclonal antibody CM6 directed against the G domain of the α3 subunit of LN5 has been shown to destabilize certain cell–matrix connectors called hemidesmosomes as well as to inhibit the ability of an LN5-rich matrix to nucleate the assembly of hemidesmosomes (Baker et al., 1996). Here we have analyzed the proliferation of CM6 antibody-treated 804G cells over 120 h (Figure 1). 804G cells secrete a matrix whose major component is LN5 (Langhofer et al., 1993). This matrix contains very little, if any, LN6 or LN7, both of which, like LN5, contain an α3 subunit (Langhofer et al., 1993; Kuhn, 1997). Thus, in these particular studies, CM6 antibody most likely perturbs LN5 function.
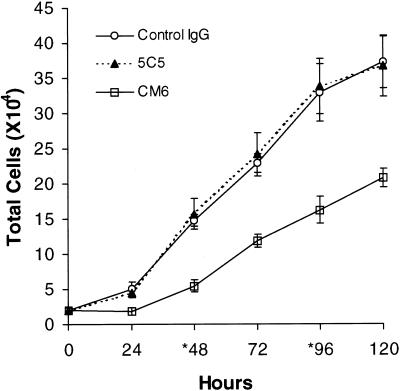
Figure 1.
804G cells (2 × 104 cells per well of a 24-well tissue culture dish) were plated into complete medium containing 50 μg/ml CM6, 5C5, or control IgG antibody. Every 24 hr cells from three wells were trypsinized and counted. Cells were fed every 48 hr with fresh medium containing the same concentration of antibody (asterisks). Note the reduced growth of the CM6 antibody-treated cells.
A known number of 804G cells were plated in medium containing CM6 antibody and, at 24-h intervals, cells were collected and counted (Figure 1). As a control we treated 804G cells with either mouse IgG or the rat α3 LN chain-specific monoclonal antibody 5C5 (Baker et al., 1996). This antibody does not inhibit LN5 function (Baker et al., 1996). At 48 and 120 h the number of cells in the CM6 antibody-treated cultures increases by ∼37 and 56% compared with cultures treated with control IgG and by 34 and 57% compared with cultures treated with 5C5 antibody (Figure 1). Based on this analysis we selected 48 h as the time point for our subsequent assays.
To confirm that CM6 antibodies impact 804G cell division, we also assessed BrdU labeling of the antibody-treated cells (Table 1). In this assay, only 12% of the CM6 antibody-treated cells stained, compared with 44% of control IgG-treated 804G cells (Table 1).
Table 1.
804G cells
| BrdU assay
|
BM28 assay
|
|||||
|---|---|---|---|---|---|---|
| Cells counteda | Labeling index (%) | Cells counteda | G1 (%) | S (%) | G2 (%) | |
| Control IgG | 349 | 44.4 | 371 | 43.8 | 33.7 | 22.4 |
| Anti-rat LN5 | 357 | 12.0 | 210 | 44.3 | 38.5 | 17.2 |
Total number of cells counted in three trials.
It should be noted that the CM6 antibodies do not prevent 804G cells attaching to or, in many instances, partially spreading onto their substrate (Figure 2). In addition, there is no significant detachment of cells during the course of the studies, as determined by counting any floating cells in the medium from the antibody-treated cell cultures each day. Furthermore, even though 804G cell growth is inhibited in CM6 antibody-containing medium, the treated cells are viable for up to 10 d in the presence of antibody and can be induced to start to proliferate normally upon trypsinization and plating onto a fresh substrate in fresh medium (our unpublished results). This indicates that the cells have not undergone terminal differentiation or apoptosis and anoikis.
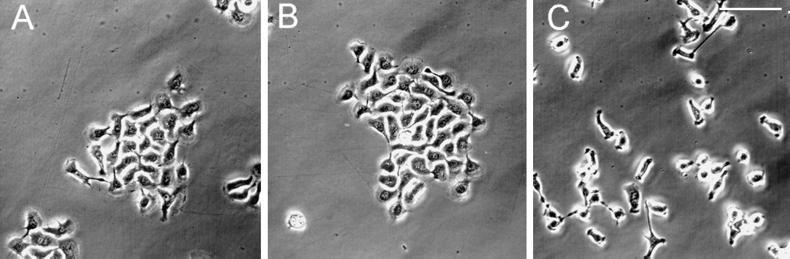
Figure 2.
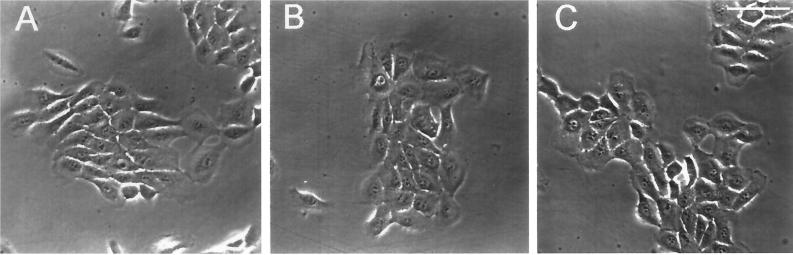
Phase-contrast images of 804G cells (A–C) are shown at 48 hr after plating into normal medium (A), medium containing 50 μg/ml control IgG (B), or medium supplemented with 50 μg/ml LN α3 subunit function-inhibiting antibody CM6 (C). Note that in all cases the cells have attached to and spread onto their substrate, although in C the cells are less well spread than in A and B. Bar, 125 μm.
We next investigated whether CM6 antibody blocks the growth of 804G cells at a particular stage in the cell cycle. For these studies we made use of an antibody against BM28/hMCM2, which is a member of the recently defined family of MCM proteins thought to play an essential role in the regulation of DNA replication (Kearsey et al., 1996). The BM28 protein as well as other members of the MCM family are found tightly bound to chromatin during the G1 phase of the cell cycle and are gradually released during S phase (Todorov et al., 1995; Krude et al., 1996). BM28 is detected using BM28 antibody after mild detergent extraction before cell fixation. The BM28 antibodies generate distinctive nuclear stains, which are dependent on the phases of the cell cycle (Todorov et al., 1995). For example, cell nuclei are stained uniformly bright in G1 cells, show a spotty pattern in S phase cells, and are practically unstained in G2 and in mitotic cells. Thus BM28 staining, in combination with mild detergent extraction, provides a useful tool to visualize the cell cycle distribution of a given cell population (Todorov et al., 1995).
804G cells maintained in the presence of CM6 or control IgG antibody were extracted in mild detergent conditions and stained with BM28 antibody in combination with DAPI. The nuclei of both the CM6- and IgG-treated cells show the full range of BM28 staining patterns (Figure 3). Furthermore, the percentage of nuclei at different cell cycle stages as indicated by antibody staining was similar in 804G cell populations maintained in normal medium, in medium supplemented with CM6 antibody, and in medium containing control IgG antibody (Table 1). This suggests that CM6 antibody treatment blocks cell growth randomly during the cell cycle.
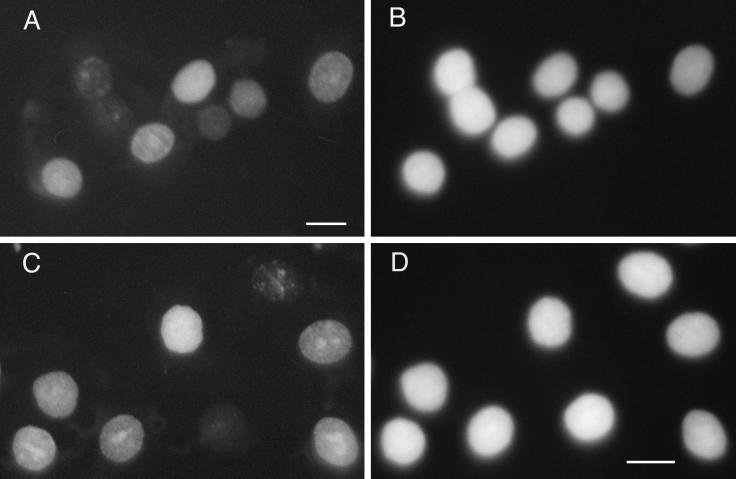
Figure 3.
804G cells were maintained in the presence of CM6 antibody (A and B) or control IgG (C and D). After 24 hr the cells were extracted in detergent and then fixed and stained using a BM28 antibody preparation (A and C) and DAPI (B and D). The same range of BM28 staining patterns is observed in the nuclei of the 804G cells in both A and C. Bar, 10 μm.
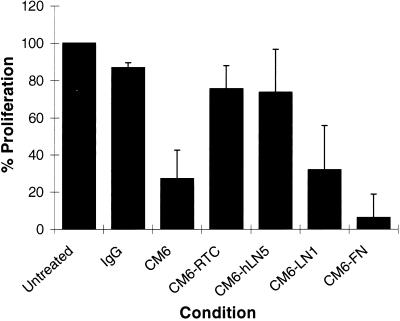
Can growth of CM6 antibody-treated 804G cells be restored by plating the cells on defined extracellular matrices including FN, LN1, RTC, or LN5? For studies involving LN5, we used human protein (hLN5), which the rat-specific CM6 antibodies fail to recognize (Baker et al., 1996). Inhibition of division of 804G cells treated with CM6 antibody is not reversed by maintaining the cells on LN1 or FN, because the increase in number of cells is only 32 and 6.2%, respectively, of that observed in control antibody cell populations (Figure 4). In contrast, proliferation of 804G cells that had been plated onto RTC- and hLN5-coated substrates and treated with CM6 antibody is 75.5 and 73.8%, respectively, of that of control antibody-treated cells (Figure 4).
Figure 4.
804G cells were plated into the wells of a 24-well tissue culture dish (2 × 104 cells per well) or similar wells coated with 50 μg/ml RTC, 1 μg/ml hLN5, 25 μg/ml LN1, and 25 μg/ml FN for 48 hr. As indicated, the 804G cells were maintained in medium supplemented with either 50 μg/ml IgG control antibody or 50 μg/ml antibody CM6. At 48 hr the cells were trypsinized and counted. % Proliferation indicates the increase in cell number as a percentage of that observed in the untreated control cell population. At 48 hr the control cell population expanded from 2 × 104 to 1.09 × 105 cells (100%).
Antibodies against the Human α3 LN Subunit Inhibit Proliferation of MCF-10A Cells
To determine whether the above phenomenon is peculiar to 804G cells and/or the CM6 antibody, we undertook comparable studies using cells from a different species (human) as well as several different function-inhibitory LN5 antibodies, in particular the antibodies P3H9-2, BM165, and RG13. The RG13 monoclonal antibody recognizes the G domain of the α3 subunit of hLN5 (Figure 5). RG13 antibodies, like P3H9-2 and BM165 antibodies, inhibit rapid adhesion of epithelial cells to hLN5 (our unpublished results). The human line we chose is MCF-10A, which is derived from breast epithelium and which expresses LN5 in vitro (Stahl et al., 1997). Indeed, immunochemical as well as molecular analyses of MCF-10A cells reveal that these cells secrete a matrix, whose major component is LN5 (Stahl et al., 1997; Scholz, personal communication). This matrix does not contain any detectable amounts of LN6 and LN7 (our unpublished results).
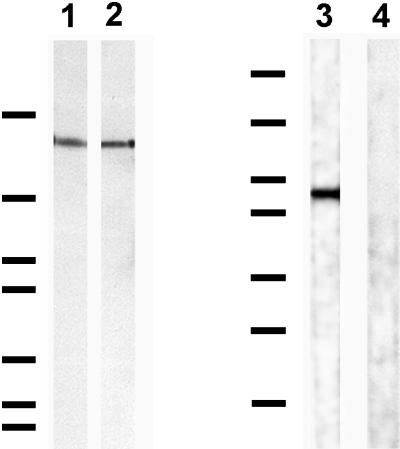
Figure 5.
LN5-rich matrices of either 804G cells (lane 1) or MCF-10A cells (lane 2) prepared according to Langhofer et al. (1993) as well as recombinant human α3 chain G domain (lanes 3 and 4) were processed for SDS-PAGE on either 6% (lanes 1 and 2) or 7.5% (lanes 3 and 4) gels. The separated proteins were then transferred to nitrocellulose and immunoblotted with CM6 (lane 1), RG13 (lane 2 and 3), and control IgG (lane 4) antibodies. CM6 antibodies recognize rat 160-kDa α3 chain (lane 1) (Baker et al., 1996), whereas RG13 recognizes human 160-kDa α3 chain (lane 2). Whereas RG13 shows reactivity with the G domain of human α3 chain in lane 3, the control IgG does not (lane 4). Molecular mass standards of 194, 120, 87, 64, 52, 39, and 26 kDa are indicated (from top to bottom).
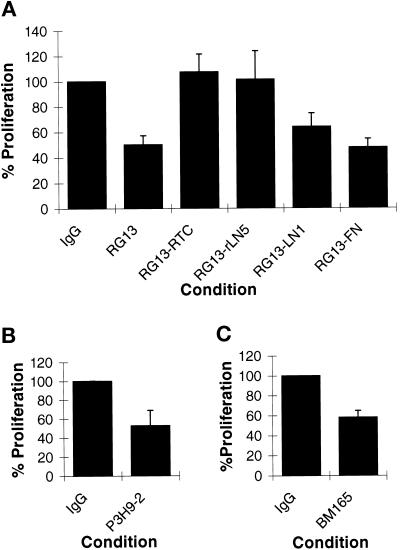
Antibodies RG13, P3H9-2, and BM165 all significantly reduce cell division of MCF-10A cells by 62, 47.4, and 41.9%, respectively, compared with IgG-treated control cell populations (Figure 6). They do so with little, if any, apparent effect on the spreading of the cells onto their substrate after 24 h (Figure 7; only MCF-10A cells treated with RG13 antibodies are shown).
Figure 6.
(A) MCF-10A cells (2 × 104 cells per well of a 24-well plate) were plated onto tissue culture plastic or surfaces coated with 50 μg/ml RTC, 2 μg/ml rtLN5, 25 μg/ml LN1, and 25 μg/ml FN for 48 hr. As indicated, MCF-10A cells were maintained in medium supplemented with either 50 μg/ml IgG control antibody or 50 μg/ml LN α3 subunit function-inhibiting antibody RG13. At 48 hr the cells were trypsinized and counted. % Proliferation indicates the increase in cell number as a percentage of that observed in the IgG-treated control cell population. The SD was determined from the data derived from three trials. At 48 hr the control cell population expanded from 2 × 104 to 1 × 105 cells (100%). (B) MCF-10A cells were maintained in medium supplemented with either 50 μg/ml IgG control antibody or 50 μg/ml LN5 function-inhibiting antibody P3H9-2. (C) MCF-10A cells were maintained in medium supplemented with either 50 μg/ml IgG control antibody or 50 μg/ml LN5 function-inhibiting antibody BM165. In B and C, the control cell populations expanded from 2 × 104 to 1.1 × 105 and from 2 × 104 to 9.1 × 104 after 48 hr (100%).
Figure 7.
Phase-contrast images of MCF10A cells (A–C) are shown at 48 hr after plating into normal medium (A), medium containing 50 μg/ml control IgG (B), or medium supplemented with 50 μg/ml antibody RG13 (C). Note that in all cases the cells have attached to and spread onto their substrate. Bar, 125 μm.
Only 39% of cells are stained by BrdU antibody in RG13 antibody-treated MCF-10A cultures compared with 52% of cells that are stained in MCF-10A cells treated with IgG control antibody (Table 2). MCF-10A cells treated with the other hLN5-inhibitory antibodies show similar staining patterns. In addition, as is the case with CM6-treated 804G cells, the hLN5 inhibitory antibodies apparently block MCF-10A cells randomly in the cell cycle, as determined using the BM28 antibody marker (Table 2).
Table 2.
MCF10A cells
| BrdU assay
|
BM28 assay
|
|||||
|---|---|---|---|---|---|---|
| Cells counteda | Labeling index (%) | Cells counteda | G1 (%) | S (%) | G2 (%) | |
| Control IgG | 302 | 52.0 | 456 | 43.2 | 34.0 | 22.8 |
| Anti-human LN5 | 301 | 39.0 | 343 | 40.8 | 33.5 | 25.7 |
Total number of cells counted in three trials.
The negative impact of RG13 antibodies on growth of MCF-10A cells is corrected by maintaining the cells on RTC and rtLN5 but not by plating the cells on LN1 or FN (Figure 6). MCF-10A cells maintained on RTC in the presence of RG13 antibodies show a proliferation of 107.4% compared with IgG-treated control cells (Figure 6). When maintained on rtLN5, the growth of MCF-10A cells in RG13 antibodies is 103.9% compared with IgG-treated control cells (Figure 6).
We next investigated potential involvement of integrin receptors in the above phenomenon using MCF-10A cells. Comparable studies using 804G cells were not possible because of lack of availability of an appropriate set of inhibitory and activating anti-rat integrin antibodies. LN5 has two known integrin receptors, α3β1 and α6β4 (Carter et al., 1990). Thus we made use of antibodies (P1B5 and GoH3) that perturb α3 and α6 integrin function, respectively. Antibody P1B5 inhibits proliferation of MCF-10A cells maintained on tissue culture plastic or RTC by 62.6 and 77.3%, respectively, compared with control IgG-treated cells (Figure 8A). In contrast, GoH3 inhibits MCF-10A cell proliferation by 30.2% (Figure 8A). In control studies, antibodies that inhibit the function of FN and α2 integrin have no obvious effect on MCF-10A cell division (Figure 8, A and B). The latter result suggests that LN5 “signals” are not transduced via some sort of “cross talk” between α3β1 and α2β1 integrin (Zhang and Kramer, 1996). It should also be noted that the proliferation of MCF-10A cells, which have been treated with P1B5 antibody for 48 h, is restored to normal after plating into fresh medium in the absence of antibody (our unpublished results).
Figure 8.
(A) MCF-10A cells were plated for 48 hr in the presence of 50 μg/ml control antibody, 25 μg/ml P1B5 (an α3 integrin-inhibitory antibody), 25 μg/ml P1B5 on RTC-coated wells, 25 μg/ml GoH3 (an α6 integrin-inhibitory antibody), a 1:250 dilution of an inhibitory antibody against FN, and a 1:50 dilution of P1E6 (an α2 integrin-inhibitory antibody). At 48 hr the cells were trypsinized and counted. To confirm that the anti-FN antibody and P1E6 are function-inhibiting in our hands, we undertook adhesion assays in which 2 × 105 MCF-10A cells and OVCA429 cells were plated onto either FN- or RTC-coated wells of a 96-well plate (B). (B) Left panel, the anti-FN antibody inhibits the attachment of MCF-10A to the FN-coated wells by 37%. Right panel, P1E6 inhibits the adhesion of OVCA429 cells to RTC by 49%. The latter confirms the results of Moser et al. (1996). The SDs indicated in the graphs in B were determined from the data derived from three trials. (C) MCF-10A cells were plated into medium containing 50 μg/ml control antibody, 50 μg/ml RG13 antibody, or 50 μg/ml RG13 antibody together with a 1:5 dilution of hybridoma medium containing the β1 integrin-activating antibody TS2/16.2.1 or 50 μg/ml 3E1 antibody. As in A, at 48 hr the cells were trypsinized and counted. In A and C, % Proliferation indicates the increase in cell number as a percentage of that observed in the control antibody-treated cell population. In A and C, the control cell population expanded from 2 × 104 to 1.025 × 105 or from 2 × 104 to 1.18 × 105 after 48 hr (100%).
The above results suggest that α3β1 integrin as well as α6β4 integrin mediate the proliferation effects of LN5 on MCF-10A cells. To provide further support for this possibility, we used antibodies (TS2/16.2.1 and 3E1) that activate β1 and β4 integrin, respectively, in the absence of ligand (van de Wiel-van Kemenade et al., 1992; Mainiero et al., 1997). MCF-10A cells were treated with a combination of RG13 and TS2/16.2.1 or RG13 and 3E1 antibodies. The proliferation of such antibody treated cells is 98.8 and 81.7% of that of control antibody-treated cell cultures (Figure 8C).
Is MAP Kinase Involved?
