Abstract
Alix (ALG-2-interacting protein X), a cytoplasmic adaptor protein involved in endosomal sorting and actin cytoskeleton assembly, is required for the maintenance of fibroblast morphology. As Alix has sequence similarity to adhesin in Entamoeba histolytica, and we observed that Alix is secreted, we determined whether extracellular Alix affects fibroblast morphology. Here, we demonstrate that secreted Alix is deposited on the substratum of non-immortalized WI38 fibroblasts. Antibody binding to extracellular Alix retards WI38 cell adhesion and spreading on fibronectin and vitronectin. Alix knockdown in WI38 cells reduces spreading and fibronectin assembly, and the effect is partially complemented by coating recombinant Alix on the cell substratum. Immortalized NIH/3T3 fibroblasts deposit less Alix on the substratum and have defects in α5β1-integrin functions. Coating recombinant Alix on the culture substratum for NIH/3T3 cells promotes α5β1-integrin-mediated cell adhesions and fibronectin assembly, and these effects require the aa 605–709 region of Alix. These findings demonstrate that a sub-population of Alix localizes extracellularly and regulates integrin-mediated cell adhesions and fibronectin matrix assembly.
Keywords: Alix, extracellular functions, fibronectin matrix assembly, integrin activation
Introduction
Mammalian ALG-2-interacting protein X (Alix), also termed as AIP1 or Hp95, is an evolutionarily conserved and ubiquitously expressed adaptor protein (Missotten et al, 1999; Vito et al, 1999; Wu et al, 2001). Studies by others have demonstrated that the yeast orthologue of Alix is a crucial component of endosomal-sorting machinery (Nikko et al, 2003; Odorizzi et al, 2003; Luhtala and Odorizzi, 2004). This function of Alix explains the involvement of Alix in viral budding (Chatellard-Causse et al, 2002; Katoh et al, 2003; Martin-Serrano et al, 2003; Strack et al, 2003; Cabezas et al, 2005; Kim et al, 2005; Lee et al, 2007), cytokinesis (Carlton and Martin-Serrano, 2007; Morita et al, 2007) and potentially its role programmed cell death (Sadoul, 2006). Our study demonstrated that Alix is an F-actin-binding protein that physically associates with multiple actin cytoskeletal structures and functionally promotes actin cytoskeleton assembly (Pan et al, 2006). This cellular function of Alix is consistent with the requirement of Alix for non-immortalized human lung WI38 fibroblasts to maintain typical fibroblast morphology. In addition to these well-defined cellular functions of Alix, Alix also directly binds lipids and regulates membrane invagination (Dikic, 2004; Matsuo et al, 2004). Alix overexpression in malignant HeLa cells restores contact inhibition and anoikis (Wu et al, 2001, 2002). Alix overexpression also reduces the strength of the static cell–matrix adhesion in HEK293 cells and inhibits endocytosis of EGF receptors in CHO cells (Schmidt et al, 2003, 2004). Mechanisms of these biological functions of Alix are yet to be understood.
Several observations led us to speculate that Alix is a member of the special class of non-transmembrane proteins that have functions on both sides of the plasma membrane (Nickel, 2003). We observed that the morphological defects of Alix-knockdown WI38 cells were much more severe after subculture than before subculture (Pan et al, 2006), which could not be satisfactorily explained by the intracellular roles of Alix in actin cytoskeleton assembly. We also demonstrated that both polyclonal and monoclonal anti-Alix antibodies consistently stained the substratum of WI38 cells (Figure 1), which was not observed with antibodies against bona fide intracellular proteins such as FAK, PYK2, cortactin, α-actinin, tensin and paxillin (data not shown). Examination of the protein databases for Alix-related proteins with defined extracellular functions revealed sequence homology between Alix and adhesin in the cytolytic enteric protozoan Entamoeba histolytica, an organism that causes amoebiasis in humans (Arroyo and Orozco, 1987; Rigothier et al, 1992; Garcia-Rivera et al, 1999; Banuelos et al, 2005). The protein sequence of the 76-kDa adhesin protein aligns with the portion of Alix containing both the N-terminal Bro1 domain (aa 1–358) and the middle V domain (aa 362–702) with 42% similarity (Banuelos et al, 2005). Most importantly, adhesin localizes both on the extracellular side of the plasma membrane and cytoplasmic vacuoles and in the cytoplasm (Garcia-Rivera et al, 1999; Madriz et al, 2004). The cell surface-localized adhesin is functionally important for heterophilic cell–cell adhesions that occur during the disease process (Garcia-Rivera et al, 1999; Martinez-Lopez et al, 2004). This finding suggests the possibility that Alix may have extracellular functions in regulating cell adhesions.
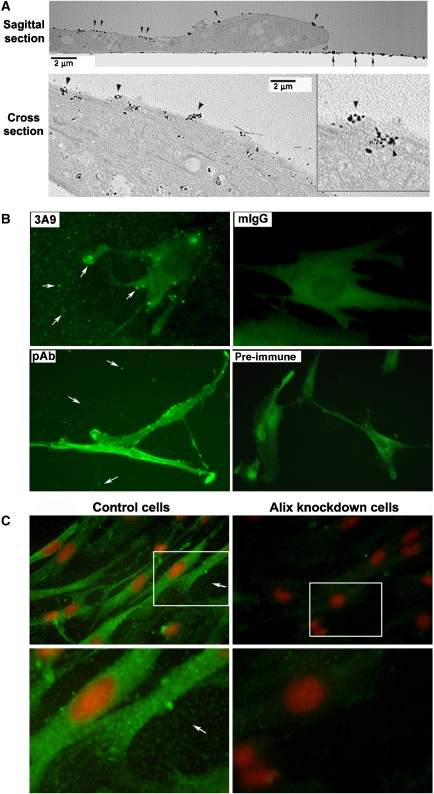
Figure 1.
Evidence that Alix is present in the extracellular compartment of WI38 cell cultures. (A) Monolayer cultures of WI38 cells were immunogold-labelled with 3A9 antibody, and electron micrographs of a sagittal section and a cross section of embedded samples are shown. Arrowheads indicate positive staining on the cell surface. Arrows indicate positive staining on the substratum. (B) Monolayer cultures of WI38 cells fixed with the EM fixative were immunostained under identical conditions with 3A9 antibody, mouse IgG (mIgG), rabbit anti-Alix immune serum (pAb) or pre-immune serum (pre-immune). Arrows indicate positive staining on the substratum and at the cell periphery. (C) Monolayer cultures of control and Alix-knockdown WI38 cells fixed with methanol were immunostained with 3A9 antibody (green) and counterstained with PI (red). Arrows indicate positive staining on the substratum.
In this study, we determined the extracellular localization of Alix in cultured mammalian fibroblasts by immunological and biochemical approaches and demonstrated the presence of Alix in the culture substratum. To investigate the biological function of extracellular Alix, we determined the effects of defined, epitope-specific anti-Alix monoclonal antibodies and recombinant Alix coated on culture substrata on adhesion, spreading and fibronectin matrix assembly of mammalian fibroblasts. Our results provide strong evidence that extracellular Alix regulates integrin-mediated cell adhesions and extracellular matrix assembly.
