Abstract
The biogenesis of endoplasmic reticulum (ER) exit sites (ERES) involves the formation of phosphatidylinositol-4 phosphate (PI4) and Sec16, but it is entirely unknown how ERES adapt to variations in cargo load. Here, we studied acute and chronic adaptive responses of ERES to an increase in cargo load for ER export. The acute response (within minutes) to increased cargo load stimulated ERES fusion events, leading to larger but less ERES. Silencing either PI4-kinase IIIα (PI4K-IIIα) or Sec16 inhibited the acute response. Overexpression of secretory cargo for 24 h induced the unfolded protein response (UPR), upregulated COPII, and the cells formed more ERES. This chronic response was insensitive to silencing PI4K-IIIα, but was abrogated by silencing Sec16. The UPR was required as the chronic response was absent in cells lacking inositol-requiring protein 1. Mathematical model simulations further support the notion that increasing ERES number together with COPII levels is an efficient way to enhance the secretory flux. These results indicate that chronic and acute increases in cargo load are handled differentially by ERES and are regulated by different factors.
Keywords: COPII, phosphatidylinositol-4 kinase IIIα, Sec16, secretory pathway, unfolded protein response
Introduction
Export of proteins from the endoplasmic reticulum (ER) is mediated by COPII-coated vesicles that form at ribosome-free ER exit sites (ERES) of the rough ER (Lee et al, 2004). COPII assembly at ERES is initiated by the activation of the small GTPase Sar1 followed by recruitment of the Sec23–Sec24 dimer to form the inner layer of the COPII coat. Subsequently, the Sec13–Sec31 tetrameric complex is recruited, forming the outer layer of the COPII coat. COPII components deform the ER membrane, leading to the budding of COPII vesicles with a diameter of 60–80 nm. ERES form de novo, move, and undergo fusion and fission (Bevis et al, 2002; Stephens, 2003).
The secretory pathway, and ERES as part of it, has to adapt to a variety of acute (within minutes) or chronic (in the range of hours to days) environmental needs. Acute stimuli require an immediate response whereas the persistence of the stimulus, that is, when cargo overload becomes chronic, might also alter gene expression and hence the two responses may be mechanistically different. The turnover rates of COPII components on ERES are known to be affected by changes in cargo load (Forster et al, 2006), and COPII assembly at ERES increases during Golgi reformation after brefeldin A (BFA)-induced Golgi breakdown, when additional cargo needs to be exported from the ER (Guo and Linstedt, 2006). The molecular mechanism controlling the biogenesis and maintenance of ERES, however, has remained elusive. Recent evidence suggests a function for Sec16 in the homoeostasis of ERES (Watson et al, 2006; Bhattacharyya and Glick, 2007; Iinuma et al, 2007).
Sec16 is a large peripheral membrane protein of ∼250 kDa. It associates with ERES and is thought to form a scaffold for the assembly of COPII (Gimeno et al, 1996). The composition of membrane lipids is also important for the function of active ERES as indicated by the observation that depletion of phosphatidylinositol-4 (PI4) phosphate inhibits COPII vesicle formation (Blumental-Perry et al, 2006). However, the kinase responsible for PI4-phosphate formation remains unknown.
Despite these findings, our understanding of the biology of ERES is sketchy. For instance, a direct comparison between the adaptive responses of ERES to acute and chronic changes in cargo load has never been performed. Moreover, although the presence of PI4-phosphate and Sec16 is important for the maintenance of ERES, it remains entirely unknown whether Sec16 and the formation of PI4-phosphate are involved in these adaptive responses. In the current study, we explored the response of ERES to acute and chronic cargo load. We report that an acute increase in cargo load causes fusion of ERES leading to an increase in ERES size. This response was dependent on a limiting amount of either PI4-kinase IIIα (PI4K-IIIα) or Sec16. By contrast, a chronic increase in cargo load resulted in the formation of additional ERES. This response was independent of PI4K-IIIα but required Sec16. The chronic increase in cargo load induced the expression of COPII and Sec16 through the unfolded protein response (UPR). The results suggest that acute and chronic cargo loads are handled in a different mechanistic way at the level of ERES.
Results
An acute increase in cargo load increases the size of ERES
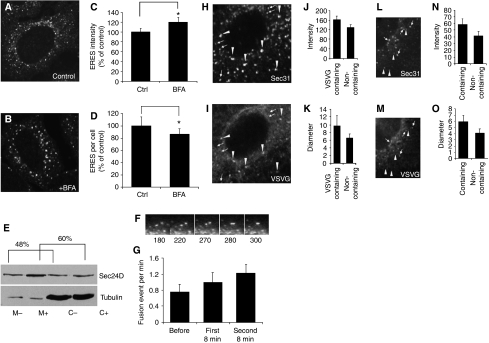
To determine the effect of acute changes on ERES, HeLa cells were treated with BFA for 40 min. BFA causes a rapid redistribution of Golgi proteins to the ER (Doms et al, 1989; Lippincott-Schwartz et al, 1989) and thereby increases acutely the load of cargo in the ER. The same experimental design was used recently by Guo and Linstedt (2006). After BFA treatment the cells were fixed, stained for Sec31 to label ERES, and analysed by immunofluorescence microscopy. The data were evaluated by counting the number of ERES and by recording the average intensity of fluorescence of ERES. The fluorescence intensity reflects the amount of COPII that assembles at ERES. Throughout the paper, this value will be referred to as intensity or ERES size.
Treatment of HeLa cells with BFA increased the intensity of ERES (Figure 1A–C). This response may reflect increased COPII assembly (Guo and Linstedt, 2006), that is, recruitment of COPII from the cytosolic reserve. To test for this, we prepared membrane and cytosolic fractions from control cells and from cells treated with BFA. There was indeed a clear increase of the relative amount of Sec24 associated with the membrane fraction in response to BFA (Figure 1E). However, we also observed a previously unrecognized ∼20%, statistically significant decrease in the number of ERES (Figure 1A, B and D). The observation that an acute increase in cargo load leads to an increase in COPII assembly does not explain the decrease in ERES number. One possible reason for this decrease may be increased ERES fusion activity. To visualize ERES fusion events, we performed live cell microscopy of HeLa cells transiently expressing YFP-tagged Sec23A. YFP–Sec23A is a widely used marker for ERES (Stephens, 2003). A representative fusion event is shown in Figure 1F and demonstrates that fusion of two ERES results in the formation of an exit site of higher intensity. We acquired images in 10-s intervals for a period of 8 min before and after the addition of BFA. Quantification shows that addition of BFA caused a time-dependent increase in the number of fusion events, which continued when fusion was monitored for another 8 min (Figure 1G).
Figure 1.
