Abstract
The homotypic fusion of yeast vacuoles, each with 3Q- and 1R-SNARE, requires SNARE chaperones (Sec17p/Sec18p and HOPS) and regulatory lipids (sterol, diacylglycerol and phosphoinositides). Pairs of liposomes of phosphatidylcholine/phosphatidylserine, bearing three vacuolar Q-SNAREs on one and the R-SNARE on the other, undergo slow lipid mixing, but this is unaffected by HOPS and inhibited by Sec17p/Sec18p. To study these essential fusion components, we reconstituted proteoliposomes of a more physiological composition, bearing vacuolar lipids and all four vacuolar SNAREs. Their fusion requires Sec17p/Sec18p and HOPS, and each regulatory lipid is important for rapid fusion. Although SNAREs can cause both fusion and lysis, fusion of these proteoliposomes with Sec17p/Sec18p and HOPS is not accompanied by lysis. Sec17p/Sec18p, which disassemble SNARE complexes, and HOPS, which promotes and proofreads SNARE assembly, act synergistically to form fusion-competent SNARE complexes, and this synergy requires phosphoinositides. This is the first chemically defined model of the physiological interactions of these conserved fusion catalysts.
Keywords: membrane fusion, reconstitution, SNARE chaperones, regulatory lipids
Introduction
Membrane fusion is required for protein trafficking, cell surface growth, hormone secretion and neurotransmission. It is catalyzed by conserved proteins and lipids that become enriched in fusion-competent microdomains (Lang et al, 2001; Miaczynska and Zerial, 2002; Wang et al, 2002; Fratti et al, 2004). Rab GTPases in their GTP-bound state cooperate with Rab:GTP-binding proteins (‘effectors') to tether organelles, providing an initial layer of specificity (Novick and Zerial, 1997; Grosshans et al, 2006). Further specificity is provided by the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins (McNew et al, 2000; Parlati et al, 2000; Jahn and Scheller, 2006), distinguished by their heptad-repeat SNARE domains that form 4-helical bundles (Poirier et al, 1998; Sutton et al, 1998), in cis (anchored to one membrane) or in trans (anchored to apposed membranes). SNARE complex assembly and disassembly is governed by distinct SNARE chaperones. αSNAP (soluble N-ethylmaleimide-sensitive factor attachment protein) (Sec17p) and NSF (N-ethylmaleimide-sensitive factor) (Sec18p) perform ATP-driven SNARE complex disassembly (Söllner et al, 1993). Sec1p/Munc18-1p family (SM) proteins associate with SNAREs and SNARE complexes to permit trans-SNARE complex assembly (Rizo and Südhof, 2002; Dulubova et al, 2007; Shen et al, 2007). Membrane fusion also requires specific lipids. Diacylglycerol (DAG), which can trigger the fusion of liposomes (Siegel et al, 1989), is required for the fusion of isolated organelles (Fratti et al, 2004; Jun et al, 2004). Sterols support fusion (Kato and Wickner, 2001; Fratti et al, 2004), perhaps by stabilizing fusion-competent microdomains (Valdez-Taubas and Pelham, 2003). Phosphoinositides can bind fusion proteins (Cheever et al, 2001; Fratti et al, 2004; Stroupe et al, 2006) in a common microdomain (Miaczynska and Zerial, 2002; Fratti et al, 2004).
SNARE proteins, discovered in the neuronal synapse, are vital for membrane fusion in all eukaryotic cells (Jahn and Scheller, 2006). They associate through their conserved SNARE domains into SNARE complexes. The buried, conserved 0-layer at the centre of the 4-helical SNARE complex has three glutamyl residues and one arginyl residue. It is the basis for classifying SNAREs as Q- or R-SNAREs (Fasshauer et al, 1998). Most SNAREs have a C-terminal trans-membrane anchor.
SNARE proteins that function together in the cell can mediate lipid mixing between proteoliposomes (Weber et al, 1998; McNew et al, 2000), suggesting that SNAREs are the engine of fusion and determinants of fusion specificity and that other factors only regulate SNARE-driven fusion. Whereas SNAREs alone can drive fusion (Nickel et al, 1999), they can also cause substantial lysis of reconstituted proteoliposomes, ‘RPLs,' or yeast vacuoles (Dennison et al, 2006; Starai et al, 2007). Synaptotagmin can associate with neuronal SNAREs in RPLs to enhance fusion and provide Ca2+ regulation (Tucker et al, 2004; Bhalla et al, 2006). However, most intracellular fusion does not use Ca2+ or synaptotagmin. The inclusion of Sec1p during the formation of RPLs with yeast exocytic SNAREs enhances their rate of lipid mixing (Scott et al, 2004), and stronger stimulation is seen with Munc18-1p and neuronal SNARE liposomes (Shen et al, 2007). Although there has been progress in reconstituting regulated neuronal SNARE-driven lipid mixing (Tucker et al, 2004; Shen et al, 2007), there has been less progress with RPL reactions with non-neuronal SNAREs.
We study membrane fusion with yeast vacuoles. Vacuole fusion (Ostrowicz et al, 2008) requires the Rab-GTPase Ypt7p, the heterohexameric HOPS (homotypic fusion and vacuole protein sorting)/Vps Class C complex (Seals et al, 2000; Wurmser et al, 2000), four vacuolar SNAREs (the Q-SNAREs Vam3p, Vti1p and Vam7p and the R-SNARE Nyv1p), SNARE disassembly chaperones (Sec17p/Sec18p) and several chemically minor but functionally vital regulatory lipids (ergosterol (ERG), DAG and phosphoinositides). The HOPS complex, which includes an SM protein (Vps33p) and acts downstream of Ypt7p (Starai et al, 2007), has been isolated in active form (Stroupe et al, 2006) and can bind SNAREs such as Vam7p (Stroupe et al, 2006), Vam3p (Sato et al, 2000; Dulubova et al, 2001) and the SNARE complex (Collins et al, 2005; CMH and WW, unpublished data) as well as phosphoinositides (Stroupe et al, 2006). HOPS and the regulatory lipids are continuously needed until fusion (Jun et al, 2006) rather than only regulating trans-SNARE complex formation. Trans-SNARE complex can undergo continuous remodelling by Sec17p/Sec18p (Jun et al, 2007), whereas HOPS permits SNARE complex assembly and proofreads its structure (Starai et al, 2008). Nevertheless, liposomes bearing only vacuolar SNAREs can undergo lipid mixing (Fukuda et al, 2000), much like liposomes with their neuronal counterparts (Weber et al, 1998). Understanding the physiological fusion machinery will require chemically defined in vitro reactions that need more than SNARE proteins.
Our current studies began by reproducing the capacity of PC/PS (phosphatidylcholine/phosphatidylserine) liposomes with vacuolar SNAREs to undergo lipid mixing (Fukuda et al, 2000). This lipid mixing is insensitive to HOPS and is blocked by Sec17p/Sec18p. To seek reconstitution conditions that require these physiological factors, we varied the lipid composition, the presence of the SNAREs on one or the other fusion-partner liposome and the addition of Sec17p/Sec18p, HOPS and regulatory lipids. With vacuolar lipid composition, rapid lipid mixing requires the four SNAREs, Sec17p/Sec18p, HOPS and regulatory lipids. Our studies of this reconstituted system reveal clear requirements for specific lipids and a novel, phosphoinositide-dependent interplay of the HOPS complex, which promotes and proofreads SNARE complex assembly (Starai et al, 2008), and Sec17p/18p, which promote disassembly, to facilitate productive SNARE pairing and fusion. This reconstituted system drives complete membrane fusion without lysis, fulfilling the definition of physiological fusion.