Results
Alix is localized, in part, on the substratum of WI38 fibroblasts
We previously generated multiple anti-Alix monoclonal antibodies to assist in the analysis of Alix biological and biochemical functions in human non-immortalized WI38 fibroblasts of fetal lung origin. By indirect immunofluorescence, 1A12 and 3A9 antibodies stained Alix associated with the actin cytoskeleton as well as with particulate structures in the cytoplasm (Pan et al, 2006). To characterize Alix-associated structures in WI38 cells in greater detail, we performed immunogold labelling of fixed and permeabilized monolayer cultures of WI38 cells with 3A9 antibody and sectioned embedded samples for electron microscopy (EM). For the immunogold labelling, WI38 cells were fixed with 2% formalin and 3% glutaraldehyde in 0.1 M sodium cacodylate (EM fixative) as compared with 4% paraformaldehyde used in our previous study for indirect immunofluorescence staining (Pan et al, 2006). Surprisingly, 3A9 antibody poorly stained the cytoplasmic Alix under the EM fixation condition; however, it stained the substratum and scattered protein aggregates near the cell surface (Figure 1A, upper panel), some of which appeared to be in the process of secretion (Figure 1A, lower panel). We then stained monolayer cultures of WI38 cells that had been fixed with the EM fixative with both monoclonal and polyclonal anti-Alix antibodies and examined the staining by indirect immunofluorescence microscopy. Both 3A9 monoclonal antibody and the rabbit anti-Alix immune serum stained the substratum, whereas the negative control antibody mouse IgG or the pre-immune serum did not (Figure 1B). We also stained control and Alix-knockdown WI38 cells that had been fixed with methanol with 1A12 anti-Alix monoclonal antibody under identical conditions. 1A12 antibody stained both the cytoplasm and the substratum, and Alix knockdown dramatically reduced the staining in both locations (Figure 1C). These findings, in conjunction with the sequence homology between the Bro1 and V domain portion of Alix and the entire length of adhesin (Supplementary Figure S1) suggested that a sub-population of Alix is secreted from WI38 cells and deposited onto the substratum.
Full-length Alix is present both in the conditioned medium and on the substratum
To test the hypothesis that a sub-population of Alix is secreted from WI38 cells, we fractionated the conditioned medium collected from WI38 cell cultures and determined whether it contained Alix that could not be accounted for by cell lysis. Figure 2A shows that although Alix was undetectable in the 1000 and 10 000 g pellets, which contained dead cells and membrane debris, respectively, full-length Alix was readily and reproducibly detected in the 100 000 g pellet, presumably containing large protein complexes and small vesicles (Odorizzi et al, 2003). Low levels of cleaved Alix were sometimes detectable in the 100 000 g supernatant, and this could be due to low levels of cell lysis. Figure 2B shows that Superose 6 gel filtration of proteins extracted from the 100 000 g pellet by multiple detergent-containing RIPA buffer resulted in one peak of Alix in the void fractions (at least 5000 kDa), whereas Superose 6 gel filtration of the postnuclear lysates of WI38 cells had the majority of Alix recovered in the 158-kDa fractions and only ∼5% of Alix in the void fractions. As cell lysis is unlikely to generate a distinct peak of full-length Alix of ∼5000 kDa, the most plausible explanation for these results is that a high molecular weight complex of Alix is secreted from WI38 cells.
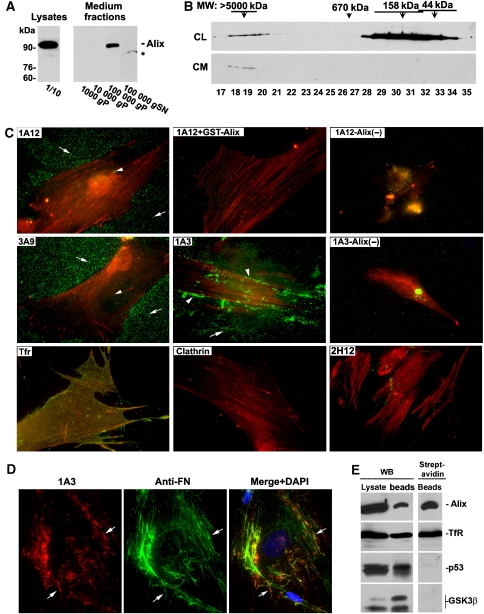
Figure 2.
Full-length Alix is present both in the conditioned medium and on the substratum of WI38 cell cultures. (A) Indicated fractions from the conditioned medium collected from WI38 cell cultures and 1/10 of cell lysates from the same cultures were immunoblotted in parallel with anti-Alix antibodies. P: pellet fraction. SN: supernatant. The asterisk indicates a cleavage product of Alix. (B) Cell lysates (CL) and protein extracts of the 100 000 g pellet fraction of the conditioned medium (CM) were fractionated by Superose 6 gel filtration, and TCA-precipitated proteins from the indicated fractions were immunoblotted with anti-Alix antibodies. (C) After live monolayer cultures of control or Alix-knockdown (Alix (−)) WI38 cells were labelled with each of the indicated antibodies, cells were fixed, permeabilized and stained with FITC-conjugated secondary antibodies (green) and TRITC-conjugated phalloidin (red). Arrows and arrowheads indicate particulate staining in the substratum and on the cell surface, respectively. (D) After live culture of WI38 cells were labelled with 1A3 antibody, fixed and permeabilized cells were labelled with anti-fibronectin (FN) antibodies. Cells were then stained with Texas-red-conjugated anti-mouse IgG for 1A3-labelled Alix (red) and FITC-conjugated anti-rabbit IgG for FN (green), and counterstained with DAPI (blue). (E) Monolayer cultures of WI38 cells were biotinylated, and derived cell lysates were immunoprecipitated with antibodies for each of the indicated proteins. Crude cell lysates and the immunoprecipitates were immunoblotted for each of the precipitated proteins (left panel) and probed with streptavidin (right panel) as indicated.
To test the hypothesis that the secreted Alix is deposited onto the substratum, we labelled live monolayer cultures of WI38 cells with each of four different anti-Alix monoclonal antibodies or control antibodies at 4°C for 30 min. By immunoblotting specific GST-tagged Alix fragments (Supplementary Figure S2A), we determined that the 1A12 and 3A9 antibodies recognize the aa 605–709 region (Supplementary Figure S2B and data not shown), and the 1A3 antibody recognizes the aa 168–436 region of Alix (Supplementary Figure S2C). In contrast to these three antibodies, 2H12 antibody had been determined to recognize the three-dimensional F676 pocket in the middle V-domain, which is hidden in the cytosolic Alix (Zhou et al, 2008). The primary antibody staining was followed by labelling fixed and permeabilized cells with fluorescence-labelled secondary antibodies and phalloidin, which decorates F-actin in the cytoplasm. We observed that both 1A12 and 3A9 antibodies stained small particles that distributed across the substratum and that the particles appeared to be more concentrated in the area adjacent to the cell periphery. These immuno-positive particles were also detectable on the cell surface but at a much lower density than on the substratum. 1A3 antibody not only stained the particles on the substratum but also fibres and clumps at the cell periphery or surrounding areas. Pre-neutralization of 1A12 antibody with recombinant Alix eliminated the ability of the antibody to stain the substratum. siRNA-mediated Alix knockdown eliminated the extracellular staining by both 1A12 and 1A3 antibodies. In contrast to 1A12, 3A9 and 1A3 antibodies, antibodies against the cell surface receptor transferrin stained the cell surface but not the substratum. 2H12 antibody, mouse IgG or antibodies against the intracellular protein clathrin stained neither the cell surface area nor the substratum (Figure 2C). Taken together, these results demonstrate that Alix is present in the substratum of WI38 cells.
