Abstract
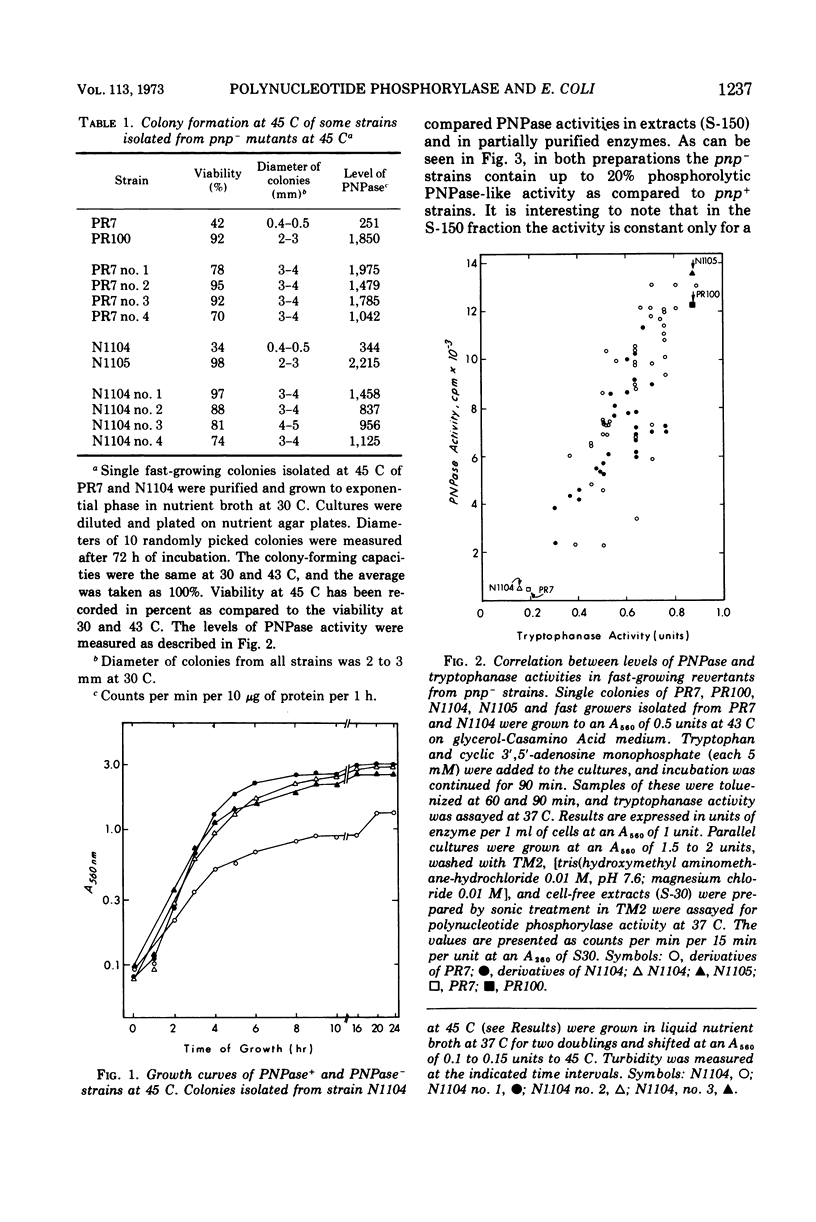
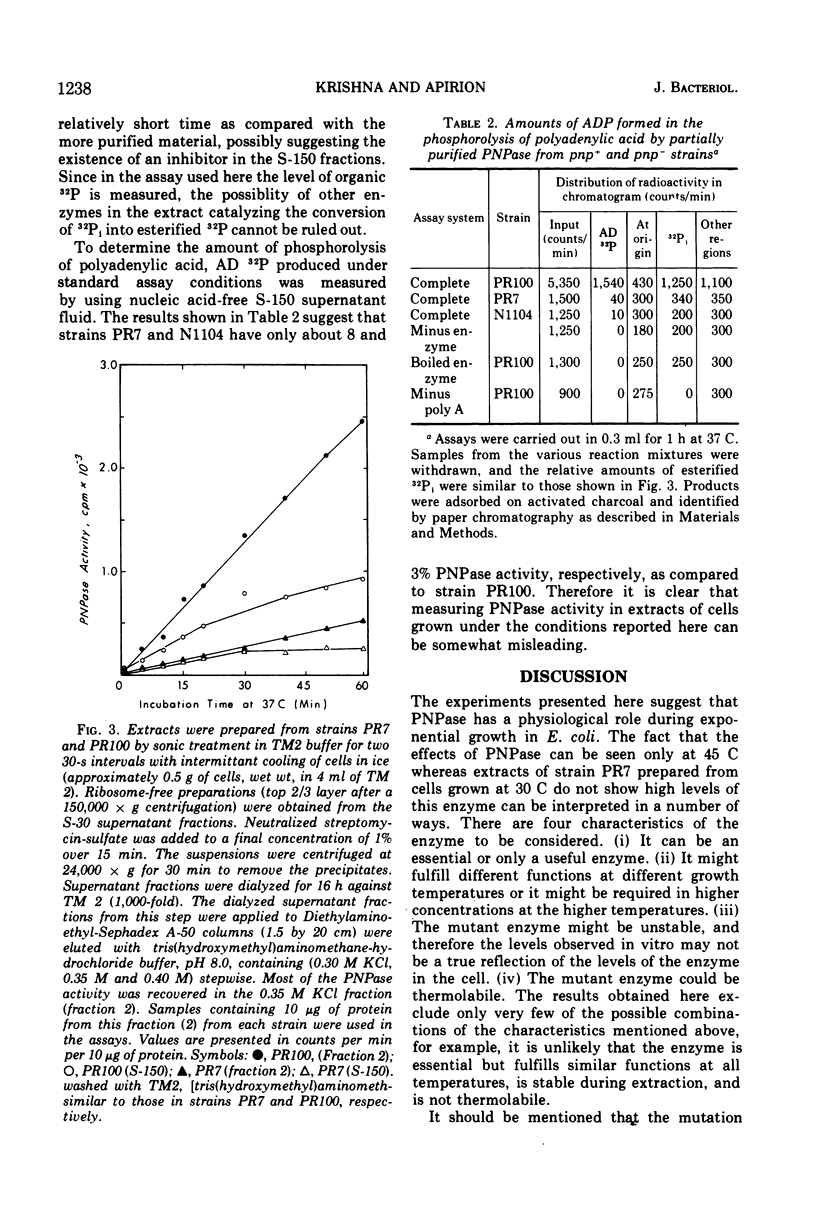
Mutants having low levels of polynucleotide phosphorylase activity grow poorly at 45 C. All revertants isolated for their ability to grow better at that temperature also regained higher levels of polynucleotide phosphorylase and the ability to be induced for tryptophanase. Thus, a physiological role is implied for the enzyme polynculeotide phosphorylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou J. Y., Singer M. F. The effect of chain length on the phosphorolysis of oligonucleotides by polynucleotide phosphorylase. J Biol Chem. 1970 Mar 10;245(5):1005–1011. [PubMed] [Google Scholar]
- GRUNBERG-MANAGO M., ORTIZ P. J., OCHOA S. Enzymic synthesis of polynucleotides. I. Polynucleotide phosphorylase of azotobacter vinelandii. Biochim Biophys Acta. 1956 Apr;20(1):269–285. doi: 10.1016/0006-3002(56)90286-4. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Kimhi Y., Littauer U. Z. Purification and properties of polynucleotide phosphorylase from Escherichia coli. J Biol Chem. 1968 Jan 25;243(2):231–240. [PubMed] [Google Scholar]
- Klee C. B., Singer M. F. The processive degradation of individual polyribonucleotide chains. II. Micrococcus lysodeikticus polynucleotide phosphorylase. J Biol Chem. 1968 Mar 10;243(5):923–927. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lennette E. T., Apirion D. Genetic analysis of an Escherichia coli syndrome. J Bacteriol. 1971 Dec;108(3):1322–1328. doi: 10.1128/jb.108.3.1322-1328.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennette E. T., Gorelic L., Apirion D. An Escherichia coli mutant with increased messenger ribonuclease activity. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3140–3144. doi: 10.1073/pnas.68.12.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Reiner A. M. Characterization of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1437–1443. doi: 10.1128/jb.97.3.1437-1443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1431–1436. doi: 10.1128/jb.97.3.1431-1436.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Apirion D. A messenger RNA from the lactose operon of Escherichia coli that can not direct the production of functional -galactosidase in absence of exogenous adenosine 3',5-cyclic monophosphate. Genetics. 1972 May;71(1):1–18. doi: 10.1093/genetics/71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Apirion D. Increased inactivation of messengers in Escherichia coli ts mutant. Nat New Biol. 1972 Sep 20;239(90):79–81. doi: 10.1038/newbio239079a0. [DOI] [PubMed] [Google Scholar]
- Weatherford S. C., Rosen L., Gorelic L., Apirion D. Escherichia coli strains with thermolabile ribonuclease II activity. J Biol Chem. 1972 Sep 10;247(17):5404–5408. [PubMed] [Google Scholar]