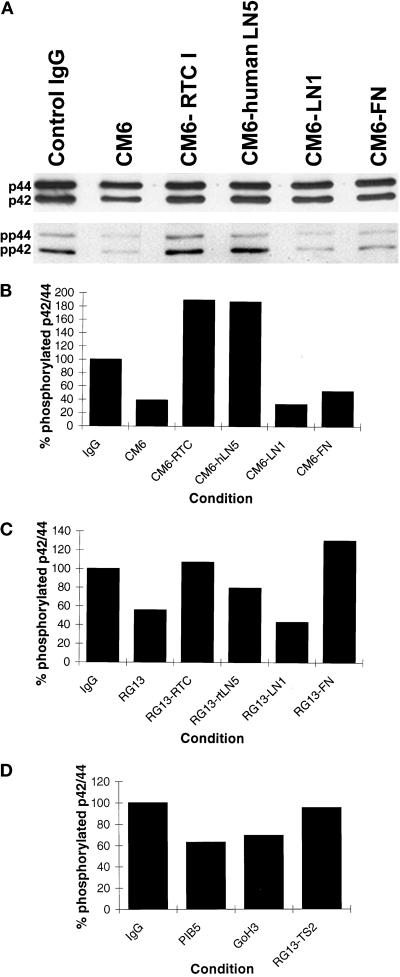
Based on data that suggest that MAP kinase mediates the regulatory effects of extracellular matrix and integrin receptors on cell cycle progression, we evaluated the degree of MAP kinase activation in both 804G and MCF-10A cells after various treatments (Rosales et al., 1995; Howe et al., 1998; Schlaepfer and Hunter, 1998). For these studies we used an antibody (anti-ACTIVE MAPK p42/p44), specific for phosphorylated, activated p42/p44 in combination with an antibody (anti-p42/p44) that recognizes p42/p44 irrespective of its activation state. Under each experimental condition, we evaluated both total MAP kinase content as well as the level of activated MAP kinase in cell extracts by immunoblotting (Figure 9A shows a typical assay result). In 804G and MCF-10A cells treated with antibodies that inhibit the function of the α3 LN subunit, MAP kinase activity is reduced by 61.1 and 44.2%, respectively, relative to MAP kinase activity in control IgG-treated cell populations (Figure 9, B and C). In contrast, in 804G cells maintained on RTC or hLN5 in the presence of CM6 antibodies, MAP kinase activity is at 189.5 and 187.1% of that in control IgG-treated cells (Figure 9B). MAP kinase activity remains down-regulated in CM6-treated 804G cells plated onto LN1- and FN-coated substrates, the level being 33.1 and 52.8%, respectively, of that observed in control IgG-treated cells (Figure 9B). In MCF-10A cells maintained on RTC or rtLN5 in the presence of RG13 antibodies, MAP kinase activity is 107.1 and 79.7% of that in control IgG-treated cells, respectively (Figure 9C). MAP kinase activity in RG13-treated MCF-10A cells plated onto LN1- and FN-coated substrate is 43.1 and 130.3%, respectively, of that observed in control IgG-treated cells (Figure 9C).
Figure 9.
(A) MAPK assay blot in which 804G cells were plated onto tissue culture plastic or surfaces coated with 50 μg/ml RTC, 25 μg/ml FN, 25 μg/ml LN1, or 1 μg/ml hLN5 for 48 hr. As indicated, the 804G cells were maintained in medium supplemented with either 50 μg/ml IgG control antibody or 50 μg/ml CM6 antibodies. After 48 hr the cells were scraped into gel sample buffer, processed for SDS-PAGE, transferred to nitrocellulose, and immunoblotted with either anti-ACTIVE MAPK p42/p44 to determine phosphorylated p42/p44 (lower panel) or a probe for total p42/p44 (upper panel). (B–D) Scan analyses of MAPK blots of 804G cells (B) and MCF-10A (C and D) were undertaken using the Bio-Rad Molecular Analyst program. The “amount” of total p42/p44 in each sample was normalized to that observed in IgG control-treated specimens, and then the levels of activated p42/p44 were appropriately adjusted. We then calculated the % phosphorylated p42/p44 for each specimen relative to that observed in the IgG control samples. The culture conditions and concentrations of antibodies used are identical to those shown in Figures 4, 6A, and 8.
We next analyzed MAP kinase activity in MCF-10A cells treated with integrin-blocking antibodies. MAP kinase activity is reduced by 37.1 and 30.7%, respectively, in MCF-10A cells treated with P1B5 and GoH3 antibodies compared with IgG-treated control cells (Figure 9D). TS2/16.2.1. antibodies restore MAP kinase activity in MCF-10A cells treated with RG13 antibodies to 95.1% of control levels (Figure 9D).
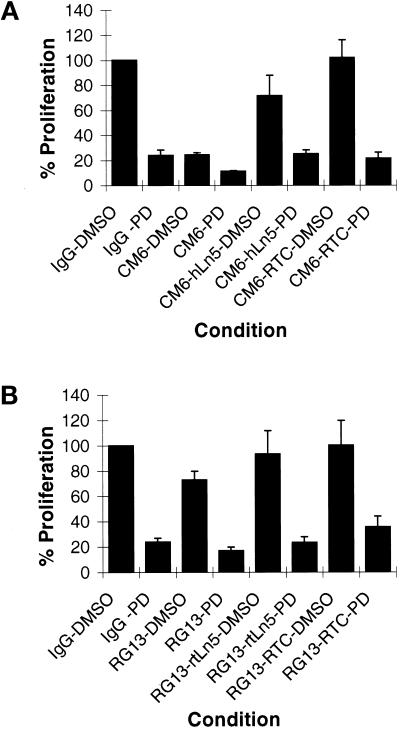
The above results reveal a correlation between MAP kinase activity and LN5 regulation of cell proliferation with the notable exception that FN is able to rescue MAP kinase activity in MCF-10A cells but not the proliferation of MCF-10A cells treated with LN5 function-inhibitory antibodies. Thus to provide additional evidence that MAP kinase is a part of the pathway by which LN5 regulates cell proliferation, we used the MAP kinase inhibitor PD98059. Cells treated with PD98059 do not die during the course of our assays, as determined by trypan blue exclusion assay (our unpublished results). MAP kinase is efficiently inactivated in 804G and MCF-10A cells treated with the inhibitor, as determined by immunoblotting using the anti-ACTIVE MAPK p42/p44 antibody probe (our unpublished results). Furthermore, in all cases PD98059 inhibits proliferation of both 804G or MCF-10A cells (Figure 10, A and B). This is true even in cells treated with LN5-perturbing antibodies and plated onto substrates that we have shown in Figures 4 and 6 “rescue” cell division (Figure 10).