To characterize the extracellular structures recognized by 1A3 antibody, we labelled live cultures of WI38 cells with 1A3 antibody and stained fixed cells with anti-fibronectin antibodies. The 1A3 antibody-decorated clumps and fibre overlapped with the clumps and fibres labelled by anti-fibronectin antibodies (Figure 2D), indicating that extracellular Alix is associated with assembled fibronectin. To biochemically characterize the Alix in the extracellular compartment, we biotinylated monolayer cultures of WI38 cells with a membrane non-permeable biotinylation agent and immunoprecipitated Alix in parallel with the cell surface receptor transferrin and abundant intracellular proteins p53 and GSK3β from cell lysates, followed by probing biotinylated proteins with streptavidin. As expected, streptavidin did not stain p53 or GSK3β. However, both Alix and transferrin were stained by streptavidin (Figure 2E), demonstrating that full-length Alix is present on the substratum of WI38 cells.
Extracellular Alix contributes to the maintenance of fibroblast morphology of WI38 cells
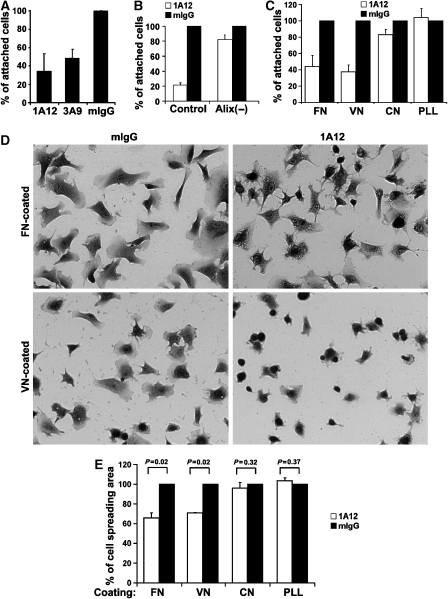
We previously reported that knockdown of Alix expression in WI38 fibroblasts led to a rounded cell morphology (Pan et al, 2006). To determine whether extracellular Alix contributes to the maintenance of WI38 fibroblast morphology, we utilized both loss-of-function and gain-of-function approaches. In the loss-of-function approach, we seeded WI38 cells in the presence of 1A12 or 3A9 antibody or, as a negative control, mouse IgG, and determined the effect of each of these antibodies on cell adhesion and spreading. Although 1A12 and 3A9 antibodies did not block cell attachment to the substratum, they reduced the rate of cell attachment within the first hour by ∼50 and 70%, respectively, whereas mouse IgG had no inhibitory effect (Figure 3A). Alix knockdown by transfection with Alix-specific siRNA almost eliminated the inhibitory effect of 1A12 antibody on the initial rate of cell attachment (Figure 3B), strongly suggesting that the inhibition was due to antibody binding to extracellular Alix. We also determined the effect of 1A12 antibody on the initial rate of WI38 cell attachment and spreading on culture substrata coated with fibronectin, vitronectin, collagen or the control polypeptide poly-L-lysine. After 1 h, 1A12 antibody caused 55 and 65% inhibition in cell attachment to fibronectin- and vitronectin-coated substrata, respectively, whereas this antibody had little or no effect on cell attachment to collagen- or poly-L-lysine-coated substrata (Figure 3C). When cell attachment neared completion, WI38 cells cultured in the presence of 1A12 antibody spread less than WI38 cells treated with mouse IgG on both vitronectin- or fibronectin-coated substrata (Figure 3D and E). As α5β1- and αvβ3-integrins are the major receptors for fibronectin and vitronectin and have key functions in determining mammalian fibroblast morphology (Akiyama, 1996; Giancotti and Ruoslahti, 1999), these results suggest that binding of extracellular Alix negatively impacts α5β1- and αvβ3-integrin-mediated cell adhesions.
Figure 3.
Anti-Alix antibodies inhibit integrin-mediated cell adhesions. (A) WI38 cells were seeded in the presence of each of the indicated antibodies, and relative cell attachments were determined at 1 h after cell seeding. Results were normalized against the value from mouse IgG (mIgG)-treated cells, and presented results are averages from three independent experiments. Error bars indicate standard errors of mean (s.e.m.). (B) Control and Alix-knockdown WI38 cells were seeded in the presence of 1A12 antibody or mIgG, and relative cell attachments were determined at 1 h after cell seeding. Presented results are averages from three independent experiments, and the error bars indicate standard errors of mean (s.e.m.). (C) WI38 cells were seeded onto the substratum that was pre-coated with FN, vitronectin (VN), collagen (CN) or poly-L-lysine (PLL) in the presence of 1A12 antibody or mIgG, and relative cell attachments on each of the coated proteins were determined at 1 h as described for (A). Results are from a representative experiment out of three, and error bars indicate standard deviations. (D, E) WI38 cells were seeded as described for (C), and cells were stained with crystal violet at 2 h after cell seeding and photographed (D). The relative spreading area per cell was determined by analysis of digitized images with Metamorph software, and the average was calculated and normalized against the value of mIgG-treated cells (E). The P-values were determined using Student's t-tests.
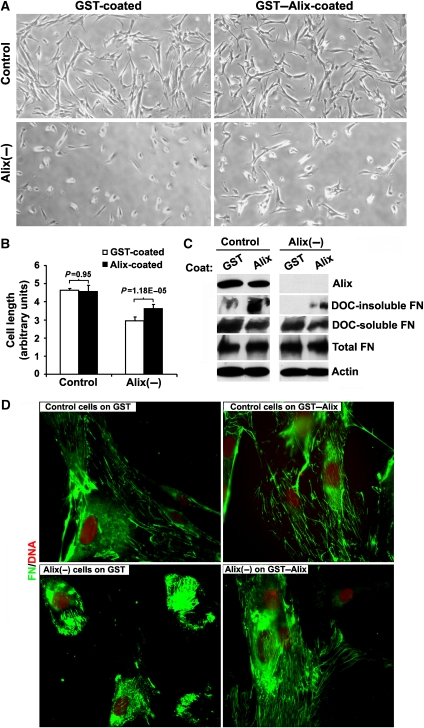
In the gain-of-function approach, we cultured control and Alix-knockdown WI38 cells on substratum pre-coated with either GST or GST–Alix, and determined the effect of extracellular addition of recombinant Alix on cell spreading and fibronectin matrix assembly. This approach was previously used to determine the effect of fibronectin on cell morphology and proliferation (Yamada et al, 1976, 1978; Ali et al, 1977; Yamada, 1978). Although the coated Alix did not produce noticeable effects on the morphology of control WI38 cells, which were well spread, it partially rescued the spreading defect of Alix-knockdown WI38 cells (Figure 4A and B). The fact that the rescue was only partial could be explained by intracellular effects of Alix knockdown on actin cytoskeleton assembly, which is closely linked to cell morphology and fibronectin assembly (Brakebusch and Fassler, 2003). Biochemical measurement of deoxycholate (DOC)-insoluble fibronectin, which represents assembled fibronectin matrix (McKeown-Longo and Mosher, 1983), in parallel with soluble and total fibronectin showed that Alix knockdown inhibited fibronectin matrix assembly of WI38 cells without inhibiting fibronectin expression. In both control and Alix-knockdown cells, Alix coated on the substratum promoted fibronectin matrix assembly without affecting fibronectin expression (Figure 4C). These observations were further confirmed by immunofluorescence staining of fibronectin in these cells (Figure 4D). Taken together, these results demonstrate that recombinant Alix coated on the substratum promotes WI38 cell spreading and fibronectin matrix assembly.
Figure 4.