Results
To reconstitute yeast vacuole membrane fusion, we purified recombinant GST-Vam3p, Vti1p, Vam7p and Nyv1p (Supplementary Figure S1A) and removed the GST moiety of GST-Vam3p by TEV protease. Proteoliposomes were reconstituted by dialysis from detergent micellar solutions of defined lipid mixtures and untagged SNAREs (Supplementary Figures S1B–I and Table SI). In addition to liposome pairs bearing 3Q or 1R SNARE (Fukuda et al, 2000), we prepared proteoliposomes bearing all four SNAREs, as in the organelle. We employed three lipid compositions (Supplementary Figure S1 and Table SI): (1) PC/PS, (2) a vacuolar lipid composition (Zinser et al, 1991; Schneiter et al, 1999) that contained PC, PE (phosphatidylethanolamine), PI (phosphatidylinositol), PS, PA (phosphatidic acid), CL (cardiolipin) and ERG and (3) vacuole lipids plus the ‘regulatory' lipids DAG, PI(3)P (phosphatidylinositol 3-phosphate) and PI(4,5)P2 (phosphatidylinositol 4,5-bisphosphate), which are required for in vitro vacuole fusion (Fratti et al, 2004). Analysis of isolated proteoliposomes showed successful reconstitution in each case (Supplementary Figure S1). We employed molar ratios of lipid/SNARE from 500:1 to 2000:1 (Supplementary Table SI), as proteoliposomes with higher SNARE densities, for example, less than 100:1 lipid/SNARE, may lose membrane integrity and leak lumenal contents (Dennison et al, 2006).
Lipid mixing with PC/PS proteoliposomes requires separate reconstitutions of 3Q-SNAREs and 1R-SNARE
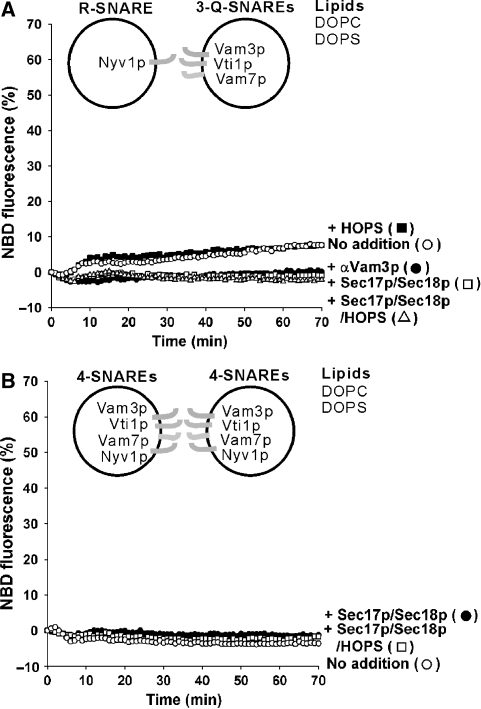
To study fusion, we employed a lipid-mixing assay using NBD-PE (N-(7-nitro-2,1,3-benzoxadiazole-4-yl)-phosphatidylethanolamine) and Rh-PE (N-(lissamine rhodamine B sulfonyl) phosphatidylethanolamine) (Struck et al, 1981; Weber et al, 1998). As reported (Fukuda et al, 2000), PC/PS liposome pairs where one partner has three vacuolar Q-SNAREs and the other partner has the R-SNARE undergo lipid mixing (Figures 1A and Supplementary Figure S2A; the Supplementary data presents statistics for the total dequenching) after a short lag for the components to warm to temperature (this lag is not seen when the system is warmed before an essential missing component is added, as in Figures 2D, 5 and 6). This does not require Sec17p, Sec18p and HOPS, which are needed for fusion of the organelle. Lipid mixing was inhibited by antibody to Vam3p (Figure 1A, αVam3p) and by antibodies to the other SNAREs but not by pre-immune IgG proteins (data not shown), indicating that all four SNAREs are involved in lipid mixing. Lipid mixing was blocked by Sec17p/Sec18p (Figure 1A and Supplementary Figure S2A), suggesting that they disassemble either trans-SNARE complexes or the 3Q-SNARE complexes on the acceptor liposomes. As the HOPS complex is required for SNARE complex assembly on isolated vacuoles (Collins et al, 2005), we expected that HOPS addition would enhance the lipid mixing. However, it had little effect on the PC/PS liposome pairs and did not restore lipid mixing in the presence of Sec17p and Sec18p (Figure 1A and Supplementary Figure S2A). In contrast to these PC/PS proteoliposomes, vacuoles bear all four SNAREs, which are disassembled from cis-SNARE complexes by Sec17p, Sec18p and ATP (Ungermann et al, 1998). PC/PS proteoliposomes bearing all four SNAREs did not undergo lipid mixing, even when incubated with Sec17p, Sec18p, ATP and HOPS (Figure 1B and Supplementary Figure S2B). Thus PC/PS proteoliposomes are not suitable for studying the functions of the HOPS, Sec17p and Sec18p SNARE chaperones.
Figure 1.
SNARE-mediated lipid mixing of PC/PS liposomes. (A) Lipid mixing between PC/PS liposomes with vacuolar 3Q-SNAREs (270–560 nM) or R-SNARE (94 nM). (B) Lipid mixing between PC/PS liposomes bearing all the four SNAREs (480–700 nM). Sec17p (400 nM), Sec18p (400 nM) and HOPS (10 nM) were added where indicated. All data were from one experiment and are representative of five independent experiments.
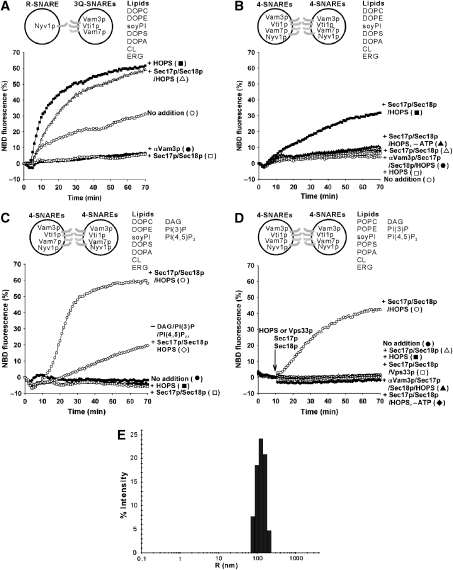
Figure 2.
Lipid mixing between SNARE proteoliposomes with vacuolar lipids, regulatory lipids, Sec17p/Sec18p and HOPS. (A) Effect of Sec17p (400 nM), Sec18p (400 nM) and HOPS (10 nM) on lipid mixing between vacuolar-lipid liposomes bearing either the 3Q-SNAREs (220–580 nM) or the R-SNARE (85 nM). (B) Lipid mixing with vacuolar-lipid liposomes bearing all four SNAREs (380–630 nM) in the presence of Sec17p (400 nM), Sec18p (400 nM) and HOPS (10 nM). (C) Regulatory lipids (DAG, PI(3)P and PI(4,5)P2) stimulated the lipid mixing of liposomes bearing the four SNAREs (450–550 nM) in the presence of Sec17p (400 nM), Sec18p (400 nM) and HOPS (10 nM). All liposomes, except those depicted with open diamonds, had regulatory lipids. (D) More physiological PO-lipids. Assays of lipid mixing used liposomes bearing vacuolar lipids including PO lipids, regulatory lipids and all the four SNAREs (310–450 nM), in the presence of Sec17p (1 μM), Sec18p (1 μM) and either HOPS (50 nM) or Vps33p (250 nM). Soluble components were added to the reactions after 10 min at 27 °C. All data (A–D) was from one experiment and is typical of more than three independent experiments. (E) Size distribution of SNARE liposomes. Dynamic light scattering experiments were performed (Araç et al, 2006), with the SNARE liposomes used in (D) (450 μM lipids in RB150 with 1 mM ATP and 6 mM MgCl2) and 50% laser intensity.
