Abstract
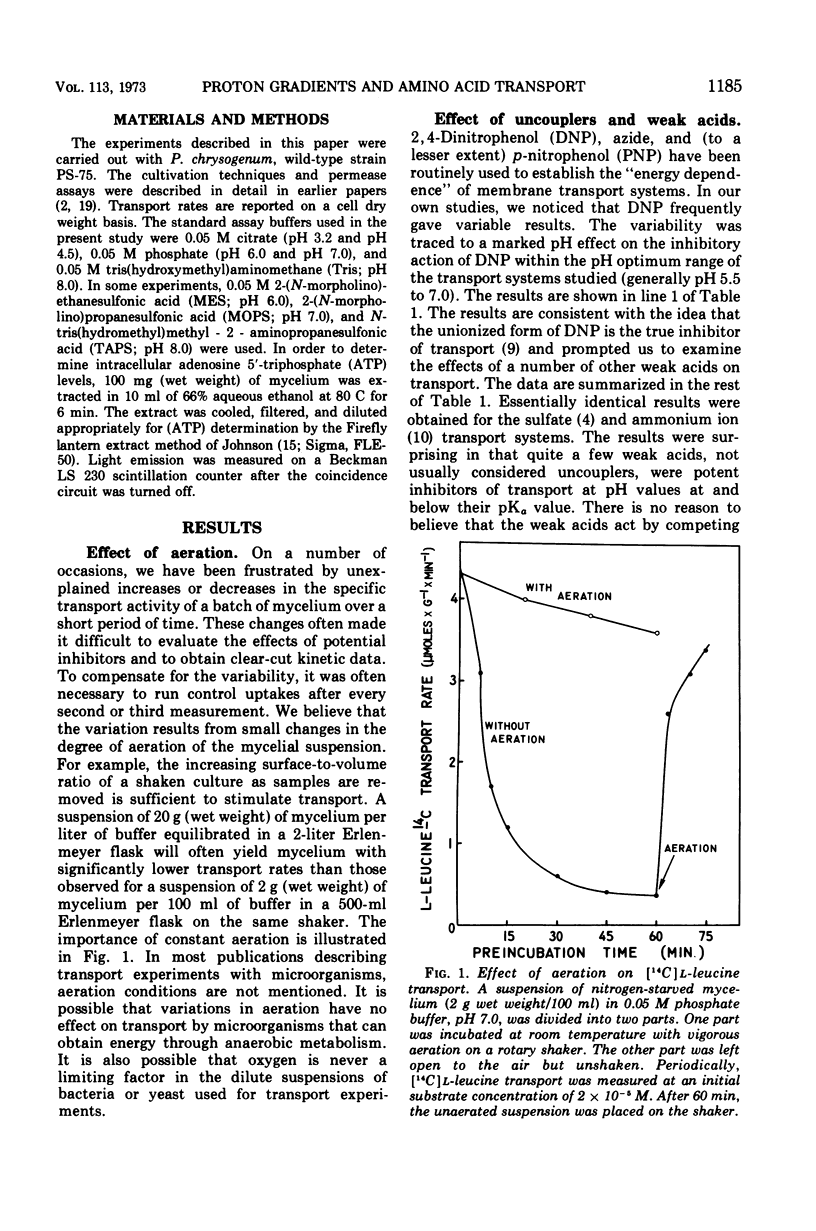
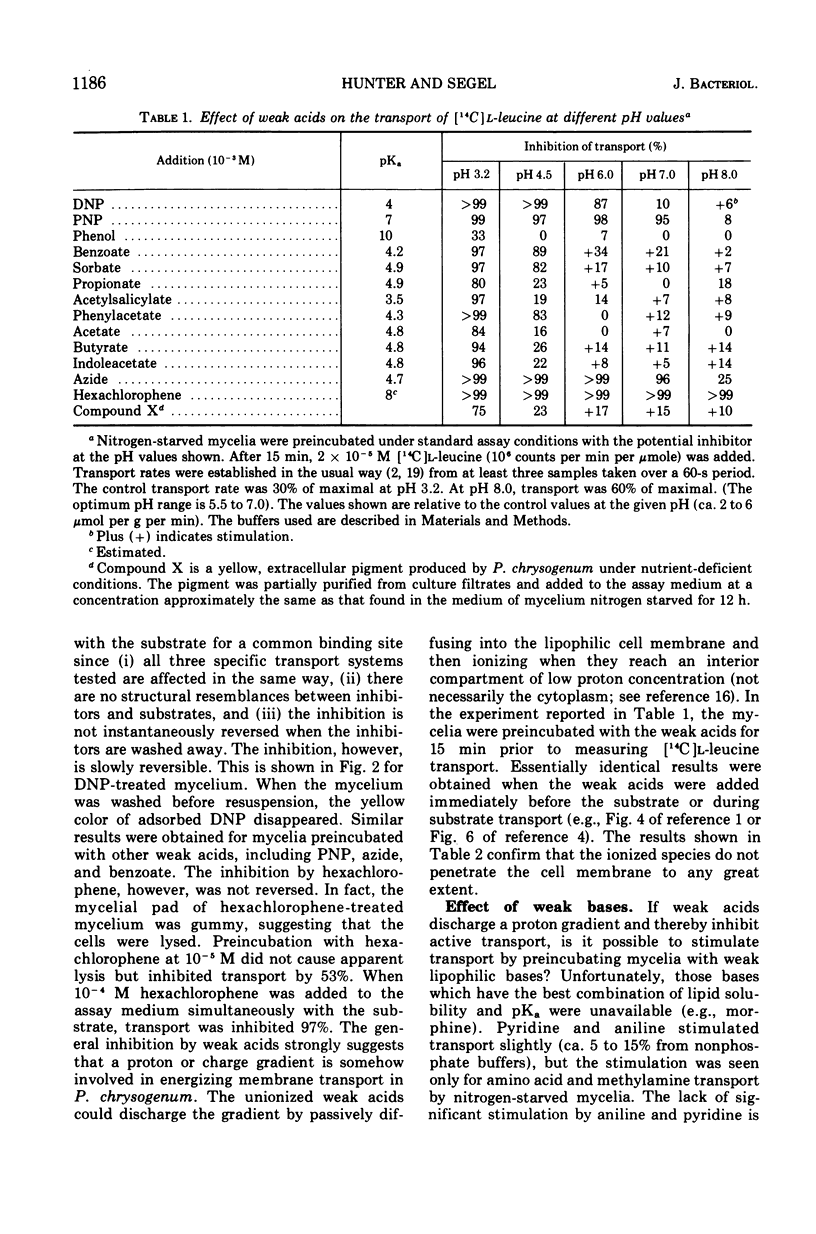
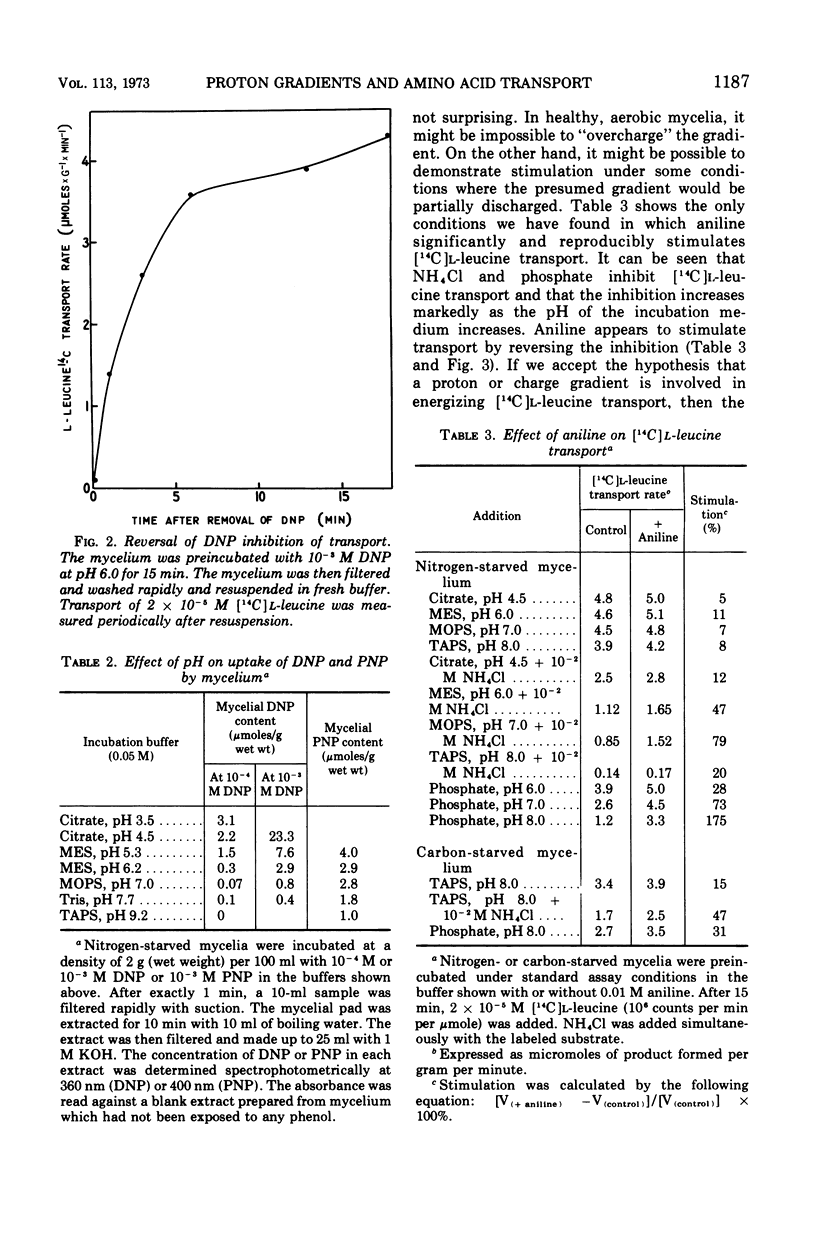
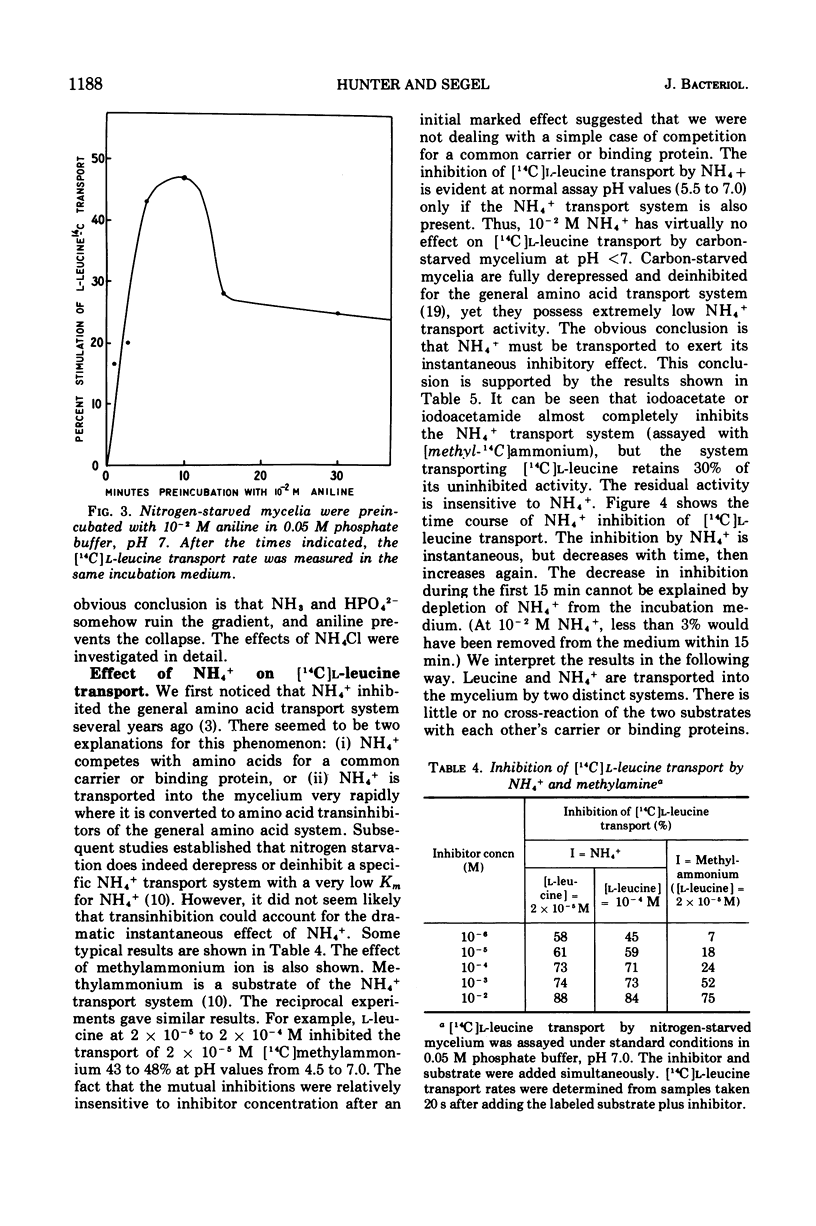
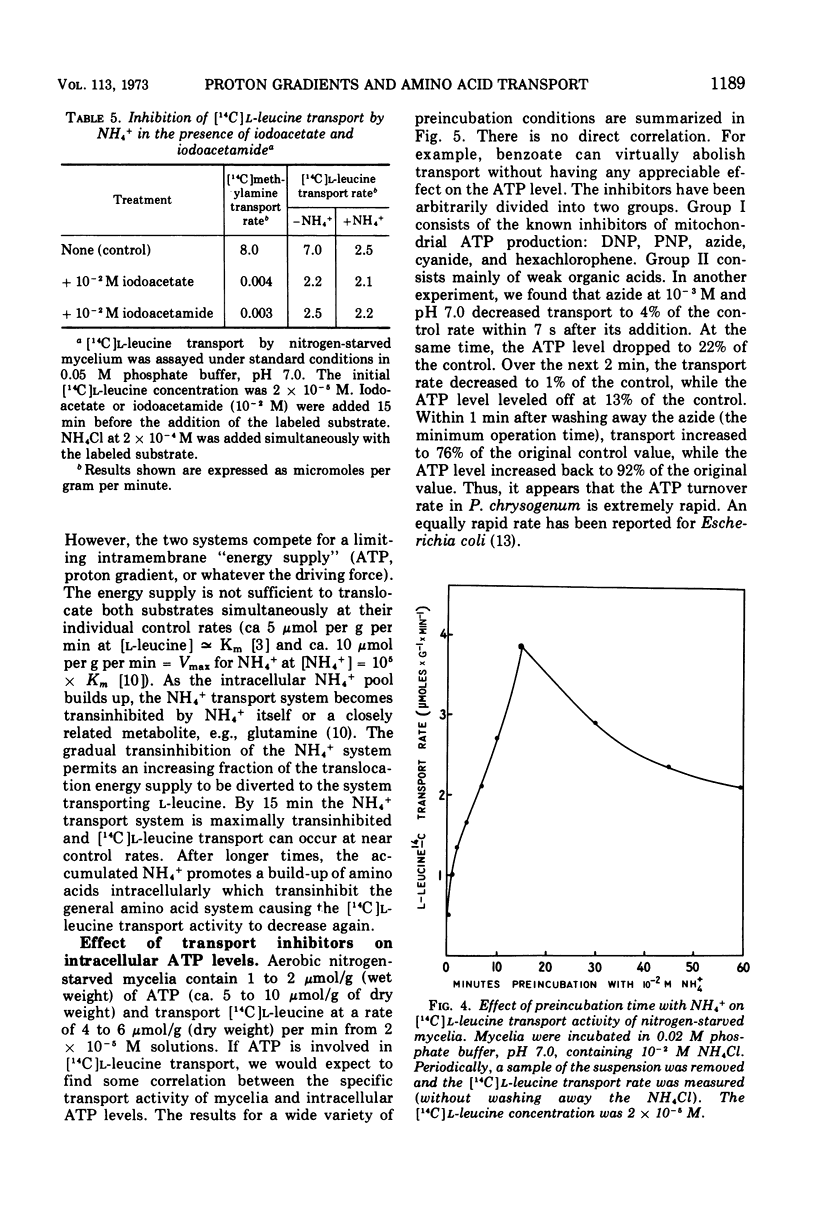
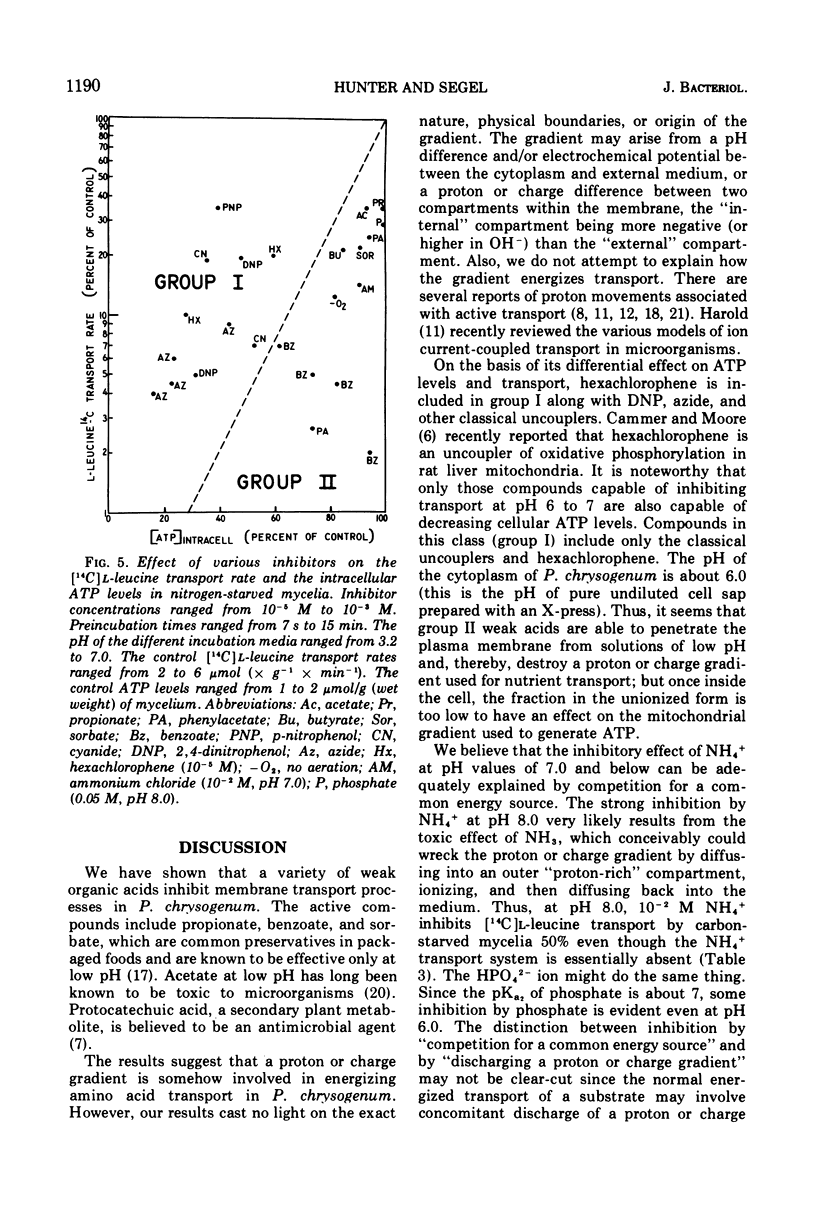
A variety of weak acids at and below their pKa are potent inhibitors of transport in Penicillium chrysogenum. The effective compounds include sorbate, benzoate, and propionate (common antifungal agents), indoleacetate (a plant hormone), acetylsalicylate (aspirin), hexachlorophene, and a yellow pigment produced by the mycelia under nutrient-deficient conditions, as well as the classical uncouplers 2,4-dinitrophenol, p-nitrophenol, and azide. The results suggest that a proton gradient or charge gradient is involved in energizing membrane