Recombinant Alix coated on the substratum partially rescues the defects of Alix-knockdown WI38 fibroblasts in cell spreading and fibronectin matrix assembly. (A) After control or Alix-knockdown WI38 cells were grown on GST- or GST–Alix-coated coverslips for 24 h, cells were observed under a phase-contrast microscope and images were taken at × 100 magnification. (B) After five fields of cells randomly photographed from each treatment in (A) were enlarged and printed, individual cell lengths were manually measured and the relative average cell length and s.e.m. among different fields (error bars) were calculated. Statistical analysis of the significance was performed by Student's t-tests. (C) After control or Alix-knockdown WI38 cells were grown on GST- or GST–Alix-coated coverslips for 48 h, total proteins or DOC-soluble and DOC-insoluble proteins were extracted. Whereas total proteins were immunoblotted with anti-Alix, anti-FN and anti-actin antibodies, DOC-soluble and DOC-insoluble proteins were immunoblotted with anti-fibronectin antibodies. (D) Control or Alix-knockdown WI38 cells grown on GST- or GST–Alix-coated coverslips for 48 h were immunostained with anti-FN antibodies (green) and counterstained with PI (red).
Extracellular recombinant Alix promotes fibronectin matrix assembly of NIH3T3 cells
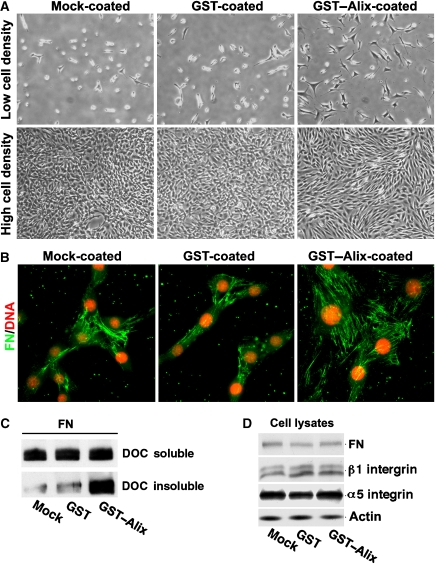
To investigate the mechanism by which extracellular Alix promotes spreading, integrin-mediated cell adhesions and fibronectin matrix assembly of mammalian fibroblasts, we chose immortalized mouse NIH/3T3 fibroblasts due to their characteristic morphology, that is, less elongation, failure to align at high density and assembly of fewer fibronectin fibres at the cell–matrix interface relative to WI38 cells (Supplementary Figure S3A and B). Also, growing NIH/3T3 cells on fibronectin-coated substrata promotes both cell spreading and alignment (Supplementary Figure S3C). Although NIH/3T3 cells express similar levels of Alix as WI38 cells (Supplementary Figure S3D), three-fold less Alix was detected on the substratum of NIH/3T3 cells as compared with WI38 cells (Supplementary Figure S3E). Alix overexpression in immortalized mouse fibroblast NIH/3T3 cells promoted cell spreading and alignment (Wu et al, 2002). When NIH/3T3 cells were grown on non-coated, GST-coated or GST–Alix-coated substrata, the coated Alix had little effect on cell adhesion (Supplementary Figure S4A). However, the coated Alix promoted cell spreading and cell–cell alignment similar to coated fibronectin (Figure 5A; Supplementary Figure S4B). In parallel with these effects, cells grown on GST–Alix-coated substrata assembled more fibronectin fibres than cells grown on non-coated or GST-coated substrata, as determined by both immunofluorescence staining of fibronectin (Figure 5B) and biochemical measurement of DOC-insoluble fibronectin (Figure 5C). The promoting effect of the coated Alix on fibronectin assembly was observed at 2 h after cell seeding and was maintained for at least 48 h (Supplementary Figure S4C). The effect was produced in the absence of increases in the expression level of fibronectin or its receptor α5β1-integrin (Figure 5D). These results both support the conclusion that extracellular Alix promotes fibroblast cell spreading and fibronectin assembly and demonstrate that NIH/3T3 cells are a suitable experimental system to study the mechanism by which extracellular Alix performs these functions.
Figure 5.
Recombinant Alix coated on the substratum promotes NIH/3T3 cell spreading, alignment and fibronectin matrix assembly. (A) NIH/3T3 cells were seeded onto mock-, GST- or GST–Alix-coated coverslips, and cell images were then taken under a phase-contrast microscope at low and high cell densities. (B) NIH/3T3 cells were cultured on mock-, GST- or GST–Alix-coated coverslips and cultured for 48 h, and cells were immunostained with anti-fibronectin antibodies (green) and counterstained with PI (red). (C) DOC-soluble and DOC-insoluble fractions of the proteins were extracted and immunoblotted with anti-FN antibodies. (D) Total proteins extracted from NIH/3T3 cells grown on mock-, GST- or GST–Alix-coated coverslips were immunoblotted with antibodies for each of the indicated proteins.
Extracellular recombinant Alix promotes α5β1-integrin-mediated cell adhesions in NIH/3T3 cells
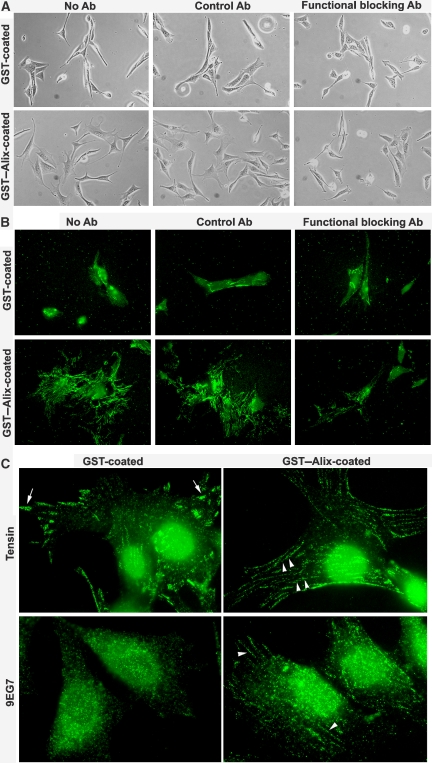
Previous studies have demonstrated that both α5β1- and αvβ3-integrins are capable of forming focal adhesions. In contrast, only α5β1-integrins that display high-affinity and translocation-competent conformations are able to translocate along with tensin from focal adhesions at cell periphery into fibrillar adhesions at cell centre. This latter conformation can be experimentally detected by conformation-sensitive antibodies such as 9EG7 and SNAKA51 monoclonal antibodies (Pankov et al, 2000; Clark et al, 2005). The translocation of α5β1-integrin ‘stretches' the fibronectin that links to α5β1-integrin and induces fibronectin matrix assembly (Zamir et al, 1999, 2000; Pankov et al, 2000; Geiger et al, 2001; Mao and Schwarzbauer, 2005). Thus, to characterize the role of extracellular Alix in cell spreading and fibronectin matrix assembly, we determined the effect of blocking α5β1-integrin functions on adhesion, spreading and fibronectin assembly of NIH/3T3 cells on GST- or GST–Alix-coated substrata. As shown in Figure 6A and B, the α5β1-integrin functional blocking antibody did not inhibit adhesion, spreading and fibronectin assembly of NIH/3T3 cells grown on GST–coated substrata, indicating that NIH/3T3 cells have defects in α5β1-integrin-mediated cell adhesions. However, the α5β1-integrin functional blocking antibody did block the ability of coated GST–Alix to induce spreading and fibronectin assembly, indicating that activation of α5β1-integrin is critical for coated GST–Alix to promote NIH/3T3 cell spreading and fibronectin assembly. We also immunostained NIH/3T3 cells grown on GST- or GST-coated substrata with anti-tensin and 9EG7 antibodies. As shown in Figure 6C, anti-tensin antibodies detected focal adhesions in NIH/3T3 cells grown on GST-coated substrata. In contrast, these antibodies detected fibrillar adhesions in NIH/3T3 cells grown on GST–Alix-coated substrata. Although 9EG7 antibody detected little, if any cell adhesion structures in NIH/3T3 cells grown on GST-coated substrata, this antibody detected fibrillar adhesions in NIH/3T3 cells grown on GST–Alix-coated substrata. These results demonstrate that extracellular Alix promotes α5β1-integrin-mediated fibrillar adhesions. Further, we immunostained control and Alix-knockdown WI38 cells with both 9EG7 antibody recognizing high-affinity α5β1-integrin and anti-α5β1-integrin antibodies at 1 h after cell seeding. The 9EG7 antibody stained focal adhesions in control cells but not in Alix-knockdown cells. In contrast, anti-α5β1-integrin antibodies stained focal adhesions in both control and Alix-knockdown cells (Supplementary Figure S5), suggesting that extracellular Alix promotes high-affinity and translocation-competent conformations of α5β1-integrin.
