Abstract
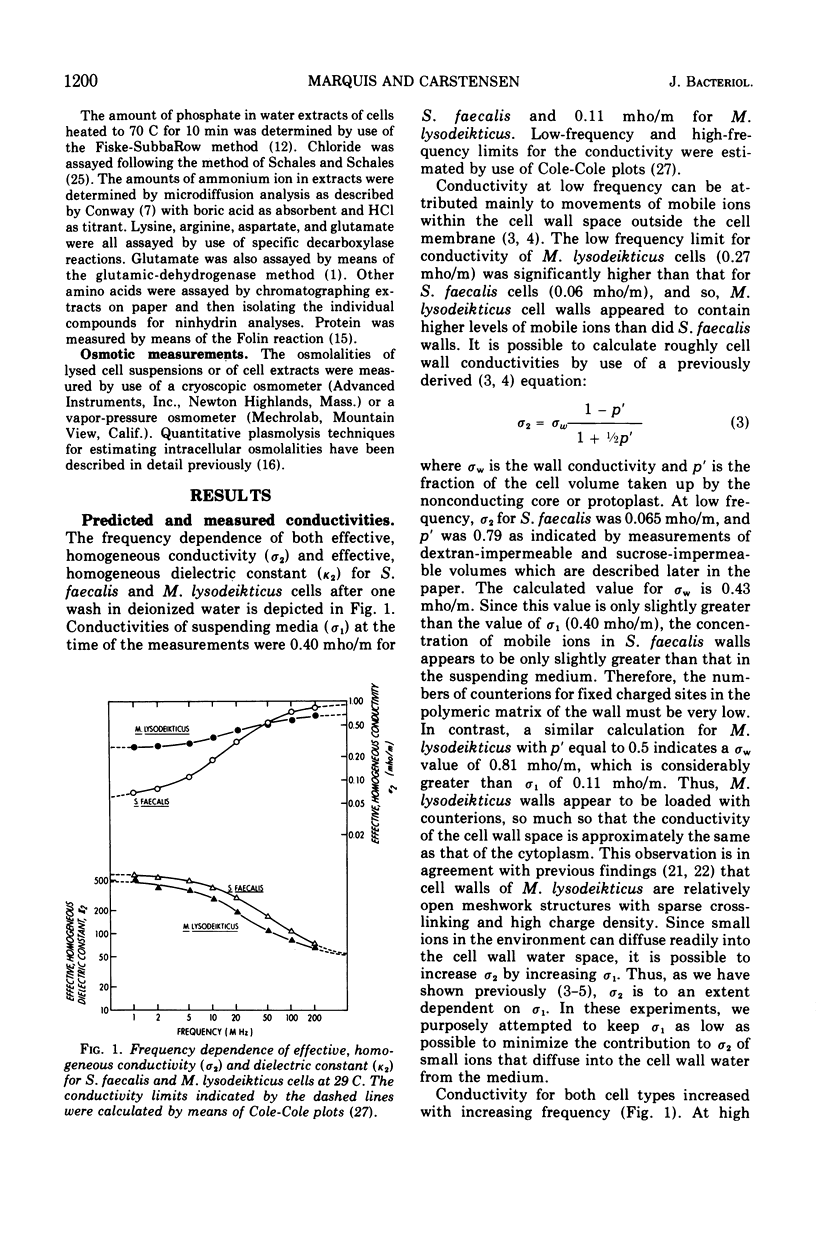
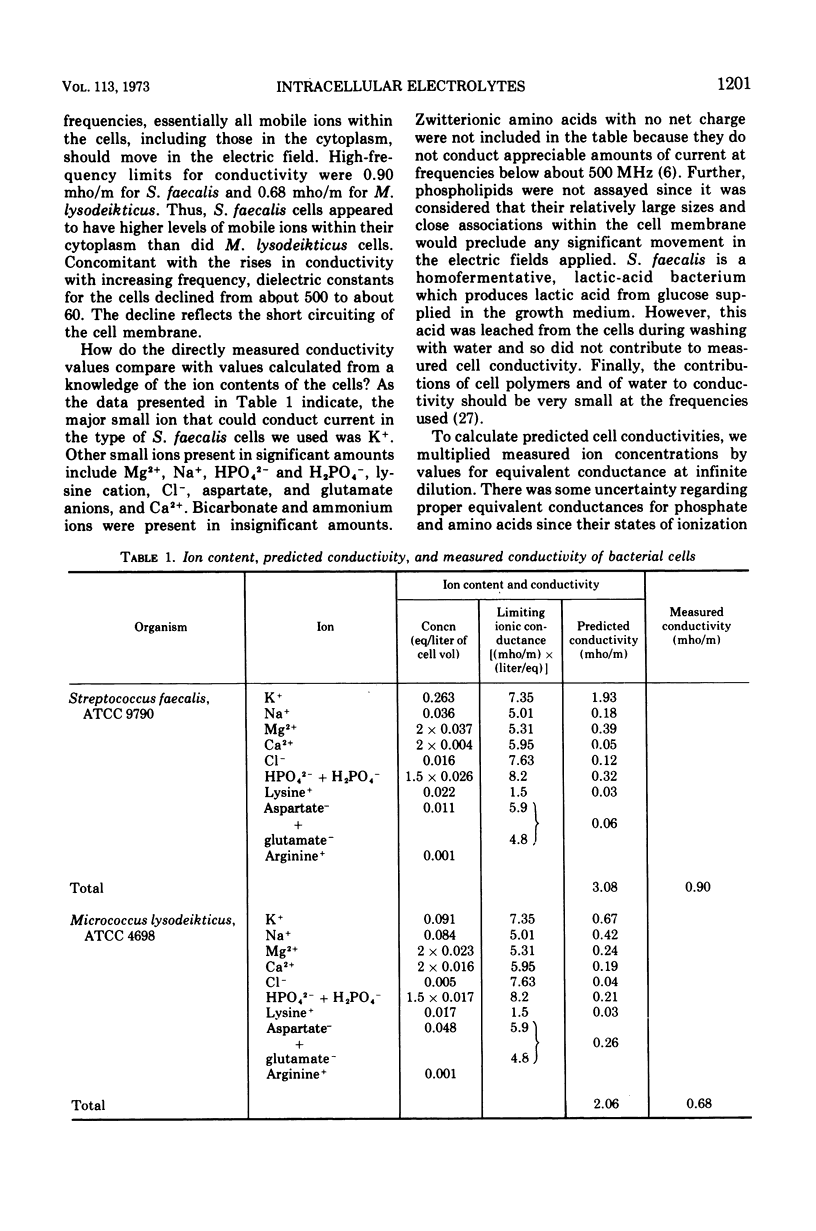
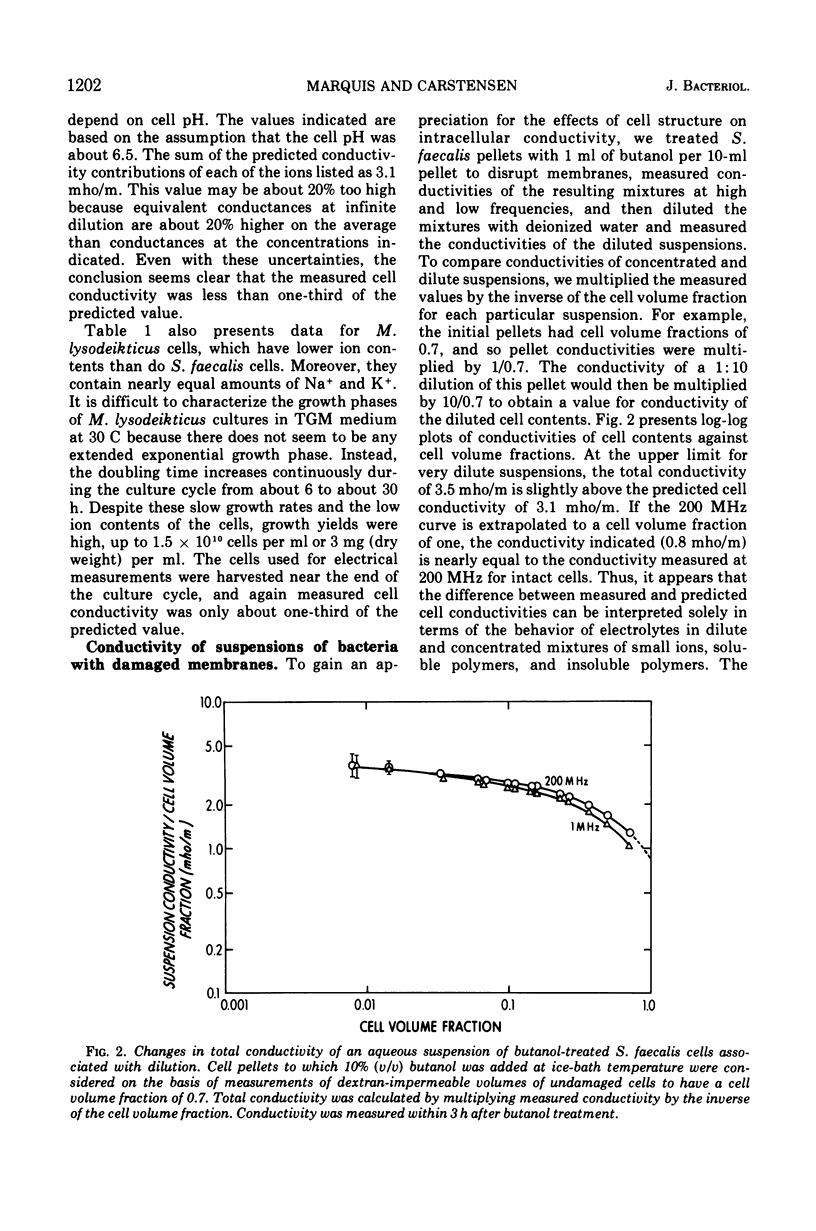
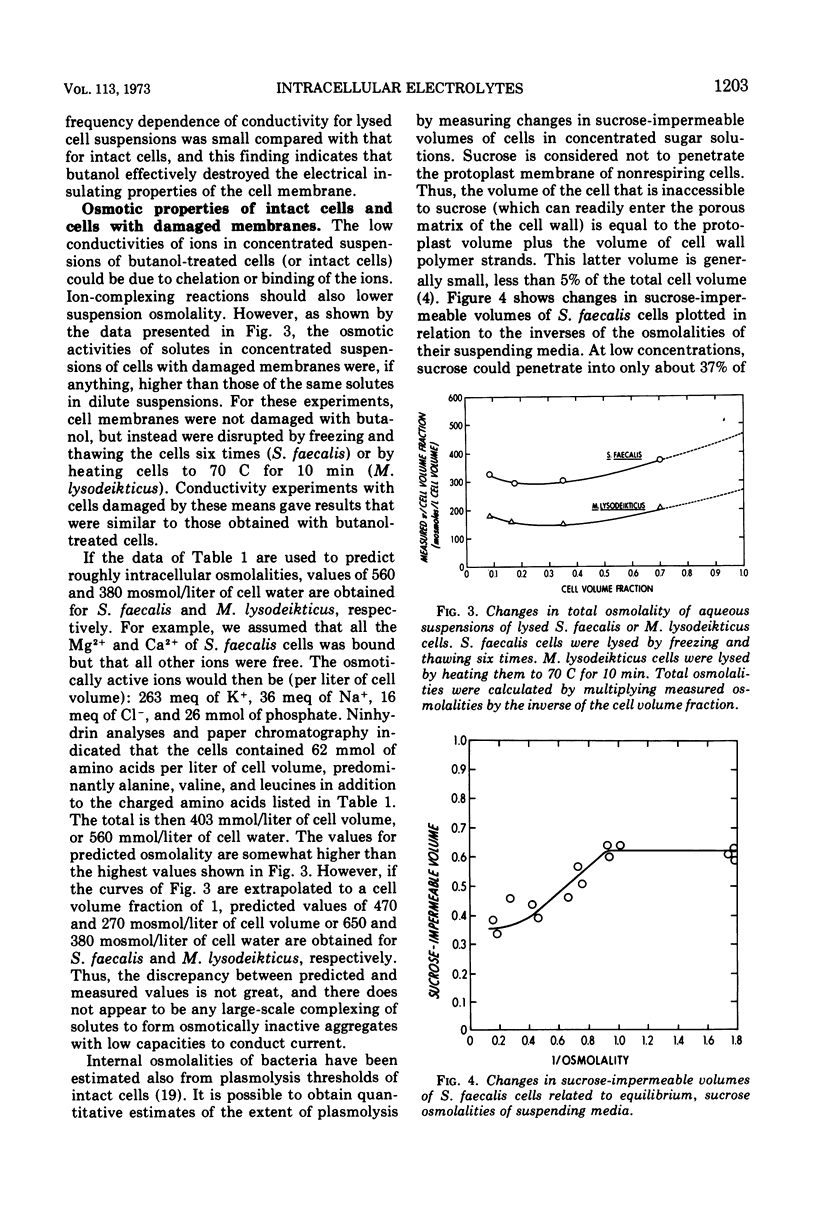
Intact cells of Streptococcus faecalis and Micrococcus lysodeikticus were found to have high-frequency electric conductivities of 0.90 and 0.68 mho/m, respectively. These measured values, which reflect movements of ions both within the cytoplasm and within the cell wall space, were only about one-third of those calculated on the basis of determinations of the amounts and types of small ions within the cells. Concentrated suspensions of bacteria with damaged membranes showed similarly large disparities between measured and predicted conductivities, whereas the conductivities of diluted suspensions were about equal to predicted values. Thus, the low mobilities of intracellular ions appeared to be interpretable in terms of the physicochemical behavior of electrolytes in concentrated mixtures of small ions and cell polymers. In contrast to the low measured values for conductivity of intact bacteria, values for intracellular osmolality measured by means of a quantitative plasmolysis technique were