Figure 6.
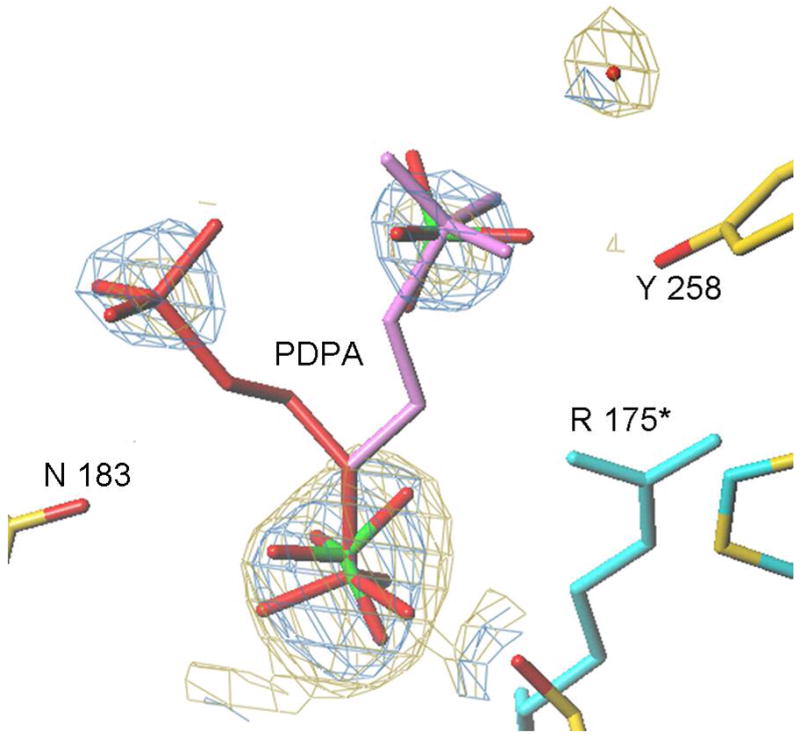
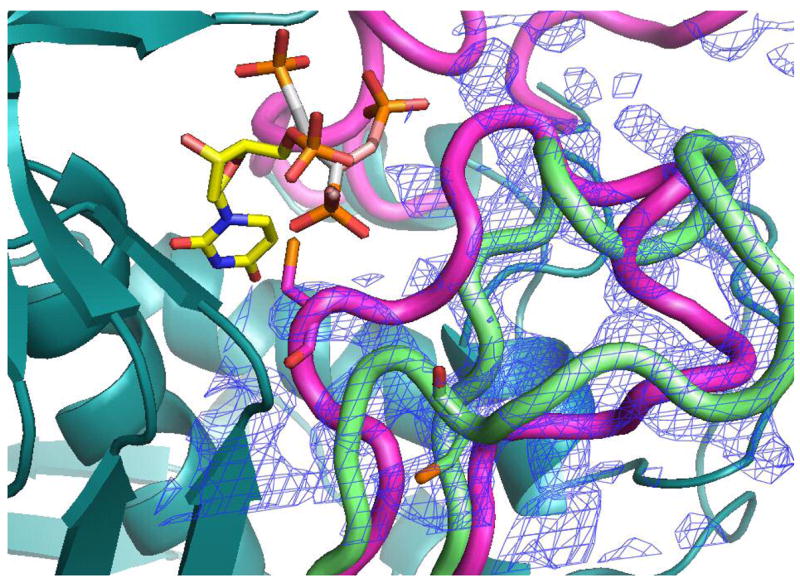
The PDPA binding site. A) One of the phosphonates occupies the same position as one the phosphate ions observed previously bound to the inactive conformer (15). The flexible 3-carbon linker allows the other phosphonate to occupy two positions as indicated. Residues from the other subunit are indicated by an asterisk (*) at the residue number. B) Superposition of the structures of hTS with bound PDPA (in brown) and with bound dUMP (in yellow). Constant parts of the molecule are in malachite. Loop 181–197 in the inactive conformation, observed in the PDPA complex, is shown in light green; corresponding electron density is represented by basket conturing in blue. In purple are loop 181–197 and loop 108–129 as observed in the active conformation, in complex with dUMP and raltitrexed (14).
