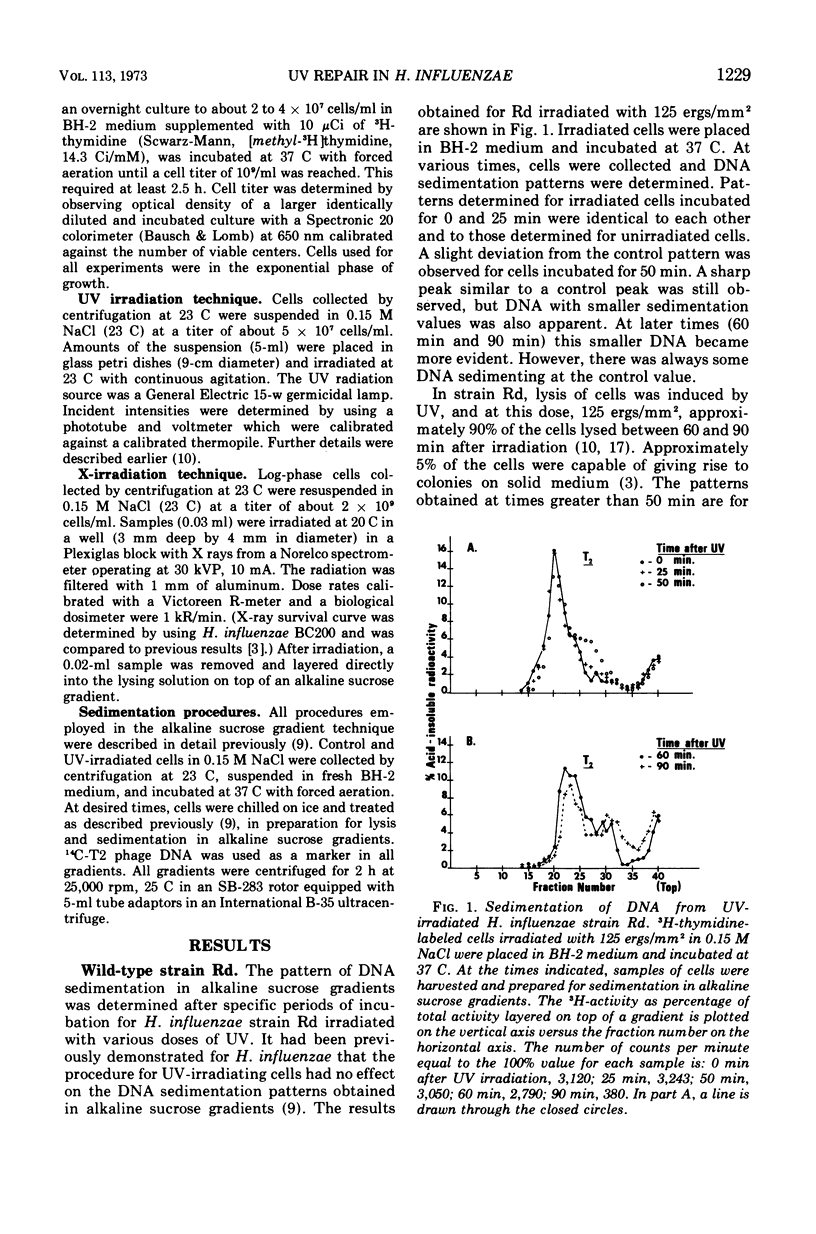
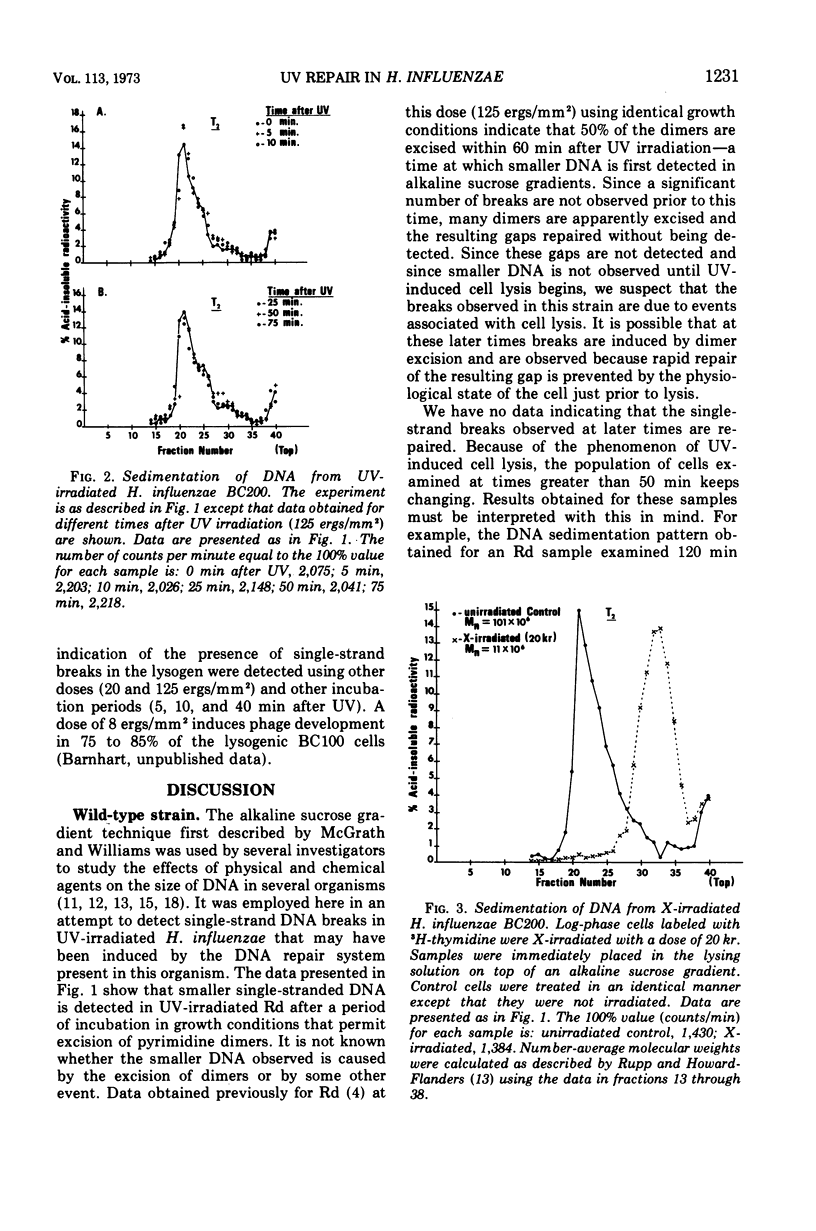
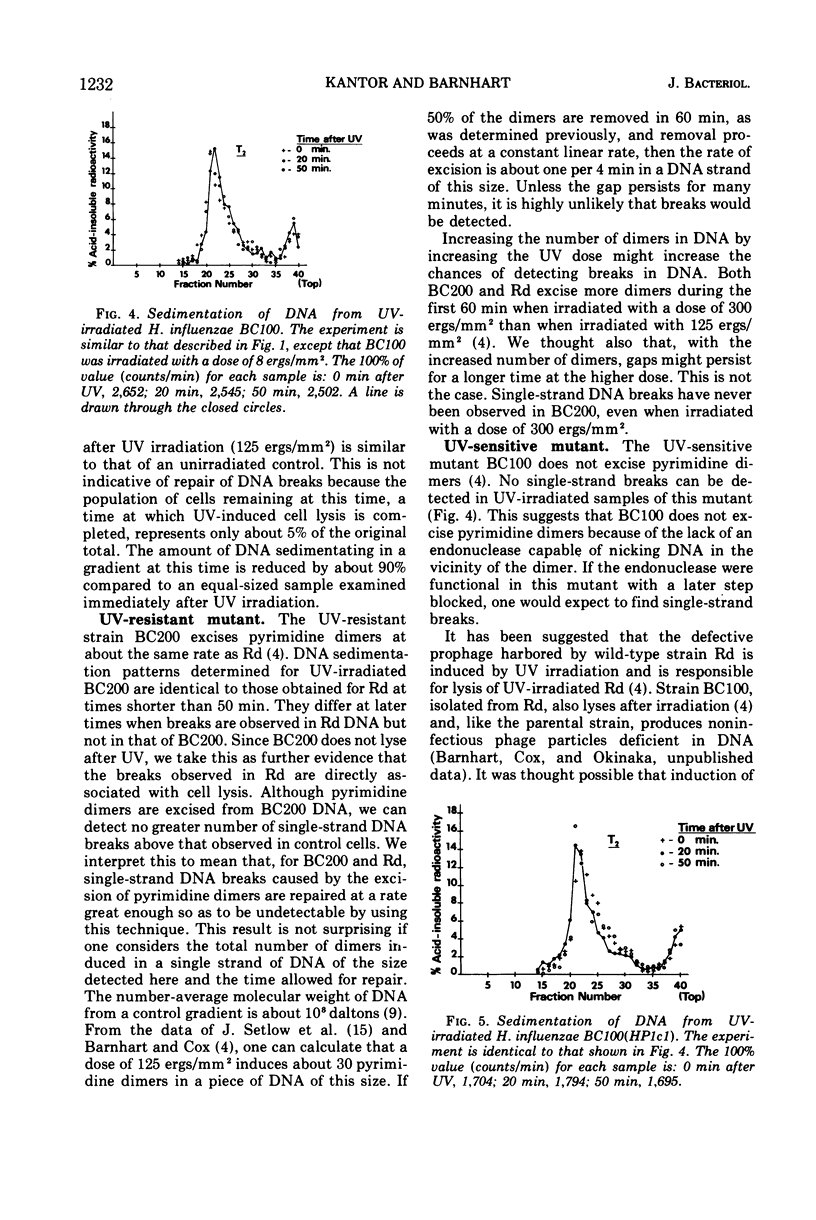
Abstract
The wild-type strain and mutants of Haemophilus influenzae, sensitive or resistant to ultraviolet light (UV) as defined by colony-forming ability, were examined for their ability to perform the incision and rejoining steps of the deoxyribonucleic acid (DNA) dark repair process. Although UV-induced pyrimidine dimers are excised by the wild-type Rd and a resistant mutant BC200, the expected single-strand DNA breaks could not be detected on alkaline sucrose gradients. Repair of the gap resulting from excision must be rapid when experimental conditions described by us are employed. Single-strand DNA breaks were not detected in a UV-irradiated sensitive mutant (BC100) incapable of excising pyrimidine dimers, indicating that this mutant may be defective in a dimer-recognizing endonuclease. No single-strand DNA breaks were detected in a lysogen BC100(HP1c1) irradiated with a UV dose large enough to induce phage development in 80% of the cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Induction of streptomycin resistance in sensitive Hemophilus influenzae by extracts containing desoxyribonucleic acid from resistant Hemophilus influenzae. J Exp Med. 1953 Jan;97(1):17–31. doi: 10.1084/jem.