Figure 6.
Recombinant Alix coated on the substratum promotes α5β1-integrin-mediated fibrillar adhesions. (A, B) NIH/3T3 cells were seeded on GST- or GST–Alix-coated coverslips in the presence of the indicated antibody and cultured for 24 h. After phase-contrast images were taken (A), fixed cells were immunostained with anti-fibronectin antibodies (B). (C) NIH/3T3 cells grown on GST- or GST–Alix-coated substrata for 24 h were immunostained with either anti-tensin antibodies (upper panel) or 9EG7 antibody (lower panel). Arrows indicate focal adhesions at the cell periphery and arrowheads indicate fibrillar adhesions at cell body.
The aa 605–709 region of Alix is sufficient to extracellularly promote fibronectin assembly
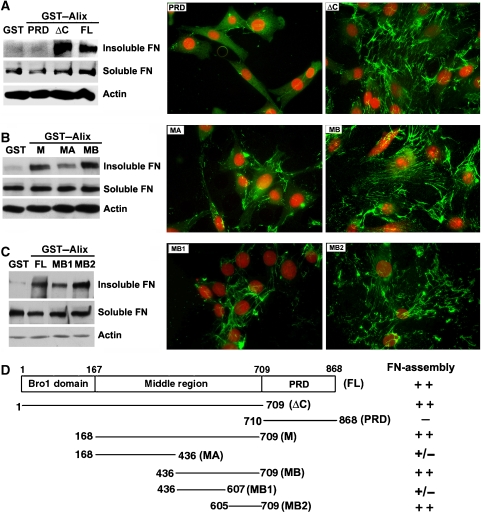
Alix consists of an N-terminal Bro1 domain (aa 1–358), a middle V domain (aa 362–702) and a C-terminal proline-rich domain (PRD, aa 703–868), each of which mediates protein–protein interactions important for endosomal sorting and/or viral budding (Kim et al, 2005; Odorizzi, 2006; Fisher et al, 2007). To determine which region(s) of Alix is/are critical to its extracellular function in promoting fibroblast spreading and fibronectin matrix assembly, we examined the effects of specific Alix fragments on fibronectin matrix assembly of NIH/3T3 cells by both biochemical measurement of DOC-insoluble fibronectin and immunofluorescence staining. As shown in Figure 7A, PRD-deleted Alix (AlixΔC) promoted fibronectin matrix assembly as efficiently as the full-length Alix, whereas PRD had no effect. To further dissect the critical regions in AlixΔC, the N-terminal 168 residues (comprising the small Bro1 domain defined by the Pfam protein domain database; Bateman et al, 2002) were deleted. This deletion also resulted in no loss of activity (data not shown). Next, the remaining middle region of Alix was divided equally into two halves and tested as described above. Only the aa 436–709 fragment (MB) retained the full activity (Figure 7B). Finally, we divided MB into two fragments, and found that only the aa 605–709 fragment (MB2), which is the region of Alix recognized by 1A12 and 3A9 antibodies (Supplementary Figure S2B) and also has a counterpart in adhesin (Supplementary Figure S1), retained the full activity (Figure 7C). These results, which are summarized in Figure 7D, indicate that the aa 605–709 region of Alix is important for Alix's functions in regulating fibroblast spreading and fibronectin matrix assembly.
Figure 7.
The aa 605–709 region of Alix promotes fibronectin matrix assembly. (A–C) NIH/3T3 cells were cultured in duplicate on coverslips coated with GST or each of the indicated GST-tagged Alix proteins for 48 h. After one set of samples were extracted, the DOC-soluble and DOC-insoluble proteins were immunoblotted with anti-FN antibodies, and the total proteins were immunoblotted with anti-actin antibodies (left panel). The other set of samples were immunostained with anti-fibronectin antibodies (green) and counterstained with PI (red) (right panel). (D) A schematic illustration of Alix fragments used (left panel) and a summary of their effects on fibronectin assembly in NIH/3T3 cell (right panel).
Recombinant Alix coated on the substratum binds assembled fibronectin
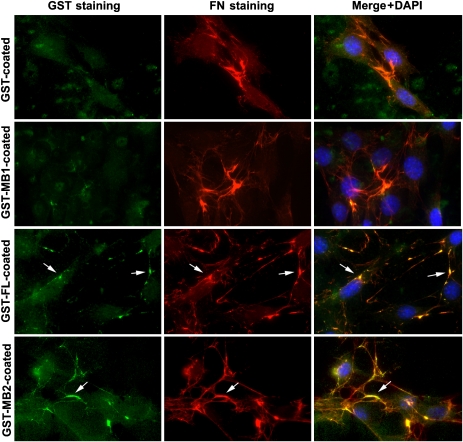
As Alix in the substratum of WI38 cells can associate with assembled fibronectin, we next determined whether both recombinant Alix and Alix-MB2 that promote fibronectin matrix assembly also associate with assembled fibronectin. For this objective, NIH/3T3 cells were grown on substrata pre-coated with GST or GST-tagged Alix, Alix-MB1 or Alix-MB2 and then double stained with anti-GST and anti-FN antibodies. Although anti-GST antibodies did not stain fibres/clumps labelled by anti-FN antibodies in the cultures on GST- or GST–Alix-MB1-coated substrata, the same antibodies stained some of these structures in the cultures on substrata coated with GST–Alix or GST–Alix-MB2 (Figure 8). However, we did not detect any interactions between fibronectin and Alix or Alix-MB by co-immunoprecipitation or GST pull-down assays (data not shown), suggesting that the fragments of Alix that promote fibronectin assembly indirectly interact with assembled fibronectin.
Figure 8.
The coated Alix recombinant proteins associate with assembled fibronectin. NIH/3T3 cells grown on coverslips pre-coated with each of the indicated recombinant proteins were fixed, immunostained with anti-GST (green) and anti-FN (red) antibodies and counterstained with DAPI. Arrows indicate colocalization of GST-tagged Alix proteins with assembled FN.
In our pilot efforts to identify proteins that may directly interact with extracellular Alix, biotinylated extracellular proteins of NIH/3T3 cells were absorbed with either GST or GST–Alix-MB, followed by blotting the bound proteins with streptavidin. One biotinylated cellular protein of ∼65 kDa was specifically found in the GST–Alix-MB complex (Supplementary Figure S6A). When biotinylated extracellular proteins of WI38 cells were immunoprecipitated with anti-Alix antibodies or mouse IgG, the Alix immunoprecipitates also seemed to contain a biotinylated protein of ∼65 kDa that did not itself react with anti-Alix antibodies (Supplementary Figure S6B). Further, when the Alix immunoprecipitates from WI38 cell lysates were silver stained after SDS–PAGE (Supplementary Figure S6C), a low abundant 65-kDa band was detected. Sequence analysis of a tryptic peptide from this band by mass spectrometry determined that this band contained a protein disulphide isomerase (PDI) (Noiva, 1999) (Supplementary Figure S6D), which is identical to the β-subunit of prolyl 4-hydroxylase (Koivu et al, 1987). These results raise the possibility that extracellular Alix interacts with PDI.