Vacuolar lipids
As lipid mixing between liposomes bearing 3Q-SNAREs and those with 1R-SNARE was promoted by vacuolar lipids (Fukuda et al, 2000), we asked whether this lipid composition might promote a more physiological response to HOPS, Sec17p and Sec18p. Although lipid mixing between these liposomes bearing the 3Q- or 1R- SNAREs was still inhibited by Sec17p/Sec18p/ATP, it was stimulated by HOPS, which overcame the Sec17/Sec18p/ATP inhibition (Figure 2A and Supplementary Figures S2C and S3A). Earlier studies of the effects of SM proteins on lipid mixing with SNARE liposomes (Scott et al, 2004; Shen et al, 2007) used only PC/PS and required lengthy preincubation at 4° C and amounts of SM proteins which were comparable with the SNARE levels to stimulate lipid mixing. The effects of HOPS in our current studies require no preincubation, and HOPS was present at 1/50 the molar concentration of the Qa-SNARE Vam3p.
Liposomes with four vacuolar SNAREs require Sec17p, Sec18p, HOPS and vacuolar lipids for lipid mixing
Sec17p/Sec18p can disassemble 4-SNARE complexes in cis. Vacuolar lipid proteoliposomes bearing the four SNAREs exhibited lipid mixing which required Sec17p/Sec18p, ATP and HOPS and was blocked by αVam3p (Figure 2B and Supplementary Figure S2D). Lipid mixing was proportional to the concentrations of Sec17p/Sec18p and HOPS (Supplementary Figures S3B and C). Thus the synergistic actions of Sec17p, Sec18p, HOPS and vacuolar lipid composition are necessary for lipid mixing between 4-SNARE proteoliposomes.
Stimulation by vacuole fusion regulatory lipids
We have reconstituted vacuole fusion with defined components: the cognate SNAREs, SNARE chaperones, HOPS and bulk vacuolar lipids. However, these lipids lack the regulatory lipids DAG and the phosphoinositides PI(3)P and PI(4,5)P2 (Fratti et al, 2004). ERG is also a regulatory lipid (Fratti et al, 2004), but was reported (Zinser et al, 1991; Schneiter et al, 1999) as part of the vacuole membrane bulk lipid composition and thus was included in our bulk vacuolar mixture. We prepared two sets of vacuolar lipid donor and acceptor proteoliposomes with the four SNAREs, one without further supplement and one with 1% of each of DAG, PI(3)P and PI(4,5)P2 (Figure 2C and Supplementary Figure S2E). Regulatory lipids strongly enhanced the initial rate of lipid mixing when compared with vacuolar lipids alone (Figure 2C, open circles versus diamonds). This stimulated lipid mixing was blocked by omission of either Sec17p/Sec18p or HOPS (Figure 2C and Supplementary Figure S2E). These findings establish that the regulatory lipids are important components of the fusion machinery.
For comparison with earlier studies (Fukuda et al, 2000), we employed dioleoyl (DO) lipids in these liposomes, yet a 1-palmitoyl 2-oleoyl (PO) fatty acyl chain composition is more physiological and may yield more stable bilayers. Liposomes with all four SNAREs and with PO- lipids exhibit similar lipid mixing to their DO-lipid counterparts (Figure 2D), and this lipid mixing still requires HOPS, Sec17p/Sec18p, ATP and the SNAREs. Vps33p, the SM subunit of HOPS, will not substitute for HOPS (Figure 2D). We therefore employed PO lipids for the rest of the study. Dynamic light scattering shows that these proteoliposomes are 200–400 nm in diameter (Figure 2E).
Topology of lipid mixing
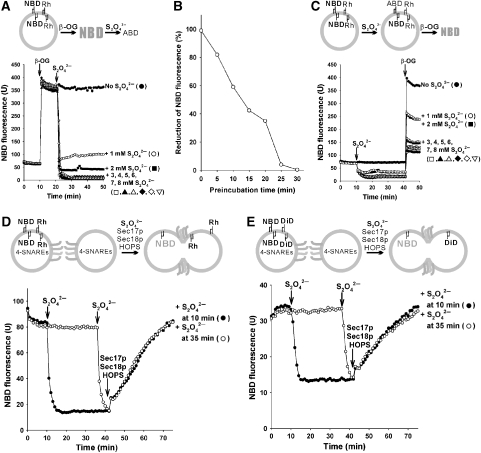
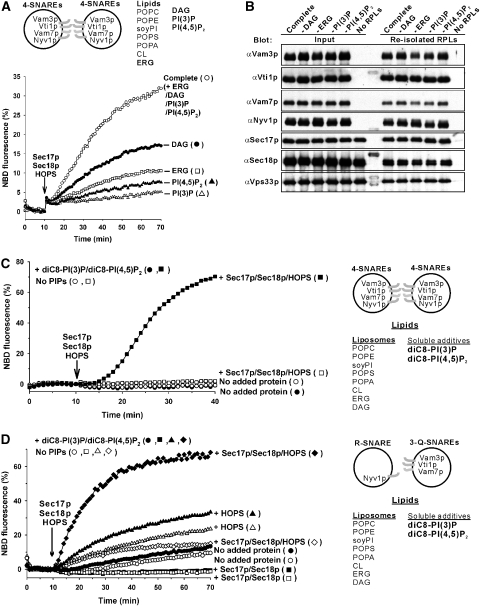
Liposomes can undergo fusion, lysis/reannealing and hemifusion (Düzgünes et al, 1987; Meers et al, 2000; Dennison et al, 2006). To distinguish these, we employed dithionite (S2O42−), a membrane-impermeable reducing agent that inactivates the fluorescence of exposed NBD (McIntyre and Sleight, 1991) but not Rh (Meers et al, 2000). The fluorescence of the NBD-PE in our liposomes is quenched by Rh-PE, as both fluorophores are present in the lumenal and outer monolayers. Added β-octylglucoside (β-OG) dilutes the fluorophores and thereby relieves quenching (Figure 3A). Dithionite concentrations of 3 mM or higher cause complete inactivation of the fluorescence of the fully accessible NBD-PE (Figure 3A). Dithionite itself is labile, and it loses potency after 30 min under the conditions of our lipid-mixing assay (Figure 3B). When intact SNARE proteoliposomes bearing NBD-PE and Rh-PE were treated with dithionite, the fluorescence of accessible NBD-PE was inactivated, whereas dithionite-inaccessible NBD-PE remained quenched by Rh-PE (Figure 3C). After 30 min of further incubation, when the dithionite had lost its potency, β-OG was added to assay the fluorescence of the remaining NBD-PE that had faced the lumen. Approximately 30–40% of the NBD-PE had been inaccessible to dithionite, showing that it had been on the lumenal monolayer of intact, sealed proteoliposomes (Figure 3C).
Figure 3.
Reconstituted membrane fusion without lysis. Topology of lipid mixing was analysed with dithionite (S2O42−), a membrane-impermeable reductant that inactivates the fluorescence of accessible NBD. All liposomes had four SNAREs (310–450 nM), vacuolar lipids (PO-lipids) and regulatory lipids, as shown in Figure 2D. (A) Dithionite reduction of NBD. 4-SNARE liposomes (450 μM lipids) were incubated in RB150 with 1 mM ATP and 6 mM MgCl2 at 27 °C, with addition of 100 mM β-OG at 10 min and 0–8 mM sodium dithionite at 20 min. (B) Lability of dithionite at 27 °C. Dithionite (40 mM in RB150 with 1 mM ATP, 6 mM MgCl2) was preincubated at 27 °C for 0–30 min, then added (4 mM final concentration) to mixtures of the SNARE liposomes and β-OG, as in (A), followed by further incubation at 27 °C while monitoring NBD fluorescence. The plateau fluorescence value was compared with the value before dithionite addition to derive the percent reduction. (C) Liposomes bear dithionite-inaccessible NBD-PE. 4-SNARE liposomes, as in (A), were incubated at 27 °C, and dithionite was added to 4 mM at 10 min. After 30 min further incubation, when dithionite had lost its potency (B), 100 mM β-OG was added to relieve Rh-PE quenching and assay the NBD-PE that had remained dithionite inaccessible. (D) Sec17p (1 μM), Sec18p (1 μM) and HOPS (50 nM) trigger lipid mixing among the four SNARE liposomes without causing lysis. Dithionite (4 mM) was added to the four SNARE liposomes at either 10 min (closed circles) or 35 min (open circles), before adding Sec17p/Sec18p and HOPS at 40 min. (E) Lysis-free inner monolayer mixing of four SNARE liposomes was triggered by Sec17p, Sec18p and HOPS. Lipid mixing was assayed with dithionite as in (D), except that donor liposomes bore NBD-PS and a quencher, diI(5)C18ds, which is rendered nonfluorescent by dithionite. The fluorescent moiety of diI(5)C18ds is indicated as DiD in the scheme. All data (A–E) were from one experiment and are representative of data from more than three independent experiments.