Figure 10.
804G cells (A) and MCF10A cells (B) were maintained in medium containing 50 μg/ml control IgG as well as 50 μg/ml CM6 or RG13 antibodies. In some studies the treated cells were plated onto substrate coated with 50 μg/ml RTC or 1 μg/ml hLN5 or rtLN5. The MAPK inhibitor PD98059 in DMSO was added to cells at a concentration of 50 μM. An equal amount of DMSO lacking PD98059 was used as a control as indicated. At 48 hr the cells were trypsinized and counted. % Proliferation was determined by evaluating the increase in cell number as a percentage of that observed in the IgG control cell population. The SD was determined from the data derived from three trials. In A and B, the control cell populations expanded from 2 × 104 to 1.08 × 105 and from 2 × 104 to 1.05 × 105 after 48 hr (100%).
DISCUSSION
LN5 is now considered to play an important role in the establishment of substrate attachment in a variety of epithelial cell types. One of the major pieces of evidence in support of this conclusion comes from recent analyses of the human inherited blistering skin disease junctional epidermolysis bullosa and the autoimmune disease cicatricial pemphigoid. In certain families afflicted by junctional epidermolysis bullosa there are defects in or a loss of LN5 (reviewed in Borradori and Sonnenberg, 1996; Jones et al., 1998). The latter results in the dysadhesion of keratinocytes, thereby bringing about blistering in the epidermis in the region of keratinocyte–extracellular matrix interaction. In cicatricial pemphigoid, autoantibodies against LN5 appear to be pathogenic, and antibody binding to the basement membrane zone induces epithelial blistering (Domloge-Hultsch et al., 1992).
Yet even though LN5 is clearly involved in adhesion, its expression at the leading edges of migrating tumor populations suggests that this heterotrimer may play roles in cell motility and cell proliferation (Pyke et al., 1994, 1995). There is already published evidence that LN5 can enhance epithelial cell motility under certain circumstances (Zhang and Kramer, 1996; Giannelli et al., 1997; Goldfinger et al., 1998). Here we show that LN5 has the potential to impact cell proliferation through its α3 subunit. Using an immunological approach we have inhibited the function of the LN5-rich matrix produced by two distinct cell lines generated from two different organs of two different species. In both instances, the division of cells is significantly inhibited.
CM6 antibody-treated 804G cells only partially flatten onto their substrate compared with their control counterparts, although they clearly attach. The function-inhibitory antibodies against hLN5 have no obvious effects on MCF-10A cellular morphology. The cells appear to spread fully onto their substrate in the presence of the inhibitory antibodies. This indicates that the inhibition of cell division induced by inhibiting LN5 function in both 804G and MCF-10A cells is not a secondary consequence of the loss of cell–substrate contact, which is known to trigger the differentiation of many epithelial cell types (for example, see Adams and Watt, 1989). Rather, it is the result of a block in a signal “encoded” by LN5, which is transduced via cell surface receptors to the overlying cells and which can directly modulate the progress of the cell cycle.
Proliferation of 804G and MCF-10A cells, treated with LN5 inhibitory antibodies, can be restored to normal levels not only if they are provided with exogenous LN5 but also when they are plated onto collagen-coated substrates, suggesting that the latter matrix molecule can substitute for LN5. This result gave us a clue as to the nature of the receptor involved in LN5 regulation of cell growth, because cell interaction with both of these particular matrix molecules can be mediated by the α3β1 integrin heterodimer (Carter et al., 1990; Elices et al., 1991; Hynes, 1992). The use of human cells enabled us to further this aspect of our study, because we could assess whether a well-characterized β1 integrin antibody that activates its human antigen in the absence of ligand could rescue the proliferation of MCF-10A cells treated with an inhibitory LN5 antibody (van de Wiel-van Kemenade et al., 1992). The integrin-activating antibody TS2/16.2.1 does rescue proliferation, supporting the idea that the mitogenic effects of LN5 are transduced to the cell cycle machinery via a β1-containing integrin receptor. Evidence that this is the α3β1 heterodimer comes from our studies using the α3 integrin function-inhibiting antibody P1B5. P1B5, like the function-inhibitory LN5 antibodies, reduces MCF-10A cell proliferation.
It should be noted that a function-perturbing antibody against the α6 component of the α6β4 integrin heterodimer, a second LN5 receptor, also impacts MCF-10A proliferation, although to a lesser degree than the α3 integrin antibody P1B5. In addition, the β4 integrin antibody 3E1 partially rescues the proliferation of MCF-10A cells treated with an inhibitory LN5 antibody. Thus α6β4 integrin may also be involved in the ability of LN5 to modulate the proliferation of MCF-10A cells. These results are consistent with a recent publication by Mainiero et al. (1997), who reported that α6β4 integrin is a component of a pathway that regulates epidermal cell proliferation, although it is interesting that these same authors claim that α3β1 integrin is not involved in such regulation, a result inconsistent with the study we present here.