Acute cargo overload enlarges ERES and induces ERES fusion activity. (A–D) HeLa cells were treated with a solvent (A) or with 5 μg/ml BFA (B) for 40 min at 37°C and ERES were visualized by confocal immunofluorescence microscopy with an antibody against Sec31. Image intensity (C) and number (D) of ERES were evaluated. The data are displayed as a percentage of control to normalize for inter-assay variance. Asterisks indicate statistically significant differences (P<0.05). (E) HeLa cells were treated with a solvent (−) or 5 μg/ml BFA (+) for 40 min. Microsomes (M) and cytosol (C) were prepared and 8 μg of each sample was separated by SDS–PAGE. Sec24D and tubulin were detected by immunoblotting. Sec24D was quantified by densitometry and displayed as percent membrane-bound of total. (F, G) HeLa cells were transfected with a plasmid encoding YFP-tagged Sec23A. Live cell imaging was performed and fusion events were counted. A typical fusion event is shown in (E). Fusion events were counted in a period of 8 min prior (before, n=11) and after the addition (first 8 min, n=11) of 5 μg/ml of BFA. In some cases (n=4), fusion events were also scored for a second period of 8 min (second 8 min) after BFA addition. (H–K) HeLa cells were transfected with a plasmid encoding GFP-tagged VSVG-tsO45 and cultured overnight at 40°C. The cells were shifted to 32°C for 5 min and immunostained for Sec31. Images were acquired for Sec31 (H) and for GFP-tagged VSVG-tsO45 (I), and the intensity (J) and diameter (K) of ERES were determined. (L–O) HeLa cells were transfected with a plasmid encoding GFP-tagged VSVG-tsO45 and cultured overnight at 40°C. The cells were shifted to 10°C for 40 min and immunostained for Sec31. Images were acquired for Sec31 (L) and for GFP-tagged VSVG-tsO45 (M), and the intensity (N) and diameter (O) of ERES were determined.
Although the response to BFA can be considered an acute adaptation to an increase in cargo load, BFA is also known to severely disturb the early secretory pathway. To overcome this limitation, we studied the acute response by reversibly accumulating an overexpressed protein in the ER. To this end, YFP-tagged vesicular stomatitis virus glycoprotein-tsO45 (VSVG–YFP) was transiently expressed in HeLa cells. This protein has a temperature-sensitive phenotype. It is misfolded and thus retained in the ER at 40°C but rapidly folds and enters the secretory pathway at 32°C. The cells were cultured at 40°C overnight to accumulate VSVG–YFP in the ER and shifted to 32°C for 3–5 min, followed by fixation and immunostaining for Sec31. The brief shift to the permissive temperature allows entry of VSVG–YFP into ERES (Figure 1H and I; Nishimura et al, 1999). We termed VSVG–YFP-positive ERES ‘VSVG-containing' (arrowheads in Figure 1H and I) and those that were negative ‘non-containing' (arrows in Figure 1H and I). We suggest that the VSVG-containing ERES represents the condition of an acute increase in cargo load. A direct comparison between VSVG-containing and non-containing ERES revealed that VSVG-containing ERES had higher intensities (Figure 1J) and larger diameters (Figure 1K). Note that VSVG-containing ERES often showed signs of fusion (arrowhead in Figure 1H).
The 3–5 min shift to the permissive temperature has the advantage that it is short enough not to fill all ERES with VSVG. This facilitates a comparison between ERES that do and those that do not contain VSVG. A disadvantage of this approach is that some VSVG-containing structures may represent ERGIC clusters rather than ERES, as no budding block was applied. Such a budding block can be achieved, however, by cooling cells to 10°C, a temperature that still allows concentration of cargo into ERES (Mezzacasa and Helenius, 2002). When cells were shifted from 40 to 10°C for 40 min, the results were comparable to those obtained with a brief shift to 32°C. VSVG-containing ERES were bigger and brighter than those not containing VSVG (Figure 1L–O).
Taken together, these results suggest that an acute increase in cargo load is associated with an increase in ERES intensity and a decrease in ERES number due to the combined effect of increased COPII assembly and homotypic fusion.
A chronic increase in cargo load increases the number of ERES
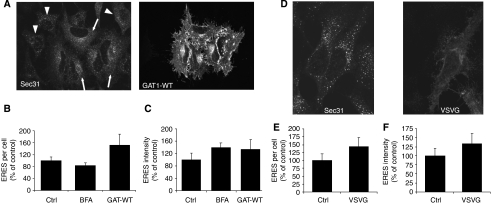
To study the response of ERES to a chronic increase in cargo load, we choose to overexpress the anterograde cargo GABA transporter 1 (GAT1). GAT1 is a polytopic plasma membrane protein and its ER export is dependent on a motif in its carboxyl terminus that mediates binding to the COPII subunit Sec24D (Farhan et al, 2007). Overexpression of GAT1 in HeLa cells resulted in a robust increase in both the number and intensity of ERES (Figure 2A–C). Thus, the chronic and acute responses are different. The chronic response increases both the number and size of ERES very much in contrast to the acute response that increases the size, but reduces the number of ERES. We also studied the chronic response to VSVG overload. Upon expression of VSVG–YFP at the permissive temperature for 24 h, both the number and intensity of ERES increased (Figure 2D–F). Thus, the adaptive response of ERES to chronic cargo overload does not seem to depend on the nature of cargo.
Figure 2.
Overexpression of cargo proteins increases the size and number of ERES. (A) HeLa cells were transfected with a plasmid encoding YFP-tagged wild-type GAT1. After 24 h, the cells were stained for Sec31, and images were acquired by confocal microscopy. Arrows, transfected cells; arrowheads, non-transfected cells. Graphic representation of the number (B) and intensity (C) of ERES of control (ctrl) cells, cells treated with 5 μg/ml BFA for 40 min (BFA) or cells overexpressing wild-type GAT1 (GAT-WT). (D) HeLa cells were transfected with plasmids encoding GFP-tagged VSVG-tsO45 and cultured at the permissive temperature. After 24 h, the cells were fixed, stained for Sec31, and analysed by confocal microscopy. (E, F) Graphic representation of the number (E) and intensity (F) of ERES of control (ctrl) cells or cells overexpressing VSVG-tsO45 (VSVG).