transport in P. chrysogenum. The unionized form of the weak acids could discharge the gradient by diffusing through the membrane and ionizing when they reach an interior compartment of higher pH. Experiments with 2,4-dinitrophenol and p-nitrophenol established that the ionized species are not absorbed by the mycelium to any great extent. The transport inhibitors also caused a decrease in cellular adenosine 5′-triphosphate (ATP) levels, but there was no constant correlation between inhibition of transport and suppression of cellular ATP. A decrease in aeration of the mycelial suspension had the same effect on transport and ATP levels as the addition of a weak organic acid. The effects on transport rates and ATP levels were reversible. The instantaneous inhibition of [14C]l-leucine transport by NH4− (and vice-versa) in nitrogen-starved mycelia at pH values of 7 or below can be explained by competition for a common energy-coupling system. The inhibition is not observed in carbon-starved mycelia in which the NH4+ transport system is absent or inactive (but the general amino acid transport is fully active), or in iodoacetate-treated mycelia in which the NH4+ transport system has been differentially inactivated. At pH values greater than 7.0, NH3 and HPO42− inhibit transport, presumably by discharging the membrane proton or charge gradient. Aniline counteracts the inhibitory effect of NH3 and HPO42− possibly by acting as a proton reservoir or buffer within the membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellenger N., Nissen P., Wood T. C., Segel I. H. Specificity and control of choline-O-sulfate transport in filamentous fungi. J Bacteriol. 