We tested whether SNARE, HOPS and Sec17p/Sec18p-mediated fusion was accompanied by lysis. Donor and acceptor proteoliposomes, each with four SNAREs and vacuolar and regulatory lipids, were incubated in portions that received 4 mM dithionite after 10 min (Figure 3D, filled circles) or after 35 min (open circles). At 40 min, the dithionite that had been added at 10 min would be inactive (Figure 3B), whereas the dithionite added at 35 min largely retains activity. At 40 min, Sec17p, Sec18p and HOPS were added, inducing rapid dequenching. Had this dequenching been due to, or even accompanied by, lysis/reannealing, the lumenally oriented NBD would have been reduced by dithionite added at 35 min and inactivated as a fluorophore. However, comparable dequenching was seen in each sample (Figure 3D, open and filled circles), establishing that Sec17p, Sec18p and HOPS promote fusion but do not promote lysis/reannealing. The addition of β-OG at 100 min showed that the same amount of NBD had remained inaccessible to dithionite added at 35 min regardless of Sec17p, Sec18p and HOPS being added or not (125 and 122 fluorescence units, respectively).
To test whether the dequenching of lumenally oriented NBD-PE reflected full fusion or only the dilution of quenching Rh-PE in the outer monolayer upon hemifusion, we employed 4-SNARE proteoliposomes bearing NBD-PS, which is less prone to translocation than NBD-PE (Meers et al, 2000) and a quenching DiD lipid, dil(5)C18ds (1,1′-diocradecyl-3,3,3′3′-tetramethylindodicarbocyanine-5′,5′-disulfonic acid), which is reduced and inactivated by dithionite (Meers et al, 2000). Fluorescent and nonfluorescent liposomes were mixed and incubated, then dithionite was added to inactivate any accessible NBD-PS or dil(5)C18ds either 30 or 5 min before the addition of Sec17p, Sec18p and HOPS. Dequenching was the same whether the dithionite remained active or had become inactivated (Figure 3E, filled and open circles) and thus reflects full fusion with its accompanying inner monolayer mixing, but not lysis.
Roles of SNARE chaperones
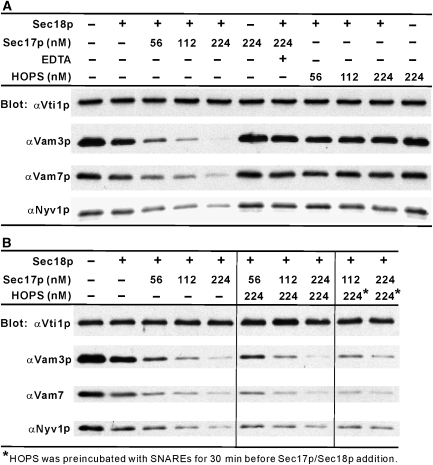
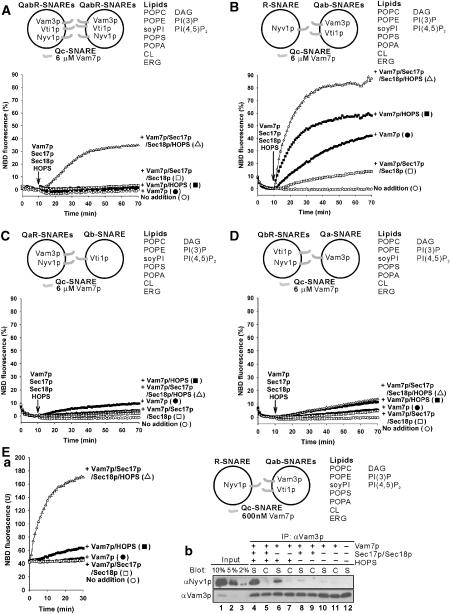
SNARE complexes on isolated vacuoles are associated with Sec17p or HOPS but not both (Collins et al, 2005). With pure chaperones and SNAREs, Sec17p allows Sec18p to disassemble SNARE complexes but HOPS does not (Figure 4A). The role of HOPS is not simply to stabilize SNARE complexes in the presence of Sec17p/Sec18p, as HOPS stimulates lipid mixing in the absence of Sec17p/Sec18p (Figure 2A) and does not prevent Sec17p/Sec18p-dependent disassembly of SNARE complexes (Figure 4B), as Sec17p can displace HOPS (Collins et al, 2005). To further explore the interplay of these SNARE chaperones, we first asked which SNARE topologies were functional. Proteoliposomes will fuse when the soluble SNARE Vam7p is added with Sec17p, Sec18p and HOPS rather than during proteoliposome assembly from detergent mixed micelles (Figure 5A). Proteoliposomes were also prepared with each single, membrane anchored SNARE in one liposome and the two complementary integral membrane SNAREs in its fusion partner (Figure 5B–D). The only combination that fused was with the R-SNARE Nyv1p on one fusion partner and the Qab-SNAREs Vam3p and Vti1p on the other, in accord with earlier studies of yeast ER to Golgi transport (Parlati et al, 2000). With the SNAREs thus artificially separated, high levels (6 μM, approximately 10-times higher concentrations than the Qab-SNAREs) of Vam7p promote a lipid mixing (Figure 5B) which is sensitive to Sec17p/Sec18p, indicating their disassembly of productive SNARE complexes that could otherwise lead to fusion. HOPS stimulates lipid mixing, and the combination of HOPS and Sec17p/Sec18p stimulates further. The synergy of SNARE chaperones was even more evident at lower levels of Vam7p (600 nM, comparable concentrations with the Qab-SNAREs), which alone could not support lipid mixing (Figure 5Ea, filled circles). In this condition, HOPS gave a modest fusion signal (filled squares), which was dramatically stimulated by the further addition of Sec17p and Sec18p (open triangles). This stimulation required each of Sec17p, Sec18p and Mg/ATP (data not shown), and thus likely reflects their concerted action of disassembling SNARE complexes. To determine whether these SNARE chaperones were regulating the formation of new SNARE complex containing both Nyv1p and Vam3p, the same samples (Figure 5Ea) were mixed with EDTA and soluble GST-Nyv1p (to block any further binding of Nyv1p to Vam3p), solubilized by detergent, and incubated with bead-bound antibody to Vam3p to assay the amount of Nyv1p that had become associated with Vam3p (Figure 5Eb; S samples are from the fusion incubations of part a; in C, donor and acceptor proteoliposomes were incubated separately and their detergent extracts mixed). Sec17p/Sec18p increased the Vam3p–Nyv1p association in the presence of HOPS (Figure 5Eb, lane 4 versus 6), consistent with its disassembly of non-productive SNARE cis-complexes to allow the formation of more productive SNARE complexes, which function with HOPS for trans-SNARE complex assembly. Sec17p/Sec18p may disassemble all SNARE complexes, whether productive or non-productive for later fusion, whereas HOPS confers selectivity for productive SNARE complex formation. This is consistent with earlier findings that Sec17p/Sec18p can disassemble cis- or trans-complexes of vacuolar SNAREs (Ungermann et al, 1998; Jun et al, 2007), whereas HOPS is selective for SNARE 0-layer composition and other structural features (Starai et al, 2008).