Role of PI4K-IIIα and Sec16 in the acute response of ERES to increased cargo load
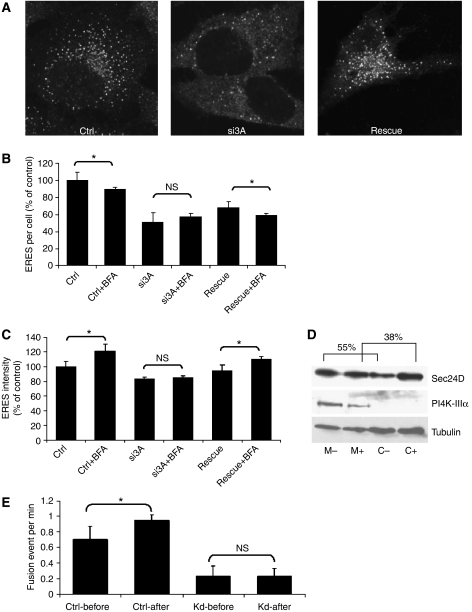
What are the factors controlling the adaptation to cargo load? It was suggested that the presence of the lipid PI4-phosphate in the ER membrane is essential for the formation of ERES (Blumental-Perry et al, 2006). As PI4K-IIIα associates with the ER (Wong et al, 1997), we reasoned that this enzyme might be responsible for generating the ER pool of PI4-phosphate. To test its role, we efficiently silenced PI4K-IIIα by siRNA (Supplementary Figure S1A), which indeed resulted in a strong reduction of ERES number (Figure 3A and B). This remarkable reduction in ERES number was not due to a reduction in the cellular COPII, as the protein level of Sec24A, used as a representative COPII indicator, was unchanged (Supplementary Figure S1A). Importantly, the drop in ERES number was prevented by overexpression of HA-tagged bovine PI4K-IIIα, which is resistant to the siRNA used (Figure 3A–C), thus excluding an off-target effect. As can be expected from the reduction in ERES number, the rate of ER-to-Golgi transport of GFP-tagged VSVG-tsO45 (determined by pulse-chase and EndoH digestion) was reduced compared with control (Supplementary Figure S1B). If the cellular level of COPII in PI4K-IIIα knockdown cells is unchanged, but the ERES number is reduced, we would expect that the cytosolic pool of free COPII is increased. To test this, membrane and cytosolic fractions were prepared and the levels of Sec24 determined. As shown in Figure 3D, the knockdown of PI4K-IIIα indeed resulted in an approximately 20% increase in Sec24 in the cytosolic fraction.
Figure 3.
Effect of knockdown of PI4K-IIIα on ERES and their response to BFA. (A) HeLa cells were transfected with a control siRNA (ctrl) and fixed 72 h later, or with an siRNA to PI4K-IIIα and fixed 72 h later (si3A), or transfected after 48 h with a plasmid encoding HA-tagged PI4K-IIIα and fixed 24 h later (rescue). Fixed cells were stained for Sec31 to label ERES. Evaluation of the number of ERES per cell (B) and their average intensity (C). The results are presented as a percentage of control to account for inter-assay variance. HeLa cells were transfected with a control siRNA. After 72 h, they were treated with a solvent (ctrl) or with 5 μg/ml BFA (ctrl+BFA). HeLa cells were transfected with an siRNA to PI4K-IIIα (si3A). After 72 h, they were treated with a solvent (si3A) or with 5 μg/ml BFA (si3A+BFA). HeLa cells were transfected with an siRNA to PI4K-IIIα. After 48 h, they were transfected with a plasmid encoding HA-tagged PI4K-IIIα and 24 h later they were treated with a solvent (rescue) or with 5 μg/ml BFA (rescue+BFA). (D) HeLa cells were transfected with an siRNA to PI4K-IIIα (+) or with a control siRNA (−). After 72 h, microsomes (M) and cytosol (C) were prepared (see ‘Materials and methods') and 8 μg of each sample was separated by SDS–PAGE. Sec24D, PI4K-IIIα and tubulin were detected by immunoblotting. Sec24D was quantified by densitometry and displayed as percent membrane-bound of total. (E) HeLa cells were transfected with a control siRNA (ctrl) or with an siRNA against PI4K-IIIα (Kd). After 48 h, the cells were transfected with a plasmid encoding YFP-tagged Sec23A as a marker for ERES. Live cell imaging was performed 24 h later. Fusion events were monitored during a period of 8 min before (-before) and 8 min after (-after) addition of 5 μg/ml BFA. *Statistically significant differences (P⩽0.01; non-paired Student's t-test); NS, nonsignificant differences.
Does the silencing of PI4K-IIIα affect the acute response of ERES induced by BFA? As shown above, BFA reduced the number of ERES and increased their intensity. Remarkably, this effect was completely inhibited in cells in which PI4K-IIIα was knocked down (Figure 3B and C) and the response to BFA was rescued by overexpression of the bovine PI4K-IIIα (Figure 3B and C). The results of Figure 1F indicate that BFA increases ERES fusion events. By contrast, the knockdown of PI4K-IIIα reduced ERES fusion activity (Figure 3E). There was a clear reduction in the number of fusion events when PI4K-IIIα was silenced and the number of fusion events did not change to any appreciable amount after treatment with BFA. We can exclude the trivial possibility that the absence of the response of ERES to BFA is due to insensitivity of the Golgi to this drug. Giantin was similarly redistributed to the ER both in control and PI4K-IIIα-depleted cells (Supplementary Figure S1C). We conclude that an adequate response of ERES to an acute increase in cargo load is dependent on PI4K-IIIα.
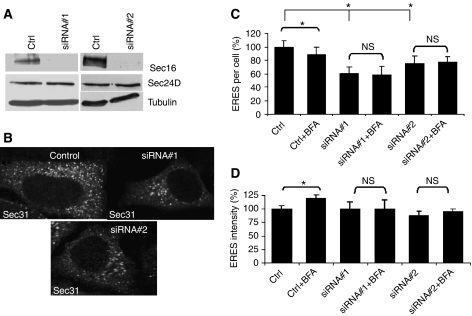
Next, we studied the role of Sec16 using a silencing approach. We used two siRNAs both of which efficiently knocked down Sec16 (Figure 4A) and reduced the number of ERES (Figure 4B and C). This effect was not due to reduced stability of COPII as judged by the levels of Sec24D, which were unchanged (Figure 4A). The knockdown results are consistent with those of Watson et al (2006); Bhattacharyya and Glick (2007) and Iinuma et al (2007). We wondered whether Sec16 is a limiting factor for the acute response. Knockdown of Sec16 indeed inhibited the response of ERES to BFA. Neither the number nor the intensity of ERES was changed (Figure 4C and D). How does this response of ERES compare to other blocks in the early secretory pathway? We choose to study a golgin-84 knockdown, which is known to severely affect Golgi morphology and to impair ER–Golgi transport (Diao et al, 2003). Only ∼20% of the cells displayed a scattered Golgi under control conditions. This value increased to ∼90% in cells treated with the golgin-84 siRNA and to ∼39% in cells treated with PI4K-IIIα siRNA. However, whereas knockdown of PI4K-IIIα reduced the number ERES, no such effect was observed in golgin-84 knockdown cells (Supplementary Figure S1D). Collectively, the results show that both Sec16 and PI4K-IIIα are limiting factors needed for the response of ERES to an acute increase of cargo load.
Figure 4.
