Abstract
The lifelong self-renewal of the epidermis is driven by a progenitor cell population with high proliferative potential. To date, the upstream signals that determine this potential have remained largely elusive. Here, we find that insulin and insulin-like growth factor receptors (IR and IGF-1R) determine epidermal proliferative potential and cooperatively regulate interfollicular epidermal morphogenesis in a cell autonomous manner. Epidermal deletion of either IR or IGF-1R or both in mice progressively decreased epidermal thickness without affecting differentiation or apoptosis. Proliferation was temporarily reduced at E17.5 in the absence of IGF-1R but not IR. In contrast, clonogenic capacity was impaired in both IR- and IGF-1R-deficient primary keratinocytes, concomitant with an in vivo loss of keratin 15. Together with a reduction in label-retaining cells in the interfollicular epidermis, this suggests that IR/IGF-1R regulate progenitor cells. The expression of dominant active Rac rescued clonogenic potential of IR/IGF-1R-negative keratinocytes and reversed epidermal thinning in vivo. Our results identify the small GTPase Rac as a key target of epidermal IR/IGF-1R signalling crucial for proliferative potential and interfollicular morphogenesis.
Keywords: IGF-1 receptor, insulin, interfollicular progenitor cells, K14-Cre, transgenic mice
Introduction
Continuous renewal of the interfollicular epidermis (IFE) is crucial for organisms to maintain and restore the skin barrier that protects them from external challenges and dehydration. This lifelong process of self-renewal is driven by a high proliferative capacity of progenitor cells, which under physiological conditions reside in the basal layer of the IFE (Ito et al, 2005; Levy et al, 2005; Kaur, 2006; Watt et al, 2006; Clayton et al, 2007).
Hair follicle stem cells reside in the bulge of the hair follicle and have been well characterized at the molecular marker level (Morris et al, 2004; Tumbar et al, 2004). In contrast, the spatial localization and molecular identity of murine IFE progenitor cell are less clearly defined (Kaur, 2006; Jones and Simons, 2008). In human skin, interfollicular progenitor cells in the basal layer are distinguