Abstract
Objective
To determine if children with white coat hypertension (WCH) have evidence of target-organ damage by comparing left ventricular mass index (LVMI) of subjects with WCH to that of matched normotensive and hypertensive controls.
Study design
Each WCH subject was matched by body mass index (± 10%), age (± 1 year), and sex to a normotensive control and to a hypertensive control. Echocardiograms were reviewed to determine LVMI for each subject. These triple matches were analyzed using repeated measures analysis of variance to detect differences in LVMI between the three groups.
Results
Twenty-seven matched triplets were established. The groups were comparable for sex, age, and body mass index (BMI). Mean LVMI was 29.2, 32.3, and 35.1 g/m2.7, for normotensives, WCH, and sustained hypertensives, respectively (normotensive vs. WCH, p = 0.028; WCH vs. sustained hypertensive, p = 0.07). Left ventricular hypertrophy was not present in any subject in the normotensive or WCH groups, but was present in 26% of the sustained hypertensive subjects (p < 0.001).
Conclusions
After controlling closely for BMI, children with WCH had a LVMI which was intermediate between that of normotensives and sustained hypertensives, suggesting that WCH may be associated with hypertensive end-organ effects.
White coat hypertension (WCH) occurs when blood pressure is elevated in the medical office but is normal outside the office setting. In adults, there is concern that WCH may be an independent risk factor for cardiovascular disease or that it may represent a prehypertensive state, with increased risk of progression to sustained hypertension.1 Numerous adult studies have evaluated left ventricular mass index (LVMI) in subjects with WCH. Many, but not all, of these studies have demonstrated increased LVMI in WCH subjects, with LVMI intermediate between that of subjects with normotension and those with sustained hypertension.2–13 In addition, a recent study suggests that the long-term incidence of stroke may be elevated in adults with WCH.14
Little is known about the clinical significance and natural history of WCH in children. Depending on the diagnostic criteria used, approximately 20 – 50% of children and adolescents referred for evaluation of elevated clinic blood pressure have WCH, confirmed by normal 24-hour ambulatory blood pressure monitoring (ABPM).15 Few studies have evaluated LVMI in children with WCH.16–18 Furthermore, no previous study of LVMI in children with WCH has matched subjects for body mass index (BMI), a characteristic that is increased in children with WCH and is itself an independent determinant of LVMI.16,19
The objective of the current study was to determine if children with WCH have evidence of hypertensive target-organ effects by comparing LVMI in children with WCH, normotension, and sustained hypertension, closely matching subjects for BMI, sex, and age.
Methods
The study was performed at the University of Rochester Medical Center in Rochester, NY. At this center, ABPM has been used in the evaluation of pediatric primary hypertension since October 2002. The clinical purpose of ABPM has been primarily to differentiate between sustained hypertension and white coat hypertension. It has been our practice to obtain echocardiography in all children diagnosed with sustained hypertension or white coat hypertension to evaluate for target-organ damage. We retrospectively identified all patients ages 10 – 17 years referred to the Division of Pediatric Nephrology or to the Pediatric Hypertension Clinic (created during the study period) for evaluation of elevated blood pressure who underwent ABPM between October 2002 and May 2007. All subjects had a history of office systolic and/or diastolic blood pressure ≥ 95th percentile for age, sex, and height on at least three occasions and an elevated mean clinic BP at the initial visit (at least ≥ 90th percentile).20 Clinic blood pressure (BP) was taken at the initial visit, first with an automated oscillometic device and then repeated 1 – 2 times by auscultation with an aneroid BP device. For study purposes, mean clinic BP was defined as the average of the blood pressures taken by auscultation. Clinic BP index was defined as the subject’s mean clinic BP divided by the 95th percentile for age, sex, and height.20 Patients with evidence of secondary hypertension and those already on antihypertensive medication were excluded. Secondary hypertension was excluded by history, physical examination, serum chemistries, urinalysis, renal ultrasound, and other tests as indicated. To be considered to have WCH, subjects were required to have daytime and nighttime mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) less than the 95th percentile for pediatric ABPM norms and also to have 24-hr SBP and 24-hr DBP load < 25th %. To be considered to have sustained hypertension, subjects were required to have 24-hr SBP and/or 24-hr DBP = 95th percentile for pediatric ABPM norms and also a 24-hr SBP load ≥ 30%.21,22
Healthy normotensive control subjects were identified retrospectively from patients referred for a heart murmur to the Division of Pediatric Cardiology during the same time period who had the final diagnosis of innocent murmur. In the Pediatric Cardiology clinic, BP was measured 1 – 2 times by auscultation with an aneroid BP device. All normotensive control subjects had Pediatric Cardiology clinic SBP and DBP < 90th percentile for age, sex, and height.20 Medical records were reviewed to document that the control subjects were otherwise healthy. Normotensive controls did not have ABPM.
WCH subjects were matched individually to sustained hypertensive subjects and normotensive control subjects by age (± 1 year), sex, and BMI (± 10%). The goal was to establish triplets of normotensive-WCH-sustained hypertensive subjects matched closely for BMI, sex, and age. The study was approved by the Research Subject Review Board of the University of Rochester Medical Center.
ABPM was performed using Spacelabs monitors 90217 (Spacelabs Medical, Issaquah, Washington). An appropriate cuff size was placed on the non-dominant arm. Monitors were programmed to obtain BP readings every 20 minutes from 8AM – 10 PM and every 30 minutes from 10 PM – 8 AM.22 Wake and sleep periods for ABPM analyses were determined by the patient’s self report. Blood pressures were analyzed using Spacelabs software (ABP Report Management System, version 1.03.16). ABPM was considered adequate if there were a minimum of 40 readings with no more than one hour between successful readings. Blood pressure load was defined as the percent of BP readings ≥ 95th percentile, for pediatric ABPM norms.22 Ambulatory BP index was defined as the mean ambulatory BP divided by the 95th percentile for pediatric ABPM norms.22 Twenty-four hour ambulatory BP variability was defined as the within-subject standard deviation of all BP readings during the 24-h measurement period.23 Twenty-four hour ambulatory heart rate variability was defined as the within-subject standard deviation of all heart rate measurements in beats per minute (bpm) during the 24-h period.
Within one month of ABPM, two dimensional echocardiography was performed on all subjects with M-mode and Doppler study (Acuson Sequoia or Aspen, Siemens, Mountain View, California). All subjects had structurally normal hearts and none were found to have coarctation of the aorta. Two-dimensional left ventricular mass by the area-length method was measured according to standards published by the American Society of Echocardiography.24 Measurements obtained were reviewed on digitally stored images and left ventricular mass was measured by a single experienced physician echocardiographer (C.C.M.) blinded to the blood pressure status of the subject. Left ventricular mass was indexed to height2.7 to correct for the effect of body size on assessment of left ventricular hypertrophy (LVH).25 LVH was defined as left ventricular mass index (LVMI) ≥ the 95th percentile, which was 39.36 g/m2.7 for boys and 36.88 g/m2.7 for girls, respectively.26
Demographic characteristics of WCH subjects who were successfully matched and included in the study were compared to the characteristics of WCH subjects who were not successfully matched, and therefore not in the study, using two-sample t-test (for continuous variables) and Fisher exact test (for categorical variables). Results from three matched groups (Normotensive, WCH, and sustained hypertension) were compared using one-factor, repeated measures analysis of variance (ANOVA), with Tukey adjustment for multiple pairwise comparisons. Descriptive statistics of continuous variables are expressed as mean ± SD. The significance level of the data analysis is set at 0.05.
Results
Children referred for office hypertension (n=217) met inclusion criteria and had ABPM. There were 58 children who did not have ABPM performed but who otherwise met inclusion criteria. These children were similar to those who did have ABPM in age (14.8 vs. 14.8 y, p = 0.99) and sex (77.6 vs. 73.1 % male, p = 0.60), but the children without ABPM had a higher BMI (36.1 ± 10.1 vs. 28.5 ± 7.3 kg/m2, p < 0.001), higher SBP index (1.10 ± 0.08 vs. 1.07 ± 0.08, p = 0.033), and higher DBP index (0.99 ± 0.12 vs. 0.93 ± 0.12, p < 0.001).
Of the 217 subjects who did have ABPM, the ABPM was inadequate in 43 subjects. There was no difference between subjects with adequate ABPM and those with inadequate ABPM in age, height, BMI, sex, SBP index, or DBP index (data not shown). Of the remaining 174 subjects with adequate ABPM, 54 (31%) had WCH. Of these, 2 did not have an echocardiogram and were excluded from the study. Twenty-seven (52%) of the remaining 52 WCH patients were able to be individually matched to both a normotensive control and a sustained hypertensive subject. The remaining 25 WCH subjects who were unable to be matched to both were not included in the study. Compared to matched WCH subjects, the unmatched WCH subjects were similar in age and clinic DBP index, but had higher clinic SBP index, higher mean BMI, a higher proportion of subjects with BMI ≥ 95th percentile, and a lower proportion that were male (Table I). Mean LVMI was comparable between matched WCH and unmatched WCH subjects (p = 0.53). No subject in the matched group had LVH, whereas 4 subjects (16%) in the unmatched group had LVH. All unmatched WCH subjects with LVH had BMI ≥ 95th percentile. In fact, the BMI percentile for each of these subjects was ≥ 97th percentile.
Table 1.
Characteristics of matched and unmatched subjects with WCH
| Characteristic | Matched WCH n = 27 |
Unmatched WCH N = 25 |
P value |
|---|---|---|---|
| Age (y) * | 14.8 ± 1.6 | 14.2 ± 1.9 | 0.244 |
| Male (%) | 89 | 64 | 0.049 |
| BMI (kg/m2) * | 24.8 ± 4.1 | 28.3 ± 7.2 | 0.044 |
| BMI ≥ 95th percentile (%) | 30 | 64 | 0.025 |
| Clinic SBP index * | 1.03 ± 0.06 | 1.06 ± 0.06 | 0.032 |
| Clinic DBP index * | 0.92 ± 0.11 | 0.93 ± 0.10 | 0.70 |
| LVMI (g/m2.7) * | 32.3 ± 4.2 | 31.4 ± 6.6 | 0.534 |
| LVH (%) | 0 | 16 | 0.047 |
mean ± SD
Because of the selection criteria, the three groups were comparable for age, sex, mean BMI, BMI percentile, and the proportion of subjects with BMI ≥ 95th percentile, but differed by clinic BP index (Table II). By definition, the WCH and sustained hypertensive groups differed by ABPM variables (Table III). With regard to BP variability, the WCH group was comparable with the sustained hypertensive group for 24-hr heart rate (69 ± 8 vs. 72 ± 10 bpm, p = 0.25), 24-hr heart rate variability (11.5 ± 3.1 vs. 11.8 ± 3.8 bpm, p = 0.74), and 24-hr DBP variability (11.1 ± 1.7 vs. 11.8 ± 2.1 mm Hg, p = 0.17), but WCH subjects had lower 24-hr SBP variability (12.9 ± 2.0 vs. 14.4 ± 2.5 mm Hg, p = 0.026) compared with sustained hypertension subjects.
Table 2.
Characteristics of normotensive, WCH, and sustained hypertensive groups
| Characteristic | Normotensive n = 27 |
WCH n = 27 |
Sustained HTN n = 27 |
P value |
|---|---|---|---|---|
| Age (y) * | 14.7 ± 1.6 | 14.8 ± 1.6 | 14.9 ± 1.8 | 0.321 |
| Male/female (%) | 89 / 11 | 89 / 11 | 89 / 11 | N/A |
| BMI (kg/m2) * | 24.5 ± 3.8 | 24.9 ± 4.1 | 24.9 ± 3.8 | 0.249 |
| BMI percentile (%) * | 80.3 ±15 | 79.9 ± 18 | 79.6 ± 19 | 0.911 |
| BMI ≥ 95th percentile (%) | 33 | 30 | 30 | 0.999 |
| Clinic SBP index * | 0.87 ± 0.06 | 1.03 ± 0.06 | 1.10 ± 0.06 | < 0.001 |
| Clinic DBP index * | 0.81 ± 0.08 | 0.93 ± 0.10 | 0.88 ± 0.10 | < 0.001 |
mean ± SD
Table III.
ABPM variables of WCH and sustained hypertension subjects
| ABPM Variables | WCH | Sustained HTN | P value |
|---|---|---|---|
| 24-hr mean SBP (mm Hg) | 118 ± 5 | 133 ± 7 | < 0.001 |
| 24-hr mean DBP (mm Hg) | 63 ± 4 | 71 ± 6 | < 0.001 |
| 24-hr SBP index | 0.92 ± 0.04 | 1.05 ± 0.05 | < 0.001 |
| 24-hr DBP index | 0.82 ± 0.06 | 0.92 ± 0.08 | < 0.001 |
| 24-hr SBP load (%) | 13.4 ± 6.9 | 59.7 ± 15.6 | < 0.001 |
| 24-hr DBP load (%) | 7.2 ± 6.3 | 21.3 ± 16.8 | < 0.001 |
| Daytime mean SBP (mm Hg) | 124 ± 6 | 139 ± 6 | < 0.001 |
| Daytime mean DBP (mm Hg) | 68 ± 5 | 75 ±7 | < 0.001 |
| Sleep mean SBP (mm Hg) | 107 ± 6 | 121 ± 8 | < 0.001 |
| Sleep mean DBP (mm Hg) | 56 ± 5 | 61 ± 6 | 0.004 |
mean ± SD
Mean LVMI for the normotensive, WCH, and sustained hypertensive groups was 29.2 ± 4.6, 32.3 ± 4.2, and 35.1 ± 6.5 g/m2.7, respectively (overall p < 0.001). Figure shows box-plots with the mean, median, and interquartile range for LVMI for the three BP groups, and provides p values adjusted for multiple pairwise comparisons of mean LVMI. Left ventricular hypertrophy was not present in either the normotensive or WCH groups but was present in 7 (26%) of the subjects with sustained hypertension (p < 0.001).
Figure 1.
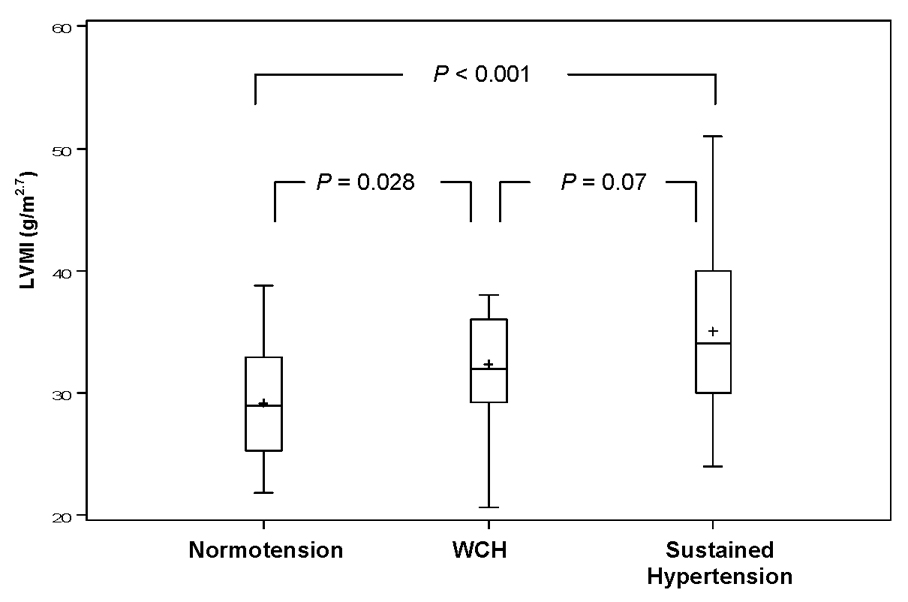
Figure Adjusted pairwise comparison of LVMI of normotensive, WCH, and sustained hypertensive triplets. Each box depicts the group interquartile range. The horizontal line and (+) symbol within each box represents the median and mean, respectively. Vertical lines depict the minimum and maximum value of each group. P values are adjusted for multiple pairwise comparisons of mean LVMI.
Discussion
Studies in adults suggest that WCH may not be a benign condition.1 A significant proportion of adults with WCH go on to progress to sustained hypertension, and a recent study of subjects closely matched for age, sex, BMI, and BP showed that adults with WCH have left ventricular measures which are intermediate between that of normotensives and sustained hypertensives.10,27 To date, there have been few studies of the cardiovascular implications of WCH in children. Similar to adults, there is preliminary evidence that children with WCH may be prone to progress to sustained hypertension.28 However, there have been only a limited number of studies of LVMI in children with WCH and the results have been inconsistent.16–18
The current study extends previous experience by comparing LVMI of children with WCH to that of children with normotension and sustained hypertension. Critical to the design is that subjects were closely matched for BMI, which is increased in both WCH and sustained hypertension and is itself an independent determinant of LVMI.16,19 Our results showed that children with WCH had a mean LVMI value which was intermediate between that of normotensives and sustained hypertensives. Subjects with WCH had mean LVMI significantly greater than that of normotensives (p = 0.028). Children with sustained hypertension had a trend toward greater LVMI than those with WCH (p = 0.07), a statistical finding likely attributable to the greater variability in LVMI among the sustained hypertensive subjects compared with the other BP groups. These results provide evidence that WCH has an effect on the cardiovascular system which is independent of BMI and supports the theory that WCH in children may represent a prehypertensive state.17,21,29
Neither children with WCH nor normotensive control subjects had LVH, whereas 26% of sustained hypertensives had LVH. Notably, four of the unmatched WCH subjects not included in the study also had LVH, but each of these subjects was significantly overweight with BMI ≥ 97th percentile. The current study design required matching of BMI of children with WCH and sustained hypertension to normotensive children with innocent murmur. These criteria resulted in a WCH study population with a significantly lower proportion of obesity compared with previous studies.16–18 The disparity in prevalence of LVH between the matched WCH group and the unmatched, more overweight, WCH group emphasizes the importance of obesity as an independent determinant of LVMI and underscores the importance of controlling for BMI in studies of WCH and LVMI.19,30
The mechanism of increased LVMI in children with WCH cannot be determined from the current study. It has been speculated that frequent increases in BP in response to stress, such as experienced in the medical office, may result in increased left ventricular mass.31 Alternatively, subjects with WCH may have average ambulatory BP values that, while in the normal range, are higher than those of normotensive children without WCH. Such mild elevations of average BP may lead to increased LVMI.10 In adults, SBP variability is increased in a stepwise fashion in patients with WCH and sustained hypertension, and elevated SBP variability is associated with increased left ventricular mass.23 In the current study, the sustained hypertension group had increased SBP variability compared with the WCH group. However, because the normotensive subjects did not have ABPM, it is not known if the WCH group had increased SBP variability compared with the normotensive group as a potential contributor to increased LVMI.
The current study has several limitations. Normotensive subjects had only a single clinic visit showing normal BP and did not have ABPM to confirm normotension. Therefore, there may have been subjects in the normotensive control group who had masked hypertension or who were otherwise not truly normotensive. In addition, while all WCH subjects had a history of office BP ≥ 95th percentile on three occasions, the inclusion criteria included a mean initial clinic BP ≥ 90th percentile. Therefore, some of the WCH subjects may have had office prehypertension rather than office hypertension. However, both of these limitations would have biased the results toward no difference in LVMI between normotensive and WCH subjects. In addition, normotensive controls were selected from patients with innocent murmurs, a population that may not be representative of the general pediatric population. Because the study was retrospective, data on ethnic group were unavailable, and could therefore not be included. Lastly, practitioners may have been less likely to obtain ABPM in patients with more severe elevation in clinic BP,32 thereby potentially biasing the sustained hypertensive group toward lower mean LVMI. However, the requirement that sustained hypertensive subjects have 24-hr BP ≥ 95th percentile on ABPM to be included in the study should have ameliorated this potential limitation.
Children with WCH should be counseled in life-style modification, and they should be monitored for the development of sustained hypertension, potentially with periodic ABPM.29 Given the increasing prevalence of hypertension in children and adolescents, early identification of risk factors for progression to hypertension and corresponding cardiovascular disease is essential for the prevention of future cardiovascular morbidity and mortality. Further study of the long-term outcome of WCH in children will help clarify its role as a potential cardiovascular risk factor.
Acknowledgments
We thank Dr. Thomas Pearson for help with the study design.
Financial support: Marc B. Lande, MD was supported, in part, by NIH grant 5K23HL080068-04 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 2.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study) Circulation. 2001;104(12):1385–1392. doi: 10.1161/hc3701.096100. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Zampi I, Gattobigio R, et al. White coat hypertension and white coat effect. Similarities and differences. Am J Hypertens. 1995;8:790–798. doi: 10.1016/0895-7061(95)00151-E. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara LA, Guida L, Pasanisi F, Celentano A, Palmieri V, Iannuzzi R, et al. Isolated office hypertension and end-organ damage. J Hypertens. 1997;15:979–985. doi: 10.1097/00004872-199715090-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cavallini MC, Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Is white coat hypertension associated with arterial disease or left ventricular hypertrophy? Hypertension. 1995;26:413–419. doi: 10.1161/01.hyp.26.3.413. [DOI] [PubMed] [Google Scholar]
- 6.Glen SK, Elliott HL, Curzio JL, Lees KR, Reid JL. White-coat hypertension as a cause of cardiovascular dysfunction. Lancet. 1996;348:654–657. doi: 10.1016/S0140-6736(96)02303-3. [DOI] [PubMed] [Google Scholar]
- 7.Pierdomenico SD, Lapenna D, Guglielmi MD, Antidormi T, Schiavoni C, Cuccurullo F, et al. Target organ status and serum lipids in patients with white coat hypertension. Hypertension. 1995;26:801–807. doi: 10.1161/01.hyp.26.5.801. [DOI] [PubMed] [Google Scholar]
- 8.Pose-Reino A, Gonzalez-Juanatey JR, Pastor C, Mendez I, Estevez JC, Alvarez D, et al. Clinical implications of white coat hypertension. Blood Press. 1996;5:264–273. doi: 10.3109/08037059609078058. [DOI] [PubMed] [Google Scholar]
- 9.Owens PE, Lyons SP, Rodriguez SA, O'Brien ET. Is elevation of clinic blood pressure in patients with white coat hypertension who have normal ambulatory blood pressure associated with target organ changes? Journal of Hum Hyperten. 1998;12:743–748. doi: 10.1038/sj.jhh.1000721. [DOI] [PubMed] [Google Scholar]
- 10.Grandi AM, Broggi R, Colombo S, Santillo R, Imperiale D, Bertolini A, et al. Left ventricular changes in isolated office hypertension: a blood pressure-matched comparison with normotension and sustained hypertension. Arch of Intern Med. 2001;16:2677–2681. doi: 10.1001/archinte.161.22.2677. [DOI] [PubMed] [Google Scholar]
- 11.Palatini P, Mormino P, Santonastaso M, Mos L, Dal Follo M, Zanata G, et al. Target-organ damage in stage I hypertensive subjects with white coat and sustained hypertension: results from the HARVEST study. Hypertension. 1998;31:57–63. doi: 10.1161/01.hyp.31.1.57. [DOI] [PubMed] [Google Scholar]
- 12.Cardillo C, De Felice F, Campia U, Folli G. Psychophysiological reactivity and cardiac end-organ changes in white coat hypertension. Hypertension. 1993;21:836–844. doi: 10.1161/01.hyp.21.6.836. [DOI] [PubMed] [Google Scholar]