Our MAP kinase analyses suggest that this enzyme is a component of a pathway that transduces signals from LN5 via the α3β1 integrin complex to the cell nucleus where they regulate cell division. We base this conclusion on the following results: 1) we observe decreased activity of MAP kinase in 804G and MCF-10A cells treated with LN5 function-inhibitory antibodies; 2) we see an inhibition of division of these same cell types when they are treated with a MAP kinase inhibitor; and 3) MAP kinase activity is restored close to, or greater than, normal levels in cells treated with LN5 antibodies when they are maintained on LN5 or RTC or when their β1 integrin is activated, under which conditions the proliferation of the cells is rescued. However, we would also like to point out one apparent anomaly in our results. MAP kinase activity appears normal in MCF-10A cells that are treated with LN5 antibodies and maintained on an FN-coated substratum. We did not observe the same phenomenon in CM6 antibody-treated 804G cells plated onto FN. Yet in both instances the proliferation of such cells is inhibited. One potential explanation for this anomalous result is that a second pathway, involving MAP kinase, is activated by MCF-10A cell interaction with FN. We suppose, based on the results presented here, that this pathway modulates an MCF-10A cellular function other than cell division. There is, in fact, precedent for this theory. In PC12 neuronal cells, both EGF and nerve growth factor signal through MAP kinase. However, whereas EGF modulates cell cycle progression, nerve growth factor regulates gene expression and cell differentiation (Marshall, 1995).
The idea that α3β1 integrin plays an important role in epithelial cell division may be considered, at first glance, inconsistent with a study by DiPersio et al. (1997). The data presented by those workers would suggest that epithelial cell division is apparently normal in an α3 integrin-deficient mouse. However, we speculate that in such mice α6β4 integrin may well “drive” epithelial cell proliferation, as suggested by Mainiero et al. (1997) and the current work. In this regard, there has been a recent report that suggests that epithelial cells in mice carrying a β4 cytoplasmic deletion have some cell cycle defects (Murgia et al., 1998). Because the epithelial tissues in the mutant mice appear relatively normal, we hypothesize that α3β1 integrin may well compensate for the functionally impaired α6β4 integrin heterodimer.
The idea that LN5, together with its α3β1 cell surface receptor, has an impact on cell proliferation is consistent with studies on LN1, which indicate that it also may impact cell growth (Panayotou et al., 1989; Mortarini et al., 1995). For example, LN and β1 integrins exert a mitogenic effect on human melanoma cells (Ocalan et al., 1988). LN1 has also been shown to stimulate the proliferation of myoblasts and bone marrow–derived macrophages in a dose-dependent manner, whereas human thymocyte proliferation is apparently influenced by LN and merosin and their receptors α3β1 and α6β1 (Ocalan et al., 1988; Ohki and Kohashi, 1994; Chang et al., 1995). In addition, the β1 cytoplasmic domain has been reported to modulate cell proliferation through an integrin signaling pathway (Pasqualini and Hemler, 1994).
Finally, the function-perturbing antibodies CM6 and RG13, which we have used in this study, recognize the LN α3 subunit (Baker et al., 1996). CM6 antibodies have been localized to the G domain of the intact LN5 molecule, whereas RG13 antibodies recognize the G domain of human α3 prepared in a bacterial expression system (Baker et al., 1996). Thus we propose that the proliferative impact of the LN α3 subunit is encoded by a sequence of amino acids in or close to the G domain of the molecule. Based on our integrin antibody studies, we speculate that this domain is likely to be the α3β1 integrin binding site. Indeed, our results would appear to confirm a recent report showing that the α3β1 integrin binding in LN5 is a sequence of 22 amino acids within the G domain of the α3 subunit (Mizushima et al., 1997). Unfortunately, we have been unable to replicate the results of Mizushima et al. (1997) with this particular peptide, and, furthermore, the α3 subunit peptide is unable to rescue the proliferation of our LN5 function-inhibited, antibody-treated cells (our unpublished results). Nonetheless, based on our observations, we propose that expression of LN5 at the leading tips of epithelial tumor populations in vivo may enhance cell proliferation and thereby may drive tumor growth.
ACKNOWLEDGMENTS
We are grateful to Dr. Erich Nigg and Dr. William Schnaper for helpful comments on this work. We thank Dr. Sharon Stack for the OVCA cells and Dr. Wolfgang Scholz for sharing unpublished observations. This work was supported by grants from the National Institutes of Health (PO1 DE12328 and RO1 GM38470 to J.C.R.J.).