Effect of knockdown of Sec16 on ERES. (A) HeLa cells were transfected with a control siRNA (ctrl) or two different siRNAs against Sec16 (siRNA#1, siRNA#2). After 72 h, the cells were lysed and subjected to SDS–PAGE followed by immunoblotting with antibodies against Sec16, Sec24D, and tubulin. (B) HeLa cells were transfected with a control siRNA (control) or with siRNAs against Sec16. After 72 h, the cells were fixed and immunostained for Sec31 to label ERES. (C) Number of ERES per cell and their average intensity (D). *Statistically significant differences (P⩽0.01, non-paired Student's t-test); NS, nonsignificant differences.
Effect of alterations of PI4K-IIIα and Sec16 levels on the chronic response of ERES to increased cargo load
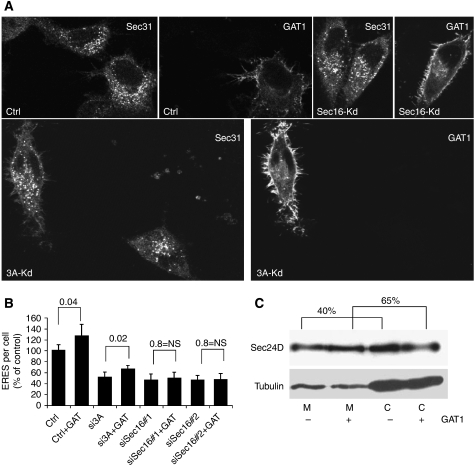
Are PI4K-IIIα and Sec16 also required for the chronic increase of cargo load? In the control sample, overexpression of GAT1 increased the number of ERES by approximately 30% (Figure 5A and B). Silencing PI4K-IIIα did not inhibit this adaptive response. The relative increase in ERES number was again in the range of 30%, although the overall number of ERES was reduced (Figure 5A and B). These results suggest that PI4K-IIIα, although important for the acute adaptation of ERES to an increase in cargo load, does not have a central function in the generation of new ERES. In contrast, knockdown of Sec16 inhibited the increase in ERES number in response to overexpression of GAT1 (Figure 5A and B). This indicates that cells require sufficient Sec16 to generate new ERES in response to higher cargo concentrations. Next, we sought to confirm the increase in ERES number biochemically by measuring membrane-bound and soluble Sec24. Overexpression of GAT1 caused a shift of Sec24 to the membrane fraction (39.9% in cells not expressing GAT1 versus 58.2% in cells overexpressing GAT1) (Figure 5C) and the same was true for Sec16. This result is in line with the increased number of ERES. Collectively, these observations suggest that the response of ERES to a chronic increase in cargo load requires Sec16 but is independent of PI4K-IIIα. This very much contrasts with the acute response that depends on both factors.
Figure 5.
Effect of PI4K-IIIα or Sec16 knockdown on the adaptation of ERES to GAT1 overexpression. HeLa cells were transfected with a control siRNA (ctrl), siRNA to PI4K-IIIα (3A-Kd) or siRNA to Sec16 (Sec16-Kd). After 48 h, the cells were either not transfected or transfected with a plasmid encoding YFP-tagged GAT1 (+GAT) and after another 24 h processed for immunofluorescence to label ERES by anti-Sec31. (A) Representative images. (B) Graphical representation of the number of ERES per cell expressed as a percentage of control. Numbers above the bars indicate P-values (paired Student's t-test). (C) HeLa cells grown in six-well plates were transfected 24 h after plating with a plasmid encoding YFP-tagged GAT1 (+) or with an empty plasmid (−). After 24 h, microsomes (M) and cytosol (C) were prepared and 8 μg protein of each sample was separated by SDS–PAGE. Sec24D and tubulin (loading control) were detected by immunoblotting. Membrane-bound Sec24 is indicated as a percentage of total. Note that GAT1 overexpression increases the membrane-bound fraction of Sec24.
A chronic increase in cargo load induces Sec24 and Sec16
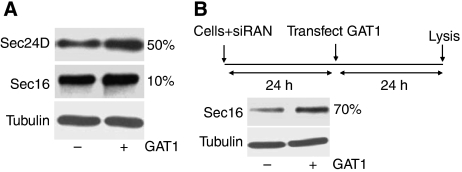
The increase in the number of ERES as part of an adaptive response to higher cargo load raises the important question of whether the steady-state levels of COPII are sufficient for the chronic response. Can the cells recruit enough COPII from the cytosol to the ER membrane, or do they require de novo synthesis of COPII? To address this question, we compared the total cellular levels of Sec24D (as a representative subunit of COPII) and Sec16 (as an organizer of ERES) between control cells and those that were forced to overexpress GAT1. Figure 6A shows that there was a clear induction of Sec24D in cells that overexpressed GAT1 by ∼50%. Sec16 levels only marginally increased (∼10%; Figure 6A), although the lack of an adaptive response in cells with reduced Sec16 levels indicated that a critical level of Sec16 is needed. The steady-state levels may largely suffice to drive the response and this may explain the relatively weak increase in Sec16 expression. To strengthen the evidence that cargo overload induces Sec16, we took a more sensitive approach. The levels of Sec16 were first lowered by an exposure to siRNA for 24 h. Thereafter, GAT1 was overexpressed for an additional 24 h and the levels of Sec16 were determined. We reasoned that when the amount of Sec16 is lowered, and thus becomes limiting, the cells must induce its expression to adapt to the increase in cargo load by generating new ERES. This was indeed the case. Overexpression of GAT1 resulted in a strong induction of Sec16 under these conditions (Figure 6B).
Figure 6.
Effect of GAT1 overexpression on Sec24D and Sec16. (A) HeLa cells were transfected with a plasmid encoding YFP-tagged GAT1 (+) or with an empty plasmid (−). After 24 h, total cell lysates were separated by SDS–PAGE. Sec24D and Sec16 were detected by immunoblotting and quantified using tubulin as a loading control. Numbers at the right margin of the immunoblots indicate the percentage of induction of expression in the sample expressing GAT1 versus the sample transfected with the empty plasmid. (B) HeLa cells were transfected with siRNA#1 against Sec16. After 24 h, the medium was removed and the cells were either transfected with a plasmid encoding YFP-tagged GAT1 (+) or with an empty plasmid (−). The cells were lysed after 24 h and 8 μg of lysate was separated by SDS–PAGE. Sec16 was detected by immunoblotting and quantified using tubulin as a loading control. The upper panel shows an outline of the experiment. Numbers at the right margin of the immunoblot indicate the percentage of induction of Sec16 by GAT1 overexpression.
