Abstract
The lipids of six strains of Bacteroides ruminicola were characterized. The nonpolar lipid accounted for 6 to 24% of the total lipid and was composed of diglycerides, triglycerides, and free fatty acids. The phospholipid fraction contained phosphatidylethanolamine, phosphosphingolipids, and trace quantities of phosphatidic acid. In three strains the phosphosphingolipid fraction made up more than half of the total lipid. The fatty acids in the nonpolar, acyl- and phosphosphingolipid consisted of a homologous series of branched and normal chains from 12 to 19 carbons. The long-chain base isolated from the phosphosphingolipids consisted of a homologous series of branched and normal chains from 14 to 24 carbons.
Full text
PDF

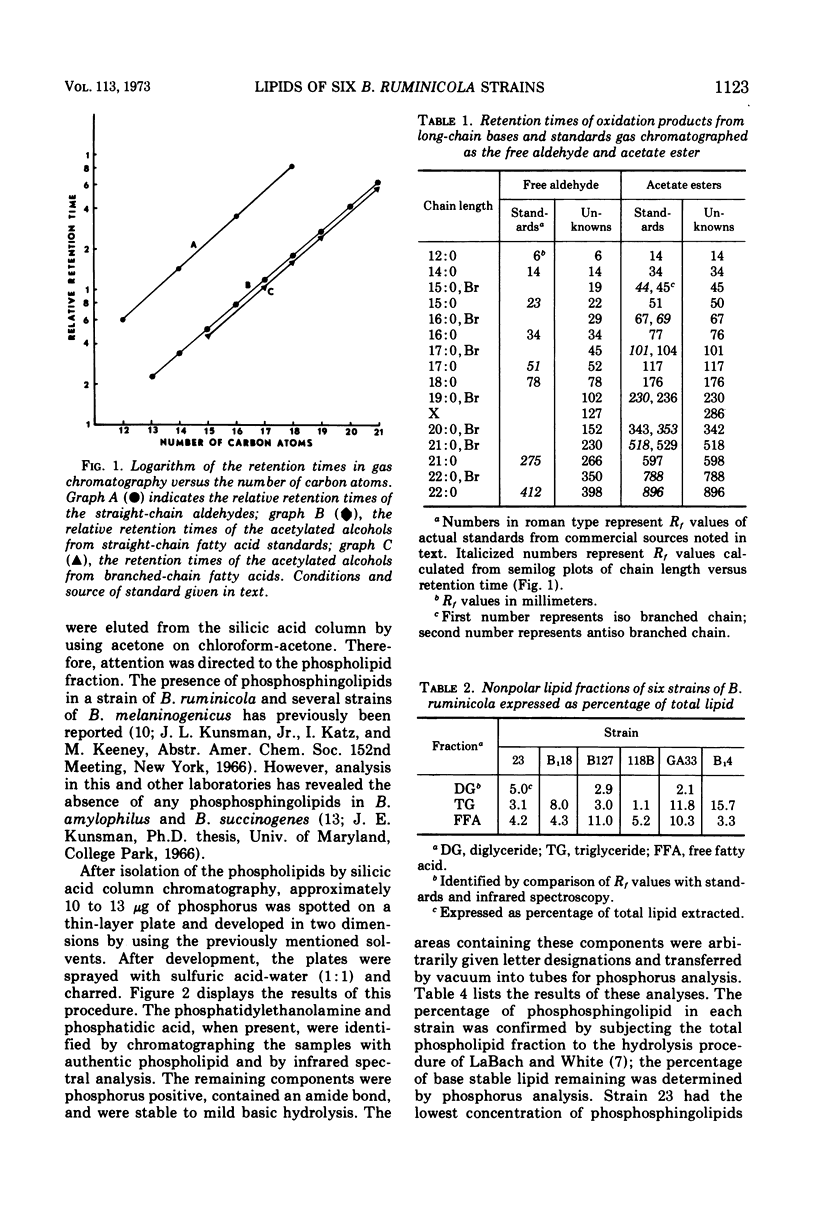



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- CARTER H. E., HENDRICKSON H. S. Biochemistry of the sphingolipids. XV. Structure of phytosphingosine and dehydrophytospingosine. Biochemistry. 1963 Mar-Apr;2:389–393. doi: 10.1021/bi00902a036. [DOI] [PubMed] [Google Scholar]
- Carter H. E., Gaver R. C. Improved reagent for trimethylsilylation of sphingolipid bases. J Lipid Res. 1967 Jul;8(4):391–395. [PubMed] [Google Scholar]
- Katz I., Keeney M. Quantitative micro determination and isolation of plasmalogen aldehydes as 2,4-dinitrophenylhydrazones. J Lipid Res. 1966 Jan;7(1):170–174. [PubMed] [Google Scholar]
- Kunsman J. E. Characterization of the lipids of Butyrivibrio fibrisolvens. J Bacteriol. 1970 Jul;103(1):104–110. doi: 10.1128/jb.103.1.104-110.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBach J. P., White D. C. Identification of ceramide phosphorylethanolamine and ceramide phosphorylglycerol in the lipids of an anaerobic bacterium. J Lipid Res. 1969 Sep;10(5):528–534. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Rizza V., Tucker A. N., White D. C. Lipids of Bacteroides melaninogenicus. J Bacteriol. 1970 Jan;101(1):84–91. doi: 10.1128/jb.101.1.84-91.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- WEGNER G. H., FOSTER E. M. Incorporation of isobutyrate and valerate into cellular plasmalogen by Bacteroides succinogenes. J Bacteriol. 1963 Jan;85:53–61. doi: 10.1128/jb.85.1.53-61.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. A., Dittmer J. C. A microanalytical technique for the quantitative determination of twenty-four classes of brain lipids. Biochemistry. 1966 Nov;5(11):3405–3418. doi: 10.1021/bi00875a004. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Ceramide phosphorylglycerol phosphate: a new sphingolipid found in bacteria. Lipids. 1970 Jan;5(1):56–62. doi: 10.1007/BF02531095. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Sweeley C. C. Characterization of the iso-branched sphinganines from the ceramide phospholipids of Bacteroides melaninogenicus. Biochim Biophys Acta. 1969 Dec 17;187(4):527–532. doi: 10.1016/0005-2760(69)90050-2. [DOI] [PubMed] [Google Scholar]