Abbreviations used:
- BrdU
bromodeoxyuridine
- DAPI
4,6-diamidino-2-phenylindole
- EGF
epidermal growth factor
- FN
fibronectin
- hLN5
human laminin-5
- Ig
immunoglobulin
- LN
laminin
- MAP
mitogen-activated protein
- MAPK
MAP kinase
- RTC
rat tail collagen
- rtLN5
rat laminin-5
REFERENCES
- Adams JC, Watt FM. Fibronectin inhibits terminal differentiation of human keratinocytes. Nature. 1989;340:307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Regulation of development and differentiation by extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Baker SE, Hopkinson SB, Fitchmun M, Andreason GL, Frasier F, Plopper G, Quaranta V, Jones JCR. Laminin-5 and hemidesmosomes: role of the α3 chain subunit in hemidesmosome stability and assembly. J Cell Sci. 1996;109:2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Hemidesmosomes: role in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Chang AC, Salomon DR, Wadswoth S, Hong MP, Mojcik CF, Otto S, Shevach EM, Coligan JE. α3β1 and α6β1 integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J Immunol. 1995;154:500–510. [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke R, Jaenisch R, Kreidberg JA, Hynes RO. α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domloge-Hultsch N, Gammon WR, Briggaman RA, Gil SG, Carter WG, Yancey KB. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J Clin Invest. 1992;90:1628–1633. doi: 10.1172/JCI116033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: fibronectin, collagen and laminin binding are differentially influenced by ARG-GLY-ASP peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG. Signal transduction by the α6β4 integrin: charting the path between laminin binding and nuclear events. J Cell Sci. 1996;109:1165–1172. doi: 10.1242/jcs.109.6.1165. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JCR. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Jones JCR. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J. 1996;10:871–880. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Asmuth J, Baker SE, Langhofer M, Roth SI, Hopkinson SB. Hemidesmosomes: extracellular matrix/intermediate filament connectors. Exp Cell Res. 1994;213:1–11. doi: 10.1006/excr.1994.1166. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Hopkinson SB, Goldfinger L. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kearsey SE, Maiorano D, Holmes E, Todorov IT. The role of MCM proteins in the control of genome duplication. Bioessays. 1996;18:183–190. doi: 10.1002/bies.950180305. [DOI] [PubMed] [Google Scholar]
- Kiatte DH, Kurpakus MA, Grelling KA, Jones JCR. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J Cell Biol, 1989;109:3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude T, Musahl C, Laskey R, Knippers R. Human replication proteins dCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S phase and are displaced locally during DNA replication. J Cell Sci. 1996;109:309–318. doi: 10.1242/jcs.109.2.309. [DOI] [PubMed] [Google Scholar]
- Kuhn K. Extracellular matrix constituents as integrin ligands. In: Eble JA, Kuhn K, editors. Integrin-Ligand Interactions. Austin: R.G. Landes; 1997. pp. 41–69. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JCR. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumenberhg M, Westwick JK, Der CJ, Giancotti FG. The coupling of α6β4 integrin to the Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Mortarini R, Gismondi A, Maggioni A, Santoni A, Herlyn M, Anichini A. Mitogenic activity of laminin on human melanoma and melanocytes: different signal requirements and role of β1 integrins. Cancer Res. 1995;55:4702–4710. [PubMed] [Google Scholar]
- Mizushima H, Takamura H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K. Identification of integrin-dependent and -independent cell adhesion domains in COOH-terminal globular region of laminin 5 α3 chain. Cell Growth & Differ. 1997;8:979–987. [PubMed] [Google Scholar]
- Moser TL, Pizzo SV, Bafetti LM, Fishman DA, Stack SM. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the α2β1 integrin. Int J Cancer. 1996;67:695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, Giancotti FG. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J. 1998;17:3940–3951. doi: 10.1093/emboj/17.14.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocalan M, Goodman SL, Kuhl U, Hauschka SD, von der Mark K. Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev Biol. 1988;125:158–167. doi: 10.1016/0012-1606(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Ohki K, Kohashi O. Laminin promotes proliferation of bone marrow-derived macrophages and macrophage cell lines. Cell Struct Funct. 1994;19:63–71. doi: 10.1247/csf.19.63. [DOI] [PubMed] [Google Scholar]
- Panayotou G, End P, Aumailley M, Timpl R, Engel J. Domains of laminin with growth factor activity. Cell. 1989;56:93–101. doi: 10.1016/0092-8674(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Hemler ME. Contrasting roles for integrin β1 and β5 cytoplasmic domains in subcellular localization, cell proliferation and cell migration. J Cell Biol. 1994;125:447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, Tryggvason K. The gamma 2 chain of kalinin/laminin-5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- Riddelle KS, Green KJ, Jones JCR. Formation of hemidesmosomes in vitro by a rat bladder cell line. J Cell Biol. 1991;112:159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C, O’Brien V, Kornberg L, Juliano R. Signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1995;1242:77–98. doi: 10.1016/0304-419x(95)00005-z. [DOI] [PubMed] [Google Scholar]
- Roskelly CD, Srebow A, Bissell MJ. A hierarchy of ECM-mediated signaling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrun GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Stahl S, Weitzman S, Jones JCR. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110:55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- Todorov IT, Attaran A, Kearsey S. BM28, a human member of the MCM2–3-5 family, is displaced from chromatin during DNA replication. J Cell Biol. 1995;129:1433–1445. doi: 10.1083/jcb.129.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov IT, Pepperkok R, Philipova RN, Kerasey S, Ansorge W, Werner D. A human nuclear protein with sequence homology to a family of early S phase proteins is required for the entry into S phase and for cell division. J Cell Sci. 1994;107:253–265. doi: 10.1242/jcs.107.1.253. [DOI] [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E, van Kooyk Y, de Boer AJ, Huijbens RJ, Weder P, van de Kasteele W, Melief CJ, Figdor CG. Adhesion of T and B lymphocytes to extracellular matrix and endothelial cells can be regulated through the β subunit of VLA. J Cell Biol. 1992;117:461–470. doi: 10.1083/jcb.117.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Gil SG, Carter WG. Anchorage mediated by integrin α6β4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane associated 80-kDa protein. J Cell Biol. 1996;132:727–740. doi: 10.1083/jcb.132.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff RV, Goldman AE, Jones JC, Steinert PM, Goldman RD. Isolation and characterization of keratin-like proteins from cultured cells with fibroblastic morphology. J Cell Biol. 1984;98:1231–1237. doi: 10.1083/jcb.98.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kramer RH. Laminin-5 deposition promotes keratinocyte motility. Exp Cell Res. 1996;227:309–333. doi: 10.1006/excr.1996.0280. [DOI] [PubMed] [Google Scholar]