Effect of an increase in ERES number on COPII vesicle production rate
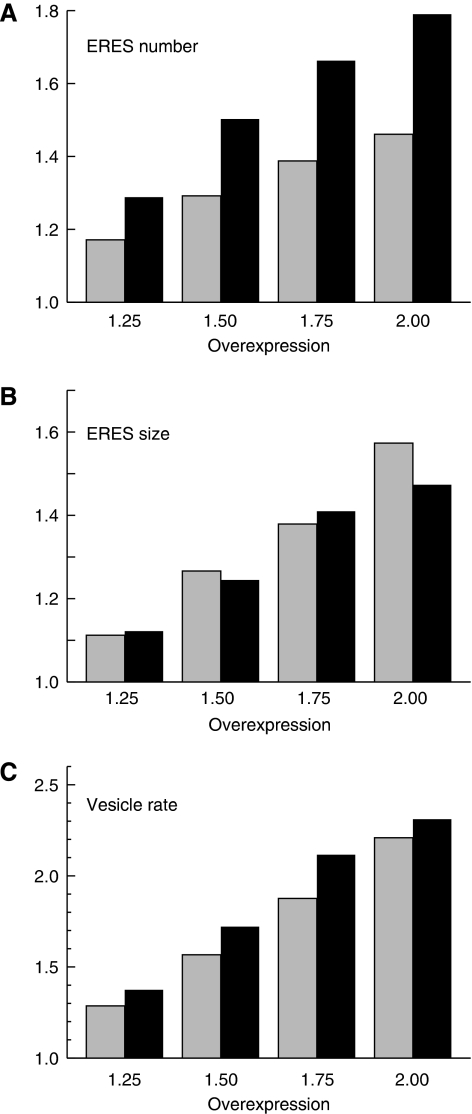
Recently, Heinzer et al (2008) developed a quantitative computational model for the process of ERES formation. In good agreement with our experimental results, this model predicts that slowing down the COPII turnover (e.g. by transiently increasing the amount of cargo) leads to an increase in the average ERES size, whereas the number of ERES slightly decreases. This is due to a longer residence time of COPII proteins on ER membranes, which induces a higher fusion probability of ERES-like clusters, in agreement with our above hypothesis. Moreover, the model predicted that an increase in ERES number decreases the rate of COPII vesicle production when the cellular COPII levels are kept constant (Heinzer et al, 2008). Thus, if a given amount of COPII components is used to generate more ERES, these ERES are expected to be smaller and less efficient. By contrast, in the current study, we observed a rise in the number of ERES after a chronic increase in cargo load. Moreover, the expression of Sec24 (and to a lesser extent Sec16) was induced. Using the Heinzer et al (2008) model, we simulated the impact of an increase in COPII levels on the number and size of ERES and on the vesicle production rate. The simulation was performed under the assumption that the rate of association and dissociation of COPII to and from the membrane is equal (kon=koff), as described by Heinzer et al (2008). In addition, we carried out the simulation with two values for kon (0.1 and 0.2/s). As shown in Figure 7, an increase in COPII levels increases both the size and number of ERES. Importantly, the vesicle production rate does not suffer from this increase in ERES number, but rather increases in parallel (Figure 7C). Our simulation supports the notion that the induction of COPII expression and the subsequent de novo biogenesis of ERES is part of an adaptive response to render ER export more efficient.
Figure 7.
Simulation of the effect of increasing COPII levels on ERES size and number, and on the rate of COPII vesicle production. Increase in the mean number (A) and size (B) of ERES with rising cellular COPII levels. The amount of overexpression is determined with regard to the putative native level investigated in Heinzer et al (2008). Grey bars represent the data for a slow COPII turnover (kon=koff=0.1/s), whereas black bars indicate the respective values for a faster turnover (kon=koff=0.2/s). The increase in ERES size and number, as a function of COPII levels, is very similar for both turnover rates. (C) The rate of vesicle production continues to increase proportionally with increasing COPII levels for both turnover rates (grey bars: slow turnover; black bars: fast turnover).
The chronic response to an increase in cargo load requires the UPR
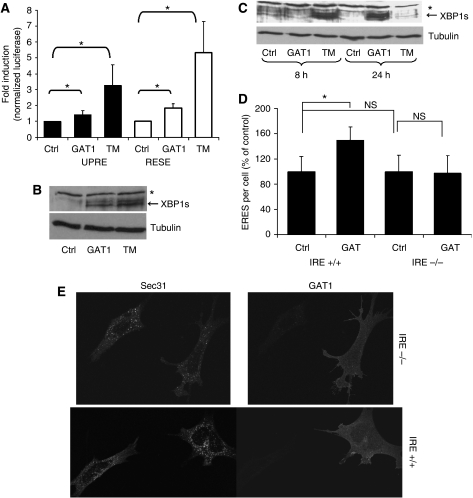
What is the molecular mechanism underlying the observed induction of Sec16 and Sec24 expression in response to GAT1 overexpression? We reasoned that forcing cells to synthesize more protein may challenge the folding machinery and thereby induce the UPR. We first tested whether overexpression of GAT1 stimulates the UPR. Two reporters were used in which either the UPR-responsive element (UPRE) or the ER stress-responsive element (ERSE) was engineered upstream of firefly luciferase. As a positive control, cells were treated with tunicamycin, which inhibits protein glycosylation and thereby exerts a strong ER stress. Overexpression of GAT1 significantly induced both UPRE and ERSE, indicating that it induced UPR although to a lower extent than tunicamycin (Figure 8A).
Figure 8.
Chronic cargo overload triggers the UPR. (A) HeLa cells grown in 12-well plates were transfected with expression vectors encoding firefly luciferase under the control of ERSE or UPRE. Transfections included an empty plasmid (ctrl) or YFP-tagged GAT1 (GAT1). Renilla luciferase was used to normalize for transfection efficiency. The ratio of plasmid to ERSE/UPRE to Renilla was always 2:2:1. The luciferase assay was performed 24 h after transfection. TM, treatment with tunicamycin (10 μg/ml) for 5 h. (B) HeLa cells grown in six-well plates were transfected with an empty plasmid (ctrl) or with a plasmid encoding YFP-tagged GAT1 (GAT1). On the following day, one well transfected with the empty plasmid was treated with tunicamycin (10 μg/ml) for 8 h (TM). The cells were lysed after 24 h in RIPA buffer and separated by SDS–PAGE. XBP1s was detected by immunoblotting. *Nonspecific band. The immunoblot was stripped and tubulin was stained to demonstrate equal loading. (C) HeLa cells grown in six-well plates were transfected with an empty plasmid or with a plasmid encoding YFP-tagged GAT1 (GAT1). Two samples transfected with an empty plasmid were treated either with solvent (ctrl) or with 10 μg/ml tunicamycin (TM) for 8 and 24 h. The cells were lysed after 8 or 24 h in RIPA buffer and separated by SDS–PAGE. XBP1s was detected by immunoblotting. *Nonspecific band. The stripped blot was re-stained for tubulin to demonstrate equal loading. (D) MEFs derived from wild-type mice (IRE+/+) or mice lacking IRE1 (IRE−/−) were electroporated with a plasmid encoding YFP-tagged GAT1. After 24 h, the cells were fixed and ERES were stained with anti-Sec31. (C) A graphic representation of the number of ERES per cells as a comparison between cells that express GAT1 (GAT1) and cells that do not (ctrl). Cells of comparable size were chosen for this analysis. (E) Representative images of ERES in cells expressing or not expressing GAT1. *Statistically significant differences (P⩽0.05; paired Student's t-test); NS, nonsignificant differences.