Figure 4.
Sec18p and Sec17p, but not HOPS, disassemble a complex of four vacuolar SNARE soluble domains (A), and HOPS does not prevent a disassembly of the 4-SNARE complex by Sec18p/Sec17p (B). The four soluble SNAREs, lacking a transmembrane domain, were purified as described (Jun et al, 2006). They were mixed at 10 μM each on ice in SSB (20 mM HEPES–NaOH, pH 7.4, 10% glycerol, 125 mM NaCl, 5 mM MgCl2, 0.008% Triton X-100), incubated at 4 °C overnight, diluted to 0.5 μM each in SSB, and mixed for 1 h with amylose beads (NEB) equilibrated in SSB. Beads were isolated by centrifugation (8000 r.p.m., 2 min, 4 °C) and washed four times in 450 μl SSB. Sec18p (2.3 μM), Sec17p (56–224 nM), HOPS (56–224 nM) and EDTA (10 mM) were mixed as indicated in SSB with 1 mM MgCl2:ATP on ice in separate tubes, then mixed with the washed beads. After incubation at 30 °C with rotation for 45 min, the beads were further washed four times as above, then bound proteins were eluted (90 °C, 0.4% SDS) and assayed by SDS–PAGE/immunoblot.
Figure 5.
Synergistic actions of Sec17p/Sec18p and HOPS. All liposomes bore vacuolar PO lipids and regulatory lipids. Vam7p (6 μM), Sec17p (1 μM), Sec18p (1 μM) and HOPS (50 nM) were added to the reactions after 10 min preincubation of liposomes at 27 °C. (A) Lipid mixing between liposomes bearing vacuolar QabR-SNAREs (850–920 nM). (B–D) Lipid mixing between liposomes bearing (B) Qab-SNAREs (580–800 nM) and R-SNARE (70 nM), (C) Qb-SNARE (820 nM) and the QaR-SNAREs (25–100 nM), and (D) Qa-SNARE (700 nM) and the QbR-SNAREs (100–120 nM). (E) Synergy of Sec17p/Sec18p and HOPS in promoting trans-SNARE pairing. (a) Lipid mixing assays had liposomes bearing either vacuolar Qab-SNAREs (580–800 nM) or the R-SNARE (70 nM), as in (B) but with 600 nM Vam7p. (b) Assay of Nyv1p bound to Vam3p. After 30 min, reaction mixtures from (a) were mixed with 560 ng of soluble GST-Nyv1p, 400 μl of ice-cold solubilization buffer (20 mM Tris–HCl, pH 7.5, 1 mM MgCl2, 150 mM NaCl, 10% glycerol, 0.5% NP-40, 0.46 μg/ml leupeptin, 3.5 μg/ml pepstatin, 2.4 μg/ml pefabloc-SC and 1 mM PMSF) and 10 mM EDTA and mixed at 4 °C for 5 min. After centrifugation (2 min, 16 000 g, 4 °C), supernatants (350 μl) were mixed with protein A agarose beads with covalently bound anti-Vam3p N-domain antibodies and incubated (4 °C, 1 h). Beads were washed with 600 μl of solubilization buffer four times. Bound proteins were eluted at 90 °C with 0.4% SDS, followed by SDS–PAGE and immunoblot. As a control, Qab-SNARE liposomes and R-SNARE liposomes were incubated and solubilized separately, mixed and assayed for Nyv1p:Vam3p association as above. The samples and their controls are labelled ‘S' and ‘C', respectively. The data in A-Ea were from one experiment and are representative of more than three independent experiments.
Regulatory lipid requirement
Each regulatory lipid has an important role in fusion. When compared with the fusion of 4-SNARE liposomes of vacuolar PO lipids and all regulatory lipids (Figure 6A), omission of DAG halved the rate of fusion, whereas omission of ERG, PI(4,5)P2 or PI(3)P caused far greater reduction. The dependence on regulatory lipids was stricter in these experiments with PO lipids than in studies with DO lipids (Figure 2C). None of the regulatory lipids was simply required for the proteoliposomal association of soluble fusion proteins, as there was similar recovery of HOPS, Sec17p, Sec18p and Vam7p with the proteoliposomes (Figure 6B). Vam7p has direct affinities for other SNAREs (Collins et al, 2005), HOPS (Stroupe et al, 2006) and PI(3)P (Cheever et al, 2001); the strict requirement for PI(3)P for liposomal fusion (Figure 6A) does not simply reflect the role of this lipid in facilitating Vam7p binding to the membrane (Figure 6B).
Figure 6.
Regulatory lipids support membrane fusion. (A) Liposomes bearing the four SNAREs (320–880 nM) with the complete lipid composition (vacuolar lipids (PO lipids) and regulatory lipids) or lacking DAG, ERG, PI(3)P or PI(4,5)P2 were assayed for lipid mixing. Sec17p (1 μM), Sec18p (1 μM) and HOPS (50 nM) were added at 10 min. (B) The binding of Sec17p, Sec18p, and HOPS to each set of liposomes was analysed by flotation (Tucker et al, 2004). Donor liposomes (450 μM) bearing the four SNAREs (200–650 nM) with the indicated lipid compositions were incubated with Sec17p, Sec18p and HOPS at 27 °C for 1 h, as shown in (A). Samples (80 μl) were mixed with 320 μl of 50% Histodenz in RB150, transferred to a 11 × 60 mm tube, then overlaid with 1.6 ml of 35% Histodenz in RB150, 2.0 ml of 30% Histodenz in RB150, and 200 μl of RB150. After centrifugation (SW60Ti (Beckman), 55 000 r.p.m., 3 h, 4 °C), liposomes were harvested and analysed by SDS–PAGE/immunoblot. (C) Liposomes lacking PI(3)P and PI(4,5)P2 and bearing four SNAREs (390–680 nM) were assayed for lipid mixing as in (A) with lower concentrations of ATP (0.5 mM) and MgCl2 (3 mM) and with Sec17p (500 nM), Sec18p (500 nM), HOPS (28 nM) and diC8-PI(3)P/diC8-PI(4,5)P2 (45 μM each) added at 0 min where indicated. (D) Liposomes lacking PI(3)P and PI(4,5)P2 and bearing 3Q-SNAREs (430–650 nM) and R-SNARE (82 nM) were assayed for lipid mixing as in (C) but with lower concentrations of diC8-PI(3)P/diC8-PI(4,5)P2 (23 μM each). The data in A, C and D were from one experiment and are representative of more than three independent experiments.
A mixture of soluble diC8-PI(3)P and diC8-PI(4,5)P2 restores Sec17p/Sec18p/HOPS-dependent full fusion to 4-SNARE proteoliposomes, which had been prepared and isolated without phosphoinositides (Figure 6C). With 4-SNARE RPLs, Sec17p/Sec18p will always be required for cis-SNARE complex disassembly. To obviate this need and facilitate study of whether phosphoinositides support a synergistic cooperation of Sec17p/Sec18p with HOPS, RPLs with 3Q-SNAREs or with R-SNARE were prepared without phosphoinositides and were assayed for fusion. Without added proteins, the low level of fusion was only slightly stimulated by phosphoinositides (Figure 6D, open versus filled circles). Sec17p/Sec18p blocked fusion, whereas the addition of HOPS without Sec17p/Sec18p stimulated fusion, with only modest enhancement by phosphoinositides. In the absence of phosphoinositides, HOPS relieved much of the inhibition by Sec17p/Sec18p (open diamonds), but there was no synergistic stimulation of fusion. Strikingly, added phosphoinositides supported a strong, synergistic stimulation of fusion by HOPS and Sec17p/Sec18p (filled diamonds). Thus phosphoinositides are required for the synergy between these SNARE chaperones, and both chaperones are essential for stimulation by phosphoinositides. This may in part reflect the affinity of these lipids for both HOPS and the Vam7p SNARE.