Discussion
Previous studies have demonstrated that Alix is a ubiquitously expressed and multi-function adaptor protein in the cytoplasm. Alix has also been detected as a component of exosomes of dendritic cells (Thery et al, 2001) and HIV-1 viral particles (Strack et al, 2003), but no previous evidence suggested localization and/or function of Alix on the extracellular side of the membrane. In this study, we demonstrate that Alix has both extracellular localizations and functions. In cultured mammalian fibroblasts, Alix is secreted into the conditioned medium, from where Alix is deposited onto the substratum. We demonstrate further that extracellular Alix is functional, promoting integrin-mediated cell adhesions and fibronectin matrix assembly. These results are consistent with our previous observation that Alix is required for the maintenance of fibroblast morphology (Pan et al, 2006). Thus, Alix is in a rare category of eukaryotic proteins that function both intracellularly and extracellularly (Nickel, 2003).
Most extracellular proteins contain a signal peptide, which links translation with transport into endoplasmic reticulum (ER) and subsequently across the plasma membrane through the Golgi system (Lodish, 1988). However, Alix does not contain a signal peptide, is synthesized in the cytoplasm and largely remains on the cytoplasmic side of the membrane system, predicting that a sub-population of Alix is transported across the plasma membrane through an unconventional mechanism. Thus far, ectocytosis (Stein and Luzio, 1991; Mehul and Hughes, 1997), externalization of MVB (Fevrier and Raposo, 2004) and membrane flip-flop (Denny et al, 2000) are known mechanisms by which such cytoplasmic proteins are transported across the plasma membrane (Nickel, 2003). Although we do not know whether Alix transports across the plasma membrane through any of these unconventional mechanisms, we observed that Alix near the cell surface and on the substratum could be differentially immunostained by 1A12 and 3A9 anti-Alix monoclonal antibodies under the EM fixation condition (Figure 1A). Also, by gel filtration, Alix in the conditioned medium has a higher apparent molecular weight than cytoplasmic Alix. Thus, we speculate that the exported Alix is present in different complexes when compared to cytoplasmic Alix.
Alix has a modular structure with an N-terminal ‘banana'-shaped Bro1 domain (Kim et al, 2005; Fisher et al, 2007), a middle ‘V'-shaped domain (Fisher et al, 2007; Lee et al, 2007) and a C-terminal PRD (Dejournett et al, 2007). The middle V domain is comprised of 11 α-helices that form two arms of unequal lengths. On the longer arm of the V domain lies a highly hydrophobic pocket termed the F676 pocket, which is formed by residues from the fourth, fifth and eleventh α-helices of the V domain and centred around the residue F676 (Fisher et al, 2007; Lee et al, 2007). The F676 pocket is the docking site for p6Gag and p9Gag viral proteins (Zhai et al, 2008). Interestingly, the MB2 fragment of Alix required for its extracellular-promoting effects on fibronectin assembly corresponds to two α-helices from different helical bundles in the middle V domain. This fragment is also recognized by 1A12 and 3A9 antibodies, which both recognize cellular Alix deposited on the substratum and retard adhesion and spreading of WI38 cells, implying that the MB2 region of Alix is exposed on the extracellular full-length Alix. These findings suggest that the middle V domain of Alix contains multiple functional motifs, which may mediate different biological functions.
In cultured mammalian cells, α5β1- and αvβ3-integrins have key functions in determining cell adhesion, morphology and fibronectin assembly (Wu et al, 1993; Ruoslahti, 1996; Wennerberg et al, 1996; Boudreau and Jones, 1999; Zaidel-Bar et al, 2004). As anti-Alix antibodies retarded adhesion and spreading of newly seeded WI38 cells on both fibronectin and vitronectin, we speculate extracellular Alix regulates functions of multiple integrins. In support of this possibility, Alix overexpression promotes flattening of both mouse fibroblast NIH/3T3 cells and human cervical carcinoma HeLa cells (Wu et al, 2002).
Previous studies demonstrated that integrin conformations have important functions in determining integrin functions (Bazzoni and Hemler, 1998). As extracellular recombinant Alix activates α5β1-integrin in NIH/3T3 cells, whereas Alix knockdown in WI38 cells reduced the high-affinity confirmation of α5β1-integrin recognized by 9EG7 antibody, extracellular Alix may promote high-affinity conformations of integrins. Integrin conformations can be extracellularly regulated by a variety of proteins, including PDI, a member in the protein disulphide isomerase family, which catalyses the oxidation of disulphide bridges of proteins (Koivu et al, 1987; Noiva, 1999). Although mainly localized in the ER, PDI has been found on the cell surface in many cell types (Essex et al, 1995; Shin et al, 2003), and cell-surface localized PDI facilitates reorganization of disulphide bonds in integrins, and as a result, promotes appearance of high-affinity conformations of integrins and formation of stable integrin-mediated cell adhesions (Lahav et al, 2000, 2003; Swiatkowska et al, 2008). In this context, our pilot result that extracellular Alix may interact with PDI is interesting as it suggests a potential mechanism by which extracellular Alix may promote high-affinity conformations of integrins. In addition to the potential Alix–PDI interaction, extracellular Alix also indirectly interacts with fibronectin, raising a possibility that this interaction may promote fibronectin assembly. In summary, our work clearly demonstrates the importance of extracellular Alix in regulating integrin-mediated cell adhesions and extracellular matrix assembly and provides an excellent example of how the so-called ‘adapter' proteins may perform distinct functions in intracellular and extracellular locations.
Materials and methods
Cell culture, preparation of cell lysates, immunoblotting, immunoprecipitation, immunostaining of cells and fractionation of the conditioned medium
Cell culture, preparation of cell lysates, immunoblotting, immunoprecipitation, immunostaining of cells were performed largely as previously described (Kloc et al, 2002; Pan et al, 2006) and are detailed in Supplementary data. Fractionation of the conditioned medium was performed as described in Supplementary data.
Live cell labelling with antibodies or biotin
Control or Alix-knockdown WI38 cells cultured on 22-mm glass coverslips for at least 24 h were washed twice with ice-cold PBS and incubated in the cold PBS for 15 min before incubation with 1 μg/ml of indicated primary antibodies or mouse IgG at 4°C for 30 min. Pre-neutralization of 1A12 antibody with GST–Alix was achieved by incubating anti-Alix antibody with 10 times molar ratio of GST–Alix recombinant protein before adding to the cells. Cells were then fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 and subsequently stained with FITC-conjugated secondary antibodies (Sigma, St Louis, MO) and TRITC-conjugated phalloidin (Sigma). The digital imaging was performed as previously described (Pan et al, 2006).
For biotinylation 100-mm subconfluent cultures of WI38 or NIH/3T3 cells were rinsed twice with ice-cold PBS and then incubated with 3 ml of 0.5 mg/ml Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) at 4°C for 30 min. For biotinylation of NIH/3T3 cells in suspension, cells were detached by trypsinization and resuspended in ice-cold PBS before incubation with 0.5 mg/ml Sulfo-NHS-LC-Biotin. In both cases, the biotinylation reaction was stopped by incubation of treated cells with 100 mM glycine in PBS for 15 min. Biotinylated proteins were detected with HRP-conjugated streptavidin (Pierce).