To further test a possible involvement of the UPR, we studied the expression of the spliced form of XBP1 (XBP1s), an endogenous UPR marker. XBP1 is a ∼30 kDa cytosolic protein. Upon induction of ER stress, its mRNA is spliced by inositol-requiring protein 1 (IRE1) that results in the synthesis of a protein of ∼55 kDa termed XBP1s. As shown in Figure 8B, XBP1s was absent in control cells but strongly induced in cells treated with tunicamycin. Remarkably, overexpression of GAT1 also induced the expression of XBP1s. In addition, we followed the time course of the induction of XBP1s. As shown in Figure 8C, tunicamycin induced XBP1s robustly after 8 h, but the levels of XBP1s were low again after 24 h. This kinetics is in line with the observations of Lin et al (2007) who reported that IRE1 activity (XBP1s is a direct readout of IRE1 activity) was attenuated by prolonged tunicamycin-induced ER stress. The situation was strikingly different for GAT1. No appreciable XBP1s was detected 8 h after transfection, but XBP1s was strongly induced after 24 h. These findings support the conclusion that overexpression of GAT1 induces UPR. We rule out the possibility that the UPR has any role in our knockdown experiments as neither the knockdown of Sec16 nor the knockdown of PI4K-IIIα induces UPR (data not shown). Furthermore, it is important to note that knockdowns of Sec16 or PI4K-IIIa did not increase the expression of Sec24 (Figure 4A; Supplementary Figure S1A).
To directly link the increase in ERES number to the induction of UPR, mouse embryonic fibroblasts (MEFs) from wild-type mice was compared to those derived from mice lacking IRE1, a key regulator of UPR. As in HeLa cells, GAT1 reached the plasma membrane in wild-type and IRE−/− MEFs, showing that there is no visible trafficking defect in these cells. Overexpression of GAT1 caused a significant increase in ERES number in wild-type MEFs (Figure 8C–E). Remarkably, no such effect was observed in MEFs lacking IRE1, where the number of ERES remained unchanged (Figure 8C and D). These results provide strong evidence that the response of ERES to a chronic increase in cargo load is dependent on IRE1 and therefore on the UPR.
Discussion
The secretory pathway has to adapt to changes in traffic load, and this response may vary depending on whether these changes are transient or permanent. In the current study, we directly compared the adaptive response of ERES to acute and chronic increases in cargo load. The major new findings can be summarized as follows: (i) an acute increase in cargo load results in larger ERES due to an increase in fusion frequency of ERES and this adaptation is dependent on PI4K-IIIα and Sec16; (ii) a chronic increase in cargo load induces the formation of new, larger ERES. This response is dependent on Sec16 but independent of PI4K-IIIα; and (iii) the generation of new ERES in response to chronic cargo overload requires the IRE1-mediated UPR.
As there are membrane-bound and free cytosolic pools of both COPII and Sec16 (Guo and Linstedt, 2006; Watson et al, 2006; Bhattacharyya and Glick, 2007; Iinuma et al, 2007; this study), one would intuitively assume that an acute need to export more cargo would induce the recruitment of cytosolic COPII to ERES. This is indeed what we observed. The accompanying decrease in ERES number, however, requires an additional explanation. Our finding that the fusion frequency increased under these conditions offers a plausible explanation. The current study also provides the first mechanistic insights into the adaptation process. We found that a critical amount of PI4K-IIIα and Sec16 is a prerequisite for the acute adaptive response of ERES. Although PI4-phosphate is required to promote COPII-mediated ER export (Blumental-Perry et al, 2006) the kinase responsible has not been identified previously. Here, we unequivocally identified PI4K-IIIα as the kinase involved in the homoeostasis of ERES based on the following observations. (i) Knockdown of PI4K-IIIα reduced the number of ERES, and (ii) this reduction was rescued by transfected bovine PI4K-IIIα. (iii) Knockdown of PI4K-IIIα inhibited the adaptive response of ERES to an acute increase in cargo load, and (iv) this response was rescued when PI4K-IIIα was reintroduced exogenously.
Why is PI4-phosphate important? Using purified yeast COPII components, Matsuoka et al (1998) showed that the presence of PI4-phosphate increases COPII binding to synthetic liposomes. This is most likely due to the presence of basic (positively charged) residues on the membrane-facing side of the Sec23–Sec24 dimer (Bi et al, 2002). The same structural consideration appears to hold true for the mammalian dimer as shown in Supplementary Figure S2, in which we labelled in yellow all lysine and arginine residues on the membrane-facing side of the Sec23A–Sec24A dimer, the structure of which has recently been established (Mancias and Goldberg, 2007). Thus, the increased presence of PI4-phosphate can be expected to favour COPII binding and thereby contribute to increased COPII vesicle production.
Why does the cell respond to an acute cargo overload by making bigger but less ERES? The computational model for the self-organization of ERES of Heinzer et al (2008) predicts that, if cells need to increase their anterograde secretory flux, the average size of ERES ought to increase. This is exactly what we observed. The model further predicts that the cooperative binding of COPII enlarges ERES. A potential candidate protein that may mediate this cooperativity is Sec16, which is thought to form a scaffold for the docking of COPII complexes (Gimeno et al, 1996). Consistent with this notion is our observation that silencing Sec16 reduced the number of ERES and inhibited the adaptation to increasing cargo load. Furthermore, our results provide evidence for the involvement of lipid modifications controlled by PI4K-IIIα. Silencing PI4K-IIIα not only reduced the number of ERES but also inhibited the acute adaptive response to higher cargo load. We propose that PI4K-IIIα contributes to the formation of PI4-phosphate at the ER, which, together with Sec16, forms a lipid–protein scaffold to maximize COPII assembly. PI4-phosphates may decrease the koff of COPII components on ERES and thereby further prolong the residence time of COPII at the ER membrane. Such a decrease in the koff was predicted by Heinzer et al (2008) to result in bigger ERES.