- 13.Kuwajima I, Suzuki Y, Fujisawa A, Kuramoto K. Is white coat hypertension innocent? Structure and function of the heart in the elderly. Hypertension. 1993;22:826–831. doi: 10.1161/01.hyp.22.6.826. [DOI] [PubMed] [Google Scholar]
- 14.Verdecchia P, Reboldi GP, Angeli F, Schillaci G, Schwartz JE, Pickering TG, et al. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension. 2005;45:203–208. doi: 10.1161/01.HYP.0000151623.49780.89. [DOI] [PubMed] [Google Scholar]
- 15.Sorof JM, Portman RJ. White-coat hypertension in children with elevated casual blood pressure. J Pediatr. 2000;137:493–497. doi: 10.1067/mpd.2000.108394. [DOI] [PubMed] [Google Scholar]
- 16.Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A, Zakopoulos N. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. 2005;20:1151–1155. doi: 10.1007/s00467-005-1979-5. [DOI] [PubMed] [Google Scholar]
- 17.Kavey RE, Kveslis DA, Atallah N, Smith FC. White coat hypertension in childhood: evidence for end-organ effect. J Pediatr. 2007;150:491–497. doi: 10.1016/j.jpeds.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 18.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, et al. Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension. 2007;50:392–395. doi: 10.1161/HYPERTENSIONAHA.107.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: The Strong Heart Study. J Am Coll Cardiol. 2006;47:2267–2273. doi: 10.1016/j.jacc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:1–22. [PubMed] [Google Scholar]
- 21.Lurbe E, Sorof JM, Daniels SR. Clinical and research aspects of ambulatory blood pressure monitoring in children. J Pediatr. 2004;144:7–16. doi: 10.1016/j.jpeds.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 23.Zakopoulos NA, Tsivgoulis G, Barlas G, Spengo K, Manios E, Ikonomidis I, et al. Impact of the time rate of blood pressure variation on left ventricular mass. J Hypertens. 2006;24:2071–2077. doi: 10.1097/01.hjh.0000244957.47114.88. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 26.Daniels SR. Hypertension-induced cardiac damage in children and adolescents. Blood Press Monit. 1999;4:165–170. [PubMed] [Google Scholar]
- 27.Ugajin T, Hozawa A, Ohkubo T, Asayama K, Kikuya M, Obara T, et al. White-coat hypertension as a risk factor for the development of home hypertensionL the Ohasama study. Arch Intern Med. 2005;165:1541–1546. doi: 10.1001/archinte.165.13.1541. [DOI] [PubMed] [Google Scholar]
- 28.Felea D, Matasaru S, Dimitriu AG. White-coat hypertension in children and adolescents. Rev Med Chir Soc Med Nat Iasi. 1998;102:103–108. [PubMed] [Google Scholar]
- 29.McNiece KL, Portman RJ. Ambulatory blood pressure monitoring: what a pediatrician should know. Curr Opin Pediatr. 2007;19:178–182. doi: 10.1097/MOP.0b013e328014671d. [DOI] [PubMed] [Google Scholar]
- 30.Urbina E. Noninvasive assessment of target organ injury in children with the metabolic syndrome. J Cardiometab Syndr. 2006;4:277–281. doi: 10.1111/j.1559-4564.2006.05799.x. [DOI] [PubMed] [Google Scholar]
- 31.Jhalani J, Goyal T, Clemow L, Schwartz JE, Pickering TG, Gerin W. Anxiety and outcome expectations predict the white-coat effect. Blood Press Monit. 2005;10:317–319. doi: 10.1097/00126097-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Sorof JM, Poffenbarger T, Franco K, Portman R. Evaluation of white-coat hypertension in children: importance of the definitions of normal ambulatory blood pressure and the severity of casual hypertension. Am J Hypertens. 2001;14:855–860. doi: 10.1016/s0895-7061(01)02180-x. [DOI] [PubMed] [Google Scholar]