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation sensitivity of Haemophilus influenzae: a composite response. J Bacteriol. 1970 Jul;103(1):9–15. doi: 10.1128/jb.103.1.9-15.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart D. J., Cox S. H. Recovery of Haemophilus influenzae from ultraviolet and x-ray damage. Photochem Photobiol. 1970 Mar;11(3):147–162. doi: 10.1111/j.1751-1097.1970.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Ginoza W. The effects of ionizing radiation on nucleic acids of bacteriophages and bacterial cells. Annu Rev Microbiol. 1967;21:325–368. doi: 10.1146/annurev.mi.21.100167.001545. [DOI] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Kantor G. J. Anomalies in the sedimentation of deoxyribonucleic acid from Haemophilus influenzae in alkaline sucrose gradients. J Bacteriol. 1972 Dec;112(3):1264–1269. doi: 10.1128/jb.112.3.1264-1269.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Barnhart B. J. Effect of ultraviolet light on division and deoxyribonucleic acid synthesis in Haemophilus influenzae. J Bacteriol. 1970 Jul;103(1):1–8. doi: 10.1128/jb.103.1.1-8.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Little J. G. Repair of x-ray damage to the DNA in Micrococcus radiodurans: the effect of 5-bromodeoxyuridine. J Mol Biol. 1970 Mar;48(3):395–408. doi: 10.1016/0022-2836(70)90053-7. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- STUY J. H. Studies on the radiation inactivation of microorganisms. V. Deoxyribonucleic acid metabolism in ultraviolet-irradiated Haemophilus influenzae. J Bacteriol. 1959 Jul;78(1):49–58. doi: 10.1128/jb.78.1.49-58.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Randolph M. L., Boling M. E., Mattingly A., Price G., Gordon M. P. Repair of DNA in Haemophilus influenzae. II. Excision, repair of single-strand breaks, defects in transformation, and host cell modification in UV-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1968;33:209–218. doi: 10.1101/sqb.1968.033.01.024. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Production and repair of radiochemical damage in Escherichia coli deoxyribonucleic acid; its modification by culture conditions and relation to survival. J Bacteriol. 1971 Jan;105(1):127–135. doi: 10.1128/jb.105.1.127-135.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