Unlike the acute response, the chronic response to cargo overload results in more ERES paralleled by an induction of COPII expression levels. We also provide evidence that Sec16 levels are induced when its amount becomes limiting. We propose that the increase in cellular COPII in conjunction with Sec16 augments the vesicle production rate. This is supported by the calculations according to the Heinzer et al (2008) computational model (Figure 7). Interestingly, a knockdown of PI4K-IIIα did not inhibit the formation of new ERES, although it reduced the total number of ERES. As we are working with knockdown and not knockout cells, we cannot be certain that cargo-induced formation of new ERES is entirely independent of PI4K-IIIα. Low levels of this enzyme may suffice. However, the clear effect of the PI4K-IIIα knockdown on the number of ERES, on ER-to-Golgi transport of VSVG and on the acute response leads us to conclude that PI4K-IIIα does not have a prominent function in chronic response. This finding is important because it contributes to our understanding of how ERES form. Although Sec16 and PI4-phosphate are important for ERES formation, it was not known previously which of them comes first in the process of ERES biogenesis. Our results suggest that Sec16 is needed to nucleate new ERES, but to maintain them both Sec16 and PI4K-IIIα are needed. This clearly places Sec16 proximal to PI4K-IIIα in the process of ERES formation. Is Sec16 the landmark on the ER for the nucleation of new ERES or is Sec16 recruited by bound COPII? Interestingly, a mutant of GAT1 (GAT1-RL/AS) that does not bind COPII (Farhan et al, 2007) is unable to induce new ERES (unpublished results). Similarly, cargo-induced formation of new ERES in plants is dependent on an intact ER export motif (Hanton et al, 2007). These findings suggest that increased COPII binding to cargo on the ER leads to the recruitment of Sec16, which stabilizes COPII on the membrane through cooperative binding.
Another remarkable finding of our study is that the generation of new ERES in response to chronic cargo overload is dependent on the IRE1-controlled UPR. This conclusion is supported by the following observations: (i) overexpression of GAT1 induced luciferase activity from a vector encoding luciferase under the control of either UPRE or ERES; (ii) overexpression of GAT1 induced the expression of the active (spliced) XBP1s; and (iii) cells lacking IRE1 did not increase the number of their ERES in response to GAT1 overexpression. The principles of the UPR are now well defined (Schröder and Kaufman, 2005; Ron and Walter, 2007). In response to ER stress due to an excess of unfolded proteins, the UPR reduces the protein load that enters the ER, increases the capacity of the ER to handle unfolded proteins, and, if homoeostasis cannot be reestablished, triggers apoptosis. IRE1 exerts an effect as one of three different ER stress transducers through the transcription factor XBP1/HAC1. Some previous studies hinted at a relationship of UPR and the secretory pathway. For instance, treatment of yeast cells with UPR-stimulating agents caused an induction of the mRNA of chaperones for the improvement of the folding capacity and of key proteins required for ER to Golgi membrane traffic (Travers et al, 2000). Moreover, overexpression of IRE1 suppresses temperature-sensitive Sec mutants defective in vesicle budding from the ER in Saccharomyces cerevisiae, including mutants in COPII components and Sec16 (Sato et al, 2002). Several lines of evidence now suggest that the UPR in higher eukaryotes contributes to the coupling of ER size and physiological demand, and augments the capacity of protein secretion. For instance, the conversion of resting B-lymphocytes to antibody-secreting plasma cells involves expansion of the secretory apparatus, a process that is strictly dependent on IRE1 (Zhang et al, 2005). XBP1-null B cells fail to upregulate many genes encoding secretory pathway components (Shaffer et al, 2004). A genome-wide screen identified XBP1-binding promoter elements in numerous genes of proteins involved in membrane trafficking including several COPII components (Acosta-Alvear et al, 2007), explaining why overexpression of the spliced (active) form of XBP1 induces the expression of COPII components and cargo receptors (Sriburi et al, 2007). Furthermore, a knockout of XBP1 in mice prevents the full development of the secretory apparatus in exocrine cells, presumably due to an imbalance of cargo load and the capacity of the ER to process and export excess cargo (Lee et al, 2005). Our current findings now clearly link the UPR to adaptive responses of the earliest station of the secretory pathway, the ERES. This link is through induction of COPII components. To the best of our knowledge, this has never been shown before and thus is an important further step in understanding the coordination of cargo load and vesicle traffic.
In summary, the findings of the current study indicate the following sequence of events in response to cargo overload. Cargo overload leads to an immediate acute reaction intended to increase the capacity of COPII-mediated ER export. To maximize this process, the scaffold protein Sec16 is recruited from the cytosol to ERES to increase the size of ERES. These ERES are further stabilized by the increased formation of PI4-phosphate by the action of PI4K-IIIα. If cargo overload persists, the UPR is triggered and new ERES are formed to cope with this chronic situation. The formation of new ERES requires the scaffold protein Sec16 but no longer PI4K-IIIα. Thus, acute and chronic adaptations of ERES to an increase in cargo load are different both in terms of phenotypic changes and the regulatory machinery.
Materials and methods
Cell culture, transfection, siRNAs, and cDNAs
HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. For reverse transfection with siRNA, HeLa cells were seeded into six-well plates and transfected with 5 nM siRNA using HiPerFect (Qiagen, Hilden, Germany). The cells were typically analysed 72 h after transfection. In the case where cDNA was transfected, the transfection was performed using FuGene6 (Roche) 24 h prior to analysis. Wild-type and Ire1a knockout MEFs were prepared from littermates bred in a C57Bl6 background (Lee et al, 2002). MEFs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, antibiotics, and non-essential amino acids. For the expression of YFP-tagged GAT1, 50% confluent MEFs were trpysinized and 2 × 106 were electroporated with 3.5 μg plasmid DNA using the Amaxa Nucleofector, program T-20, and MEF solution 2 (Amaxa Biosystems). The cells were analysed 24 h after electroporation. The following siRNAs (Qiagen) were used: siRNA against Sec16 (#1) targeting the sequence CACGGTTAGACTCACTGTTAA; siRNA against Sec16 (#2) targeting the sequence CCAGGTGTTTAAGTTCATCTA; siRNA against PI4K-IIIα: Hs_PIK4CA_11_HP validated siRNA; Cat. no. SI02777383. cDNAs: YFP–GAT1 (Farhan et al, 2007), YFP–Sec23A (kind gift of R Pepperkok), VSVG–YFP (kind gift of K Simons), ERSE- and UPRE-luciferase (kind gift of HH Sitte) and HA-PI4K-IIIα (kind gift of T Balla).
Live cell microscopy
HeLa cells were cultured on 18 mm round glass coverslips. They were then transferred to imaging medium 1 (Ham's F12 supplemented with 20 mM HEPES, pH 7.4) in a Ludin chamber (Life Imaging Services GmbH, Switzerland) and imaged with a × 100 Plan-Apochromat oil objective on a Zeiss Axiovert 135M microscope at 37°C. Images were taken with a CCD camera (SensiCam; PCO Computer Optics) every 10 s using a filter wheel to switch between excitation and emission wavelengths. ImagePro® Plus software (Media Cybernetics®) was used for both recording and movie processing.
Modelling
Simulations were performed as described previously (Heinzer et al, 2008) using kon=koff for the COPII kinetics with kon=0.1, 0.2/s and varying amounts of COPII overexpression (with respect to the previously used amount of available COPII proteins). For consistency with the experimental approach, the number of ERES was determined by considering only clusters with a size larger than three COPII vesicles. The size of an ERES-like cluster was determined by the number of participating COPII complexes (a quantity that is proportional to the experimentally used total fluorescence of an ERES). The rate of vesicle production was determined as described (Heinzer et al, 2008).
Luciferase assay
HeLa cells were plated into 12-well plates. After 24 h, the cells were transfected with plasmids for GAT1 or with pcDNA4, together with the indicated reporter plasmid (UPRE or ERSE), and a plasmid encoding Renilla luciferase as an internal transfection control. After 24 h, the luciferase assay was performed using the DualLuciferase® kit (Promega) according to the manufacturer's instructions.
Immunofluorescence microscopy and antibodies
HeLa cells were cultured on 18 mm round glass coverslips. For immunofluorescence, the cells were fixed with 3% paraformaldehyde (pH 7.4) for 30 min at room temperature. The cells were permeabilized with PBS supplemented with 20 mM glycine, 0.2% Triton X-100, and 3% bovine serum albumin. Images were acquired using a LeicaSPE confocal microscope using the × 40 oil objective. When acquisition was for the purpose of counting ERES, images were acquired with a 1.5-fold magnification. For imaging MEFs, the × 63 oil objective and a 1.5-fold magnification was used. The raw images were converted to TIF files and analysed using the ImagePro® Plus software. This software recognizes 255 potential grey values. ERES were defined as bright Sec31-positive objects that were in the top 80% of this intensity scale and had a diameter of more than 1.5 pixels. This evaluation procedure allows exclusion of objects that are too dim and too small and thereby avoids counting of false objects that may occur due to pixelization of images. Rabbit polyclonal antibodies against the following proteins were used: Sec31 (kind gift of F Gorelick; Shugrue et al, 1999), PI4K-IIIα (Cell Signaling), Sec16 (Iinuma et al, 2007), Sec24A (kindly provided by JP Paccaud), Sec24D (Wendeler et al, 2007), giantin (kind gift of AD Linstedt), and XBP1s (Biolegend). Mouse monoclonal antibodies: anti-tubulin (kind gift of K Matter), anti-GM130 (BD Biosciences). Secondary antibodies (Invitrogen): Alexa568 goat anti-rabbit for single staining; Alexa488 goat anti-rabbit and Alexa568 goat anti-mouse for co-staining.
Supplementary Material
Supplementary Figures S1 and S2
Acknowledgments
We thank T Balla (NIH, Bethesda), F Gorelick (Yale University, New Haven), R Pepperkok (EMBL, Heidelberg), K Simons (MPI, Dresden) M Freissmuth and HH Sitte (Medical University of Vienna), and J-P Paccaud (Geneva) for reagents. This study was supported by the Swiss National Science Foundation and the University of Basel. HF was supported by a Schrödinger fellowship from the Austrian Science Foundation (FWF) and an EMBO long-term fellowship. MW was supported by the Institute for Modeling and Simulation in the Biosciences (BIOMS) Heidelberg. Part of this study was supported by NIH grants RO1 DK042394, R01 HL052173, and PO1 HL057346. RJK is an Investigator of the Howard Hughes Medical Institute.
References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66 [DOI] [PubMed] [Google Scholar]
- Bevis BJ, Hammond AT, Reinke CA, Glick BS (2002) De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol 4: 750–756 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya D, Glick BS (2007) Two mammalian Sec16 homologues have nonredundant functions in endoplasmic reticulum (ER) export and transitional ER organization. Mol Biol Cell 18: 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Corpina RA, Goldberg J (2002) Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419: 271–277 [DOI] [PubMed] [Google Scholar]
- Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M (2006) Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell 11: 671–682 [DOI] [PubMed] [Google Scholar]
- Diao A, Rahman D, Pappin DJ, Lucocq J, Lowe M (2003) The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol 160: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Russand G, Yewdell JW (1989) Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol 109: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H, Reiterer V, Korkhov VM, Schmid JA, Freissmuth M, Sitte HH (2007) Concentrative export from the endoplasmic reticulum of the gamma-aminobutyric acid transporter 1 requires binding to SEC24D. J Biol Chem 282: 7679–7689 [DOI] [PubMed] [Google Scholar]
- Forster R, Weiss M, Zimmermann T, Reynaud EG, Verissimo F, Stephens DJ, Pepperkok R (2006) Secretory cargo regulates the turnover of COPII subunits at single ER exit sites. Curr Biol 16: 173–179 [DOI] [PubMed] [Google Scholar]
- Gimeno RE, Espenshade P, Kaiser CA (1996) COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol Biol Cell 7: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Linstedt AD (2006) COPII–Golgi protein interactions regulate COPII coat assembly and Golgi size. J Cell Biol 174: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Renna L, Matheson LA, Brandizzi F (2007) De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiol 143: 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzer S, Wörz S, Kalla C, Rohr K, Weiss M (2008) A model for the self-organization of exit sites in the endoplasmic reticulum. J Cell Sci 121: 55–64 [DOI] [PubMed] [Google Scholar]
- Iinuma T, Shiga A, Nakamot K, O'Brien MB, Aridor M, Arimitsu N, Tagaya M, Tani K (2007) Mammalian Sec16/p250 plays a role in membrane traffic from the endoplasmic reticulum. J Biol Chem 282: 17632–17639 [DOI] [PubMed] [Google Scholar]
- Lee AH, Chu GC, Iwakoshi NN, Glimcher LH (2005) XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J 24: 4368–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ (2002) IRE1-mediated nonconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16: 452–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20: 87–123 [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318: 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56: 801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J (2007) The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol Cell 26: 403–414 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T (1998) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275 [DOI] [PubMed] [Google Scholar]
- Mezzacasa A, Helenius A (2002) The transitional ER defines a boundary for quality control in the secretion of tsO45 VSVG glycoprotein. Traffic 3: 833–849 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch WE (1999) A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem 274: 15937–15946 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A (2002) Evidence for the intimate relationship between vesicle budding from the ER and the unfolded protein response. Biochem Biophys Res Commun 296: 560–567 [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM (2004) XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21: 81–93 [DOI] [PubMed] [Google Scholar]
- Shugrue CA, Kolen ER, Peters H, Czernik A, Kaiser C, Matovcik L, Hubbard AL, Gorelick F (1999) Identification of the putative mammalian orthologue of Sec31P, a component of the COPII coat. J Cell Sci 112: 4547–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW (2007) Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem 282: 7024–7034 [DOI] [PubMed] [Google Scholar]
- Stephens DJ (2003) De novo formation, fusion and fission of mammalian COPII-coated endoplasmic reticulum exit sites. EMBO Rep 4: 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101: 249–258 [DOI] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ (2006) Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic 7: 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler MW, Paccaud JP, Hauri HP (2007) Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep 8: 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Meyers R, Cantley LC (1997) Subcellular localization of phosphatidylinositol 4-kinase isoforms. J Biol Chem 272: 13236–13241 [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ (2005) The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest 115: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1 and S2