1968 Nov;96(5):1574–1585. doi: 10.1128/jb.96.5.1574-1585.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko P. V., Wood T. C., Segel I. H. Multiplicity and regulation of amino acid transport in Penicillium chrysogenum. Arch Biochem Biophys. 1969 Feb;129(2):498–508. doi: 10.1016/0003-9861(69)90207-0. [DOI] [PubMed] [Google Scholar]
- Bradfield G., Somerfield P., Meyn T., Holby M., Babcock D., Bradley D., Segel I. H. Regulation of sulfate transport in filamentous fungi. Plant Physiol. 1970 Nov;46(5):720–727. doi: 10.1104/pp.46.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. E., LéJohn H. B. On the involvement of calcium in amino acid transport and growth of the fungus Achlya. J Biol Chem. 1972 Aug 10;247(15):4729–4739. [PubMed] [Google Scholar]
- Cammer W., Moore C. L. The effect of hexachlorophene on the respiration of brain and liver mitochondria. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1887–1894. doi: 10.1016/0006-291x(72)90066-6. [DOI] [PubMed] [Google Scholar]
- Eddy A. A., Indge K. J., Backen K., Nowacki J. A. Interctions between potassium ions and glycine transport in the yeast Saccharomyces carlsbergensis. Biochem J. 1970 Dec;120(4):845–852. doi: 10.1042/bj1200845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. R. The effect of cycloheximide on membrane transport in Euglena. A comparative study with nigericin. J Biol Chem. 1971 Oct 25;246(20):6144–6151. [PubMed] [Google Scholar]
- Hackette S. L., Skye G. E., Burton C., Segel I. H. Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J Biol Chem. 1970 Sep 10;245(17):4241–4250. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Acidic and basic amino acid transport systems of Penicillium chrysogenum. Arch Biochem Biophys. 1971 May;144(1):168–183. doi: 10.1016/0003-9861(71)90466-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Hardman J. G., Broadus A. E., Sutherland E. W. Analysis of adenosine 3',5'-monophosphate with luciferase luminescence. Anal Biochem. 1970 May;35(1):91–97. doi: 10.1016/0003-2697(70)90014-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skye G. E., Segel I. H. Independent regulation of cysteine and cystine transport in Penicillium chrysogenum. Arch Biochem Biophys. 1970 May;138(1):306–318. doi: 10.1016/0003-9861(70)90311-5. [DOI] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]