Similarity to vacuolar Rab-bypass fusion
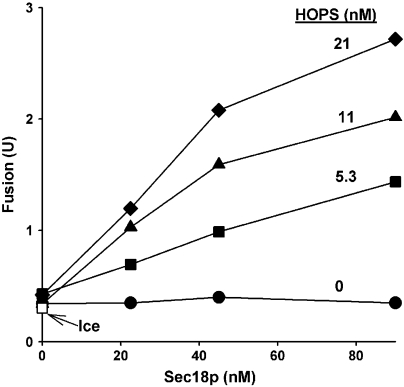
Is HOPS, a Rab-effector complex, ever seen to function without its Rab, Ypt7p, in the context of a biological membrane? To address this, we returned to assays of vacuole fusion. Vacuoles from strains that overexpress each of the four vacuolar SNAREs (termed 4SNARE++) bypass the normal requirement for Ypt7p for fusion (Starai et al, 2007). The fusion of 4SNARE++ ypt7Δ vacuoles require both Sec18p and HOPS (Figure 7). Thus, for both reconstituted 4-SNARE proteoliposomes and for vacuoles with elevated levels of SNAREs, Sec18p and HOPS synergistically promote fusion in the absence of Ypt7p.
Figure 7.
Fusion of ypt7Δ vacuoles requires Sec18p and HOPS. Standard fusion reactions (Jun et al, 2007) employed vacuoles from BJ3505 4SNARE++ ypt7Δ and DKY6281 4SNARE++ ypt7Δ (Starai et al, 2007). Vacuoles were incubated on ice (open square) or at 27 °C with recombinant Sec18p, HOPS or both. After 90 min, Pho8p phosphatase activity was assayed to measure vacuole fusion (Haas, 1995). Fusion units are μmol of P-nitrophenolate formed per min per μg of BJ3505 4SNARE++ ypt7Δ vacuoles.
Discussion
There have been three approaches to studying membrane fusion. (1) Liposome fusion can be triggered by bilayer perturbants such as calcium, polyethylene glycol, viral fusion proteins, DAG or phospholipase C (which generates DAG). These studies show how lipids rearrange for fusion, but do not reveal the roles of physiological fusion proteins. (2) Fusion is also studied with isolated organelles. These in vitro reactions rely on physiological fusion factors such as Rab GTPases, Rab effectors, SNAREs, SNARE chaperones, SM proteins and specific lipids. (3) Fusion is also studied through the lipid mixing of proteoliposomes bearing v- or t-SNAREs. This approach reveals important SNARE properties, but often employs high SNARE densities, is accompanied by substantial SNARE-dependent lysis (Dennison et al, 2006) and does not depend on other established physiological fusion factors. To connect these approaches, we began with assays of lipid mixing of v- and t-SNARE PC/PS proteoliposomes (Weber et al, 1998; Fukuda et al, 2000), but moved stepwise to more physiological conditions of lipid composition, disposition of SNAREs on fusion partners and addition of other fusion proteins.
Our current reconstitution faithfully reflects many proteins and lipids needed for vacuole fusion and shows that they cooperate to promote true fusion and not lysis. Lipid choice is crucial, as specific lipids form functional membrane microdomains (Lang et al, 2001; Fratti et al, 2004) and support the association of peripheral membrane proteins such as Vam7p (Cheever et al, 2001) or HOPS (Stroupe et al, 2006) at the vertex ring domain of yeast vacuole fusion (Wang et al, 2002). The reconstitution presented here does not require Ypt7p, yet we have shown that HOPS and Sec17p/Sec18p can bypass the need for Ypt7p, either on the intact vacuole or in proteoliposomes. Studies of this reconstituted system yield four lessons: (1) SNARE chaperones cooperate, with Sec17p/Sec18p disassembling non-productive SNARE complexes and HOPS capturing functional complexes for rapid fusion. (2) Fusion without lysis does not rely on additional protein factors, but is inherent to the complete fusion machinery. (3) The regulatory lipids (ERG, phosphoinositides and DAG), implicated in fusion from genetic studies in vivo and biochemical studies of the isolated organelle (Mayer et al, 2000; Cheever et al, 2001; Kato and Wickner, 2001; Seeley et al, 2002; Fratti et al, 2004; Jun et al, 2004), are an integral part of the core fusion pathway. (4) Phosphoinositides stimulate fusion only when both SNARE chaperone systems are present, and these act synergistically only in the presence of phosphoinositides (Figure 6D).
Both Sec17p/Sec18p, which disassemble SNARE complexes (Söllner et al, 1993; Ungermann et al, 1998; Jun et al, 2007), and HOPS, which promotes the assembly of vacuolar SNARE complexes (Collins and Wickner, 2007), are needed to fuse 4-SNARE liposomes of vacuolar lipids. Unlike Sec17p, HOPS does not function with Sec18p to promote SNARE complex disassembly (Figure 4). However, this does not explain the full HOPS function, as HOPS also promotes fusion of 3Q-SNARE and R-SNARE proteoliposomes in the absence of Sec17p/Sec18p (Figures 2A and 5B). Thus HOPS must catalyze SNARE complex assembly and/or enhance the capacity of assembled trans-SNARE complexes for fusion. This is in accord with other studies that found stimulation by Sec1p of lipid mixing between liposomes bearing yeast plasma membrane SNAREs (Scott et al, 2004) or by Munc18-1p for liposomes with neuronal SNAREs (Shen et al, 2007). In our current reconstitution, nanomolar levels of HOPS, substoichiometric with the SNAREs, support rapid and efficient fusion. Our data reveal new aspects of how Sec17p/Sec18p and HOPS cooperate to promote fusion. In the presence of phosphoinositides, Sec17p/Sec18p inhibits fusion in the absence of HOPS but stimulates fusion in its presence. The strong inhibition of fusion by Sec17p/Sec18p (Figure 5B) likely reflects the ATP-driven disassembly of the 3-Q-SNARE complex or the 4-SNARE trans-complex, intermediates on the path to fusion. Proteoliposomes bearing Vam3p and Vti1p will fuse with those with Nyv1p when given Vam7p and HOPS, now stimulated by Sec17p/Sec18p instead of being inhibited (Figure 5B and E). How is the role of Sec17/Sec18p switched, from inhibitor to activator, by HOPS and phosphoinositides (Figure 6D)? SNAREs form non-productive as well as productive complexes, and SM proteins can improve the specificity of pairing (Tsui et al, 2001; Peng and Gallwitz, 2002; Brandhorst et al, 2006). We suggest that Sec17p/Sec18p can disassemble SNARE complexes, whether they are productive or not, allowing a fresh chance for these SNAREs to form complexes that can be captured by HOPS, which proofreads SNARE complex composition and structure (Starai et al, 2008), and to thereby form more fusogenic complexes. In accord with this working model, added Sec17p and Sec18p only enhance the total level of SNARE complex between Vam3p from one fusion partner and Nyv1p from the other when HOPS is also present (Figure 5Eb). Phosphoinositides may support this synergy through binding both Vam7p and HOPS.
Fusion in model systems has often been accompanied by lysis (Kendall and MacDonald, 1982; Burgess et al, 1992; Lau et al, 2004; Dennison et al, 2006). Our reconstitution of fusion with physiological lipids, SNAREs and SNARE chaperones has little or no accompanying lysis, suggesting that other proteins of the vacuole are not required to avert SNARE-driven lysis. Vacuoles can be driven to lyse by excess SNAREs (Starai et al, 2007); further studies are required to understand whether chaperones and vacuole lipids guide the bilayer destabilization by SNAREs towards fusion and away from lysis.