Determination of the effects of antibodies on cell attachment and spreading
WI38 cells were trypsinized and resuspended at 1 × 106 cells/ml in a serum-free medium containing 40 μg/ml of anti-Alix monoclonal antibody or mouse IgG and incubated at 4°C for 30 min. These cells were then plated in triplicate into 48-well plates at 1 × 104 cells per well and incubated at 37°C for 1 h. When designated, the 48-well plates were pre-coated at 37°C for 2 h with 20 μg/ml of fibronectin, 2.5 μg/ml of collagen, 10 μg/ml of vitronectin or 0.1 mg/ml of poly-L-lysine diluted in PBS. After three washes with PBS, attached cells were stained with 0.5% crystal violet/70% ethanol for 20 min followed by three washes with double distilled H2O. A cross line was then drawn in a fixed direction on the bottom surface of the triplet wells, and the number of cells on the cross line were counted under a light microscope. The average for each triplet was then determined and standard deviation was calculated using Excel software.
Approximately 1 × 105 NIH/3T3 cells were suspended in 0.5 ml of regular culture medium or the medium that contained 10 μg/ml BMA5, or the control monoclonal antibody 33B6. The cells were seeded onto 22-mm PBS-, GST- or GST–Alix-coated coverslips, which were placed in 35-mm culture plates, and cultured for 24 h. The cells were then first examined under a phase-contrast microscope and then immunostained.
Preparation of GST-tagged recombinant proteins
Plasmids for expressing GST–Alix, GST–AlixΔC, GST–AlixM, GST–AlixMA, GST–AlixMB and GST-PRD in Escherichia coli were constructed in our previous studies (Pan et al, 2006). cDNA encoding Alix-MB1 was PCR-amplified from Alix cDNA with primers 5′-ggcggatcttgattaaagaactgcctg-3′ and 5′-atagcggccgcgactcgatctagttcagt-3′, and cDNA encoding Alix-MB2 was PCR-amplified from Alix cDNA with primers 5′-actggatccgatcgagtctatggaggt-3′ and 5′-tgttgcggccgcagtcctttaagagttcat-3′. Both products contained a BamHI site at 5′ end and a NotI site at 3′ end, and each of them was cloned in frame into pGEX4T3 vector at these restriction enzyme cleavage sites after the coding sequence for GST. All GST or GST-tagged recombinant proteins were produced and purified as previously described (Pan et al, 2006).
Measurement of DOC-soluble and DOC-insoluble fibronectin
Cells grown on glass coverslips were rinsed with PBS, and DOC-soluble and -insoluble proteins were extracted according to a commonly utilized procedure (Chernousov et al, 1998) with minor modifications. In brief, the coverslips were first rinsed with cold PBS and extracted on ice for 10 min with 100 μl of 1% DOC in 20 mM Tris–HCl, pH 8.3, containing 2 mM PMSF, 2 mM NEM, 2 mM EDTA and 2 mM iodoacetic acid, and extracts were immediately mixed with 50 μl of 3 × SDS–PAGE sample buffer. DOC-insoluble proteins remaining on coverslips were then extracted with 150 μl of 1 × SDS–PAGE sample buffer.
Supplementary Material
Supplementary Information
Acknowledgments
This study was supported by ACS grant RPG-00-071-01-DDC (J Kuang), NIH/NCI grants 1 RO1 CA93941 (J Kuang) and RO1 CA111479 (S-H Lin). DNA sequencing was performed by the DNA Analysis Facility of UT MD Anderson Cancer Center, which is supported by NCI Grant CA-16672. Mass spectrometry was performed by the Proteomics Core Facility of UT MD Anderson Cancer Center. We thank Dr WN Hittelman for instruction on image analysis.
References
- Akiyama SK (1996) Integrins in cell adhesion and signaling. Hum Cell 9: 181–186 [PubMed] [Google Scholar]
- Ali IU, Mautner V, Lanza R, Hynes RO (1977) Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell 11: 115–126 [DOI] [PubMed] [Google Scholar]
- Arroyo R, Orozco E (1987) Localization and identification of an Entamoeba histolytica adhesin. Mol Biochem Parasitol 23: 151–158 [DOI] [PubMed] [Google Scholar]
- Banuelos C, Garcia-Rivera G, Lopez-Reyes I, Orozco E (2005) Functional characterization of EhADH112: an Entamoeba histolytica Bro1 domain-containing protein. Exp Parasitol 110: 292–297 [DOI] [PubMed] [Google Scholar]
- Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL (2002) The Pfam protein families database. Nucleic Acids Res 30: 276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Hemler ME (1998) Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci 23: 30–34 [DOI] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL (1999) Extracellular matrix and integrin signalling: the shape of things to come. Biochem J 339 (Part 3): 481–488 [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R (2003) The integrin–actin connection, an eternal love affair. EMBO J 22: 2324–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas A, Bache KG, Brech A, Stenmark H (2005) Alix regulates cortical actin and the spatial distribution of endosomes. J Cell Sci 118: 2625–2635 [DOI] [PubMed] [Google Scholar]
- Carlton JG, Martin-Serrano J (2007) Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316: 1908–1912 [DOI] [PubMed] [Google Scholar]
- Chatellard-Causse C, Blot B, Cristina N, Torch S, Missotten M, Sadoul R (2002) Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J Biol Chem 277: 29108–29115 [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Stahl RC, Carey DJ (1998) Schwann cells use a novel collagen-dependent mechanism for fibronectin fibril assembly. J Cell Sci 111 (Part 18): 2763–2777 [DOI] [PubMed] [Google Scholar]
- Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, Newham P, Yamada KM, Humphries MJ (2005) A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci 118: 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejournett RE, Kobayashi R, Pan S, Wu C, Etkin LD, Clark RB, Bogler O, Kuang J (2007) Phosphorylation of the proline-rich domain of Xp95 modulates Xp95 interaction with partner proteins. Biochem J 401: 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny PW, Gokool S, Russell DG, Field MC, Smith DF (2000) Acylation-dependent protein export in Leishmania. J Biol Chem 275: 11017–11025 [DOI] [PubMed] [Google Scholar]
- Dikic I (2004) ALIX-ing phospholipids with endosome biogenesis. Bioessays 26: 604–607 [DOI] [PubMed] [Google Scholar]
- Essex DW, Chen K, Swiatkowska M (1995) Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood 86: 2168–2173 [PubMed] [Google Scholar]
- Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16: 415–421 [DOI] [PubMed] [Google Scholar]
- Fisher RD, Chung HY, Zhai Q, Robinson H, Sundquist WI, Hill CP (2007) Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128: 841–852 [DOI] [PubMed] [Google Scholar]
- Garcia-Rivera G, Rodriguez MA, Ocadiz R, Martinez-Lopez MC, Arroyo R, Gonzalez-Robles A, Orozco E (1999) Entamoeba histolytica: a novel cysteine protease and an adhesin form the 112 kDa surface protein. Mol Microbiol 33: 556–568 [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2: 793–805 [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285: 1028–1032 [DOI] [PubMed] [Google Scholar]
- Katoh K, Shibata H, Suzuki H, Nara A, Ishidoh K, Kominami E, Yoshimori T, Maki M (2003) The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J Biol Chem 278: 39104–39113 [DOI] [PubMed] [Google Scholar]
- Kim J, Sitaraman S, Hierro A, Beach BM, Odorizzi G, Hurley JH (2005) Structural basis for endosomal targeting by the Bro1 domain. Dev Cell 8: 937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M, Dougherty MT, Bilinski S, Chan AP, Brey E, King ML, Patrick CW Jr, Etkin LD (2002) Three-dimensional ultrastructural analysis of RNA distribution within germinal granules of Xenopus. Dev Biol 241: 79–93 [DOI] [PubMed] [Google Scholar]
- Koivu J, Myllyla R, Helaakoski T, Pihlajaniemi T, Tasanen K, Kivirikko KI (1987) A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem 262: 6447–6449 [PubMed] [Google Scholar]
- Lahav J, Gofer-Dadosh N, Luboshitz J, Hess O, Shaklai M (2000) Protein disulfide isomerase mediates integrin-dependent adhesion. FEBS Lett 475: 89–92 [DOI] [PubMed] [Google Scholar]
- Lahav J, Wijnen EM, Hess O, Hamaia SW, Griffiths D, Makris M, Knight CG, Essex DW, Farndale RW (2003) Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin alpha2beta1. Blood 102: 2085–2092 [DOI] [PubMed] [Google Scholar]
- Lee S, Joshi A, Nagashima K, Freed EO, Hurley JH (2007) Structural basis for viral late-domain binding to Alix. Nat Struct Mol Biol 14: 194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish HF (1988) Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem 263: 2107–2110 [PubMed] [Google Scholar]
- Luhtala N, Odorizzi G (2004) Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J Cell Biol 166: 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madriz X, Martinez MB, Rodriguez MA, Sierra G, Martinez-Lopez C, Riveron AM, Flores L, Orozco E (2004) Expression in fibroblasts and in live animals of Entamoeba histolytica polypeptides EhCP112 and EhADH112. Microbiology 150: 1251–1260 [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE (2005) Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24: 389–399 [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Yarovoy A, Perez-Caballero D, Bieniasz PD (2003) Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc Natl Acad Sci USA 100: 12414–12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez C, Orozco E, Sanchez T, Garcia-Perez RM, Hernandez-Hernandez F, Rodriguez MA (2004) The EhADH112 recombinant polypeptide inhibits cell destruction and liver abscess formation by Entamoeba histolytica trophozoites. Cell Microbiol 6: 367–376 [DOI] [PubMed] [Google Scholar]
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J (2004) Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303: 531–534 [DOI] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF (1983) Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol 97: 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehul B, Hughes RC (1997) Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci 110 (Part 10): 1169–1178 [DOI] [PubMed] [Google Scholar]
- Missotten M, Nichols A, Rieger K, Sadoul R (1999) Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ 6: 124–129 [DOI] [PubMed] [Google Scholar]
- Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI (2007) Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26: 4215–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W (2003) The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 270: 2109–2119 [DOI] [PubMed] [Google Scholar]
- Nikko E, Marini AM, Andre B (2003) Permease recycling and ubiquitination status reveal a particular role for Bro1 in the multivesicular body pathway. J Biol Chem 278: 50732–50743 [DOI] [PubMed] [Google Scholar]
- Noiva R (1999) Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin Cell Dev Biol 10: 481–493 [DOI] [PubMed] [Google Scholar]
- Odorizzi G (2006) The multiple personalities of Alix. J Cell Sci 119: 3025–3032 [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Katzmann DJ, Babst M, Audhya A, Emr SD (2003) Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J Cell Sci 116: 1893–1903 [DOI] [PubMed] [Google Scholar]
- Pan S, Wang R, Zhou X, He G, Koomen J, Kobayashi R, Sun L, Corvera J, Gallick GE, Kuang J (2006) Involvement of the conserved adaptor protein Alix in actin cytoskeleton assembly. J Biol Chem 281: 34640–34650 [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM (2000) Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol 148: 1075–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigothier MC, Garcia-Rivera G, Guaderrama M, Orozco E (1992) Purification and functional characterization of the 112 kDa adhesin of Entamoeba histolytica. Arch Med Res 23: 239–241 [PubMed] [Google Scholar]
- Ruoslahti E (1996) Integrin signaling and matrix assembly. Tumour Biol 17: 117–124 [DOI] [PubMed] [Google Scholar]
- Sadoul R (2006) Do Alix and ALG-2 really control endosomes for better or for worse? Biol Cell 98: 69–77 [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Chen B, Randazzo LM, Bogler O (2003) SETA/CIN85/Ruk and its binding partner AIP1 associate with diverse cytoskeletal elements, including FAKs, and modulate cell adhesion. J Cell Sci 116: 2845–2855 [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Hoeller D, Yu J, Furnari FB, Cavenee WK, Dikic I, Bogler O (2004) Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol 24: 8981–8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM (2003) Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem 278: 7607–7616 [DOI] [PubMed] [Google Scholar]
- Stein JM, Luzio JP (1991) Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J 274 (Part 2): 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack B, Calistri A, Craig S, Popova E, Gottlinger HG (2003) AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114: 689–699 [DOI] [PubMed] [Google Scholar]
- Swiatkowska M, Szymanski J, Padula G, Cierniewski CS (2008) Interaction and functional association of protein disulfide isomerase with alpha(V)beta(3) integrin on endothelial cells. FEBS J 275: 1813–1823 [DOI] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S (2001) Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166: 7309–7318 [DOI] [PubMed] [Google Scholar]
- Vito P, Pellegrini L, Guiet C, D'Adamio L (1999) Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J Biol Chem 274: 1533–1540 [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R (1996) Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol 132: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Bauer JS, Juliano RL, McDonald JA (1993) The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem 268: 21883–21888 [PubMed] [Google Scholar]
- Wu Y, Pan S, Che S, He G, Nelman-Gonzalez M, Weil MM, Kuang J (2001) Overexpression of Hp95 induces G1 phase arrest in confluent HeLa cells. Differentiation 67: 139–153 [DOI] [PubMed] [Google Scholar]
- Wu Y, Pan S, Luo W, Lin SH, Kuang J (2002) Hp95 promotes anoikis and inhibits tumorigenicity of HeLa cells. Oncogene 21: 6801–6808 [DOI] [PubMed] [Google Scholar]
- Yamada KM (1978) Immunological characterization of a major transformation-sensitive fibroblast cell surface glycoprotein. Localization, redistribution, and role in cell shape. J Cell Biol 78: 520–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Olden K, Pastan I (1978) Transformation-sensitive cell surface protein: isolation, characterization, and role in cellular morphology and adhesion. Ann NY Acad Sci 312: 256–277 [DOI] [PubMed] [Google Scholar]
- Yamada KM, Yamada SS, Pastan I (1976) Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci USA 73: 1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Cohen M, Addadi L, Geiger B (2004) Hierarchical assembly of cell–matrix adhesion complexes. Biochem Soc Trans 32: 416–420 [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z (1999) Molecular diversity of cell–matrix adhesions. J Cell Sci 112 (Part 11): 1655–1669 [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B (2000) Dynamics and segregation of cell–matrix adhesions in cultured fibroblasts. Nat Cell Biol 2: 191–196 [DOI] [PubMed] [Google Scholar]
- Zhai Q, Fisher RD, Chung HY, Myszka DG, Sundquist WI, Hill CP (2008) Structural and functional studies of ALIX interactions with YPX(n)L late domains of HIV-1 and EIAV. Nat Struct Mol Biol 15: 43–49 [DOI] [PubMed] [Google Scholar]
- Zhou X, Pan S, Sun L, Corvera J, Lin S, Kuang J (2008) The HIV-1 p6/EIAV p9 docking site in Alix is autoinhibited as revealed by a conformation-sensitive anti-Alix monoclonal antibody. Biochem J (advance online publication, 13 May 2008; doi:10.1042/BJ20080642). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information