higher than expected. For example, the plasmolysis threshold for S. faecalis cells indicated an internal osmolality of about 1.0 osmol/kg, compared with a value of only 0.81 osmol/liter of cell water calculated from a knowledge of the cell content and the distribution of small solutes. In all, our results indicate that most of the small ions within vegetative bacterial cells are free to move in an electric field and that they contribute to cytoplasmic osmolality.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carstensen E. L., Cox H. A., Mercer W. B., Natale L. A. Passive Electrical Properties of Microorganisms: I. Conductivity of Escherichia coli and Micrococcus lysodeikticus. Biophys J. 1965 May;5(3):289–300. doi: 10.1016/s0006-3495(65)86717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen E. L., Marquis R. E., Gerhardt P. Dielectric study of the physical state of electrolytes and water within Bacillus cereus spores. J Bacteriol. 1971 Jul;107(1):106–113. doi: 10.1128/jb.107.1.106-113.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen E. L., Marquis R. E. Passive electrical properties of microorganisms. 3. Conductivity of isolated bacterial cell walls. Biophys J. 1968 May;8(5):536–548. doi: 10.1016/s0006-3495(68)86506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen E. L. Passive electrical properties of microorganisms. II. Resistance of the bacterial membrane. Biophys J. 1967 Sep;7(5):493–503. doi: 10.1016/S0006-3495(67)86600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope F. W., Damadian R. Cell potassium by 39K spin echo nuclear magnetic resonance. Nature. 1970 Oct 3;228(5266):76–77. doi: 10.1038/228076a0. [DOI] [PubMed] [Google Scholar]
- Damadian R. Biological ion exchanger resins. 3. Molecular interpretation of cellular ion exchange. Biophys J. 1971 Sep;11(9):773–785. doi: 10.1016/s0006-3495(71)86253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Biological ion exchanger resins. I. Quantitative electrostatic correspondence of fixed charge and mobile counter ion. Biophys J. 1971 Sep;11(9):739–760. doi: 10.1016/S0006-3495(71)86251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R., Goldsmith M., Zaner K. S. Biological ion exchanger resins. II. QUERP water and ion exchange selectivity. Biophys J. 1971 Sep;11(9):761–772. doi: 10.1016/S0006-3495(71)86252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marquis R. E. Osmotic sensitivity of bacterial protoplasts and the response of their limiting membrane to stretching. Arch Biochem Biophys. 1967 Feb;118(2):323–331. doi: 10.1016/0003-9861(67)90356-6. [DOI] [PubMed] [Google Scholar]
- Matula T. I., MacLeod R. A. Mechanism of optical effects in suspensions of a marine pseudomonad. J Bacteriol. 1969 Oct;100(1):403–410. doi: 10.1128/jb.100.1.403-410.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matula T. I., MacLeod R. A. Penetration of Pseudomonas aeruginosa by sodium chloride and its relation to the mechanism of optical effects. J Bacteriol. 1969 Oct;100(1):411–416. doi: 10.1128/jb.100.1.411-416.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Coccal cell-wall compactness and the swelling action of denaturants. Can J Microbiol. 1972 May;18(5):623–629. doi: 10.1139/m72-099. [DOI] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly H., Schwan H. P. Dielectric properties and ion mobility in erythrocytes. Biophys J. 1966 Sep;6(5):621–639. doi: 10.1016/S0006-3495(66)86682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAN H. P. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