Regulatory lipids are required for vacuole fusion, whether in vivo, in vitro with the isolated organelle or with purified proteins and lipids in proteoliposomes (Figure 6). Vacuole fragmentation, a hallmark of defective fusion, is seen in strains with deletions in either ERG biosynthetic genes, in PLC1, a phospholipase C that converts PI(4,5)P2 to DAG, or in VPS34, a PI 3-kinase (Seeley et al, 2002). The fusion of isolated vacuoles is blocked by sterol ligands such as nystatin, aphotericin B or filipin or by sterol extraction by β-methycyclodextrin (Kato and Wickner, 2001; Fratti et al, 2004), by phosphoinositide ligands such as monoclonal antibodies, neomycin or recombinant FYVE or ENTH domains or by phosphoinositide phosphatases (Mayer et al, 2000; Fratti et al, 2004), or by DAG ligands such as recombinant C1b domain or inhibitors of phospholipase C that generate DAG such as U73122 or 3-nitrocoumarin (Jun et al, 2004). Biochemical studies have tied the regulatory lipids to specific subreactions, and catalysts, of fusion. ERG supports Sec17p/Sec18p-mediated priming (Kato and Wickner, 2001) and hence the ensuing steps: 4-phosphoinositides regulate priming and docking (Mayer et al, 2000), phosphoinositides contribute to the vacuole association of Vam7p (Cheever et al, 2001) and HOPS (Stroupe et al, 2006) and support the synergy of the two SNARE chaperone systems (Figure 6D) and DAG is needed for trans-SNARE pairing and fusion (Jun et al, 2004). These lipids become enriched at the vertex ring fusion microdomain, they are required for each other's vertex ring enrichment, their vertex enrichment is regulated by SNAREs and in turn they regulate the SNAREs spatial enrichment (Fratti et al, 2004). Nevertheless, it had remained possible that these lipid requirements were only indirect; for example, they might have regulated a vacuolar ion transport system that itself indirectly regulated more direct fusion catalysts. With our current finding that reconstituted fusion is also governed by each of the regulatory lipids (Figure 6), which are integral components of the fusion pathway, it is clear that the bilayer is not simply a passive substrate for protein action, but has lipids that regulate each stage of fusion.
The current reconstitution is a platform for exploring fusion mechanisms. Other proteins such as Ypt7p might further enhance or regulate the fusion rate. Components can be omitted singly or in groups, and the effects on fusion subreactions are studied in detail, avoiding indirect effects such as the alterations of vacuole content of fusion proteins when genes encoding trafficking proteins or lipid metabolism are deleted from the cell. The ability to control proteoliposome composition is an invaluable aid to studies of mechanism.
Materials and methods
Protein isolation
Protein expression and purification is described in Supplementary data.
Reconstitution of SNARE proteoliposomes
Proteoliposomes with vacuolar SNAREs were prepared as described (Weber et al, 1998; Scott et al, 2003) with modifications. Non-fluorescent lipids, except for ERG (Sigma) and phosphoinositides (Echelon), were from Avanti Polar Lipids. Fluorescent lipids (NBD-PE, Rh-PE and dansyl-PE) were from Molecular Probes. Donor lipid mixes contain 1.5% (mol/mol) NBD-PE and 1.5% Rh-PE, whereas acceptor lipid mixes contain 1.0% dansyl-PE. For PC/PS liposomes, the lipid mixes are DOPC (82 or 84% for donor or acceptor liposomes, respectively), DOPS (15%) and fluorescent lipids. Vacuolar lipid liposomes contain DOPC or POPC (45 or 47% for donor or acceptor, respectively), DOPE or POPE (18%), soyPI (18%), DOPS or POPS (4.4%), DOPA or POPA (2.0%), CL (1.6%), ERG (8.0%) and fluorescent lipids. For liposomes with vacuolar and regulatory lipids, DAG (1.0%), PI(3)P (1.0%) and PI(4,5)P2 (1.0%) are included, and DOPC or POPC is reduced to 42 or 44% for donor or acceptor, respectively. Dried films with these compositions were dissolved (final 2 mM lipids) in RB500 (20 mM HEPES–NaOH, pH 7.4, 500 mM NaCl, 10% glycerol) with 40 mM CHAPS, purified vacuolar SNAREs (GST-Vam3p, Vti1p, Vam7p, and Nyv1p) (final 2 μM each), and TEV protease (final 2 μM, only added when GST-Vam3p is present). The detergent-lipid-SNARE mixed micellar solutions were incubated (4 °C, 1 h, gentle agitation), then dialyzed against RB500 in a Slide-A-Lyzer 20 kDa cutoff dialysis cassette (Pierce) at 4 °C to remove detergent. SNARE proteoliposomes were purified by flotation through steps of 40, 30 and 0% Histodenz (Sigma) in RB150 (20 mM HEPES–NaOH, pH 7.4, 150 mM NaCl, 10% glycerol). Each dialysate (1–1.5 ml) was mixed with an equal volume of 80% Histodenz in RB150 and transferred to an 11 × 60 mm tube. Samples were overlaid with 30% Histodenz in RB150 to a total volume of 4 ml, then 200 μl RB150. After centrifugation (SW60Ti [Beckman], 55 000 r.p.m., 3 h, 4 °C), liposomes were harvested from the 0/30% Histodenz interface. Lipid concentrations were determined from the fluorescence of NBD-PE (λex=460 nm, λem=538 nm, emission cutoff=515 nm) for donor liposomes and dansyl-PE (λex=336 nm, λem=517 nm, emission cutoff=495 nm) for acceptor liposomes, in the presence of 100 mM β-OG. Liposomes were diluted with RB150 to 2 mM lipids and stored at −80 °C without loss of activity. Protein concentrations of each SNARE were determined from Coomassie-stained gels using UN-SCAN-IT gel version 5.1 (Silk Scientific Corporation) and bovine serum albumin as standard.
Lipid mixing assay
Lipid mixing assays were performed as described (Weber et al, 1998; Scott et al, 2003) with modifications. Typically, reaction mixtures in RB150 were prepared in black 96-well plates (for 100 μl reactions) or 384-well plates (for 20 μl reactions) (Corning) on ice and were composed of donor (50 μM lipids) and acceptor (400 μM lipids) SNARE proteoliposomes, 1 mM ATP, 6 mM MgCl2, and Sec17p (0.4–1.0 μM), Sec18p (0.4–1.0 μM), HOPS (10–50 nM), Vps33p (250 nM), Vam7p (0.6–6.0 μM) and αVam3p (1.0 μM) where indicated. Without preincubation, plates were placed in a SpectraMAX Gemini XPS plate reader (Molecular Devices) equilibrated at 27 °C. NBD fluorescence (λex=460 nm, λem=538 nm, emission cutoff=515 nm) was monitored at 1 min intervals, 30 reads per well on the ‘high' PMT setting (arbitrary units). In some experiments, Sec17p, Sec18p, HOPS, Vps33p and Vam7p were added to liposome reactions that had been preincubated at 27 °C for 10 min in a plate reader. After 1–2 h at 27 °C, 100 mM β-OG was added to fully dequench the NBD. For calculating maximal NBD fluorescence, the signals at 0 min were set to 0% and the signals after β-OG addition were 100%.
Topology analysis of lipid mixing
All experiments with sodium dithionite (Sigma) employed 4-SNARE liposomes with vacuolar and regulatory lipids including POPC, POPE, POPS and POPA, prepared as above, except that, in Figure 3E, NBD-PS (Avanti) and diI(5)C18ds (Molecular Probes) were present in donor liposomes instead of NBD-PE and Rh-PE. For lipid mixing assays with dithionite, reactions (24 μl) containing donor (50 μM lipids) and acceptor (400 μM lipids) liposomes, 1 mM ATP and 6 mM MgCl2 in RB150 were incubated at 27 °C in a black 384-well plate, with addition of dithionite (4 mM), Sec17p (1 μM), Sec18p (1 μM), HOPS (50 nM) and β-OG (100 mM), as described, monitoring NBD fluorescence.
Vacuole isolation and in vitro vacuole fusion assay
Yeast vacuoles were isolated from BJ3505 4SNARE++ ypt7Δ and DKY6281 4SNARE++ ypt7Δ and fusion was assayed as described (Haas, 1995; Starai et al, 2007).
Supplementary Material
Supplementary data
Acknowledgments
This work was supported by NIH grant GM23377. J.M. was supported by TOYOBO BIO Foundation long-term research grants. C.M.H. received support from T32GM08704 (NIGMS). We are especially grateful to Timothy Craig and Josep Rizo, UT Southwestern, for dynamic light scattering analysis. We thank Amy Burfeind, Nathan Margolis and Naomi Thorngren for expert assistance.
References
- Araç D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Südhof TC, Rizo J (2006) Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol 13: 209–217 [DOI] [PubMed] [Google Scholar]
- Bhalla A, Chicka MC, Tucker WC, Chapman ER (2006) Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat Struct Mol Biol 13: 323–330 [DOI] [PubMed] [Google Scholar]
- Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R (2006) Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc Natl Acad Sci USA 103: 2701–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SW, McIntosh TJ, Lentz BR (1992) Modulation of Poly (ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry 31: 2653–2661 [DOI] [PubMed] [Google Scholar]
- Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M (2001) Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol 3: 613–618 [DOI] [PubMed] [Google Scholar]
- Collins KM, Thorngren NL, Fratti RA, Wickner WT (2005) Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J 24: 1775–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KM, Wickner WT (2007) Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA 104: 8755–8760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison SM, Bowen ME, Brunger AT, Lentz BR (2006) Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J 90: 1661–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Wang Y, Südhof T, Rizo J (2001) Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol 8: 258–264 [DOI] [PubMed] [Google Scholar]
- Dulubova I, Khvotchev M, Liu S, Huryeva I, Südhof TC, Rizo J (2007) Munc18-1 binds directly to the neuoronal SNARE complex. Proc Natl Acad Sci USA 104: 2697–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzgünes N, Allen TM, Fedor J, Papahadjopoulos D (1987) Lipid mixing during membrane aggregation and fusion: why fusion assays disagree. Biochemistry 26: 8435–8442 [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R (1998) Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA 95: 15871–15876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti R, Jun Y, Merz AJ, Margolis N, Wickner W (2004) Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol 167: 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, McNew JA, Weber T, Parlati F, Engel T, Nickel W, Rothman JE, Söllner T (2000) Functional architecture of an intracellular membrane t-SNARE. Nature 407: 198–202 [DOI] [PubMed] [Google Scholar]
- Grosshans B, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A (1995) A quantitative assay to measure homotypic vacuole fusion in vitro. Methods Cell Sci 17: 283–294 [Google Scholar]
- Haas A, Wickner W (1996) Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF). EMBO J 15: 3296–3305 [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller R (2006) SNAREs- engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643 [DOI] [PubMed] [Google Scholar]
- Jun Y, Fratti RA, Wickner W (2004) Diacylglycerol and its formation by phospholipase C regulate Rab- and SNARE-dependent yeast vacuole fusion. J Biol Chem 279: 53186–53195 [DOI] [PubMed] [Google Scholar]
- Jun Y, Thorngren N, Starai VJ, Fratti RA, Collins K, Wickner W (2006) Reversible, cooperative reactions of yeast vacuole docking. EMBO J 25: 5260–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Y, Xu H, Thorngren N, Wickner W (2007) Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion. EMBO J 26: 4935–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Wickner W (2001) Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion. EMBO J 20: 4035–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DA, MacDonald RC (1982) A fluorescence assay to monitor vesicle fusion and lysis. J Biol Chem 257: 13892–13895 [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R (2001) SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 20: 2202–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WL, Ege DS, Lear JD, Hammer DA, DeGrado WF (2004) Oligomerization of fusogenic peptides promotes membrane fusion by enhancing membrane destabilization. Biophys J 86: 272–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A (2000) Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol Biol Cell 11: 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JC, Sleight RG (1991) Fluorescence assay for phospholipid membrane asymmetry. Biochemistry 30: 11819–11827 [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Söllner TH, Rothman JE (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407: 153–159 [DOI] [PubMed] [Google Scholar]
- Meers P, Ali S, Erukulla R, Janoff AS (2000) Novel inner monolayer fusion assays reveal differential monolayer mixing associated with cation-dependent membrane fusion. Biochim Biophys Acta 1467: 227–243 [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Zerial M (2002) Mosaic organization of the endocytic pathway. Exp Cell Res 272: 8–14 [DOI] [PubMed] [Google Scholar]
- Nickel W, Weber T, McNew JA, Parlati F, Söllner T, Rothman JE (1999) Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci USA 96: 12571–12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Zerial M (1997) The diversity of Rab proteins in vesicle transport. Curr Op Cell Biol 9: 496–504 [DOI] [PubMed] [Google Scholar]
- Ostrowicz CW, Meiringer CTA, Ungermann C (2008) Yeast vacuole fusion: a model system for eukaryotic endomembrane dynamics. Autophagy 4: 5–19 [DOI] [PubMed] [Google Scholar]
- Parlati F, McNew JA, Fukuda R, Miller R, Söllner TH, Rothman JE (2000) Topological restriction of SNARE-dependent membrane fusion. Nature 407: 194–198 [DOI] [PubMed] [Google Scholar]
- Peng R, Gallwitz D (2002) Sly1 protein bound to Golgi syntaxin Sec5p allows assembly and contributes to specificity of SNARE-fusion complexes. J Cell Biol 157: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin Y-K, Bennett MK (1998) The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol 5: 765–769 [DOI] [PubMed] [Google Scholar]
- Rizo J, Südhof T (2002) SNAREs and Munc18 in synaptic vesicle fusion. Nat rev Neurosci 3: 641–653 [DOI] [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell 6: 661–671 [DOI] [PubMed] [Google Scholar]
- Schneiter R, Brügger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland FT, Kohlwein SD (1999) Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol 146: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BL, vanKomen JS, Irshad H, Liu S, Wilson KA, McNew JA (2004) Sec1p directly stimulates SNARE-mediated membrane fusion. J Cell Biol 167: 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BL, Van Komen JS, Liu S, Weber T, Melia TJ, McNew JA (2003) Liposome fusion assay to monitor intracellular membrane fusion machines. Methods Enzymol 372: 274–300 [DOI] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA 97: 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley ES, Kato M, Margolis N, Wickner W, Eitzen G (2002) Genomic analysis of homotypic vacuole fusion. Mol Biol Cell 13: 782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128: 183–195 [DOI] [PubMed] [Google Scholar]
- Siegel DP, Banschbach J, Alford D, Ellens H, Lis LJ, Quinn PJ, Yeagle PL, Bentz J (1989) Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry 28: 3703–3709 [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418 [DOI] [PubMed] [Google Scholar]
- Starai VJ, Jun Y, Wickner W (2007) Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci USA 10: 13551–13558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Hickey CM, Wickner W (2008) HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell 19: 2500–2508, in press 16 April 2008; doi: 10.1091/mbc.E08-01-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Collins KM, Fratti RA, Wickner WT (2006) Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J 25: 1579–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck DK, Hoekstra D, Pagano RE (1981) Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20: 4093–4099 [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395: 347–353 [DOI] [PubMed] [Google Scholar]
- Tsui MMK, Tai WCS, Banfield DK (2001) Selective formation of Sed5p-containing SNARE complexes is mediated by combinatorial binding interactions. Mol Biol Cell 12: 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker WC, Weber T, Chapman ER (2004) Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304: 435–438 [DOI] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HRB, Wickner W (1998) A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol 140: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham HRB (2003) Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol 13: 1636–1640 [DOI] [PubMed] [Google Scholar]
- Wang L, Seeley ES, Wickner W, Merz A (2002) Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell 108: 357–369 [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92: 759–772 [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD (2000) New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol 151: 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser E, Sperka-Gottlieb CDM, Fasch E-V, Kohlwein SD, Paltauf F, Daum G (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173: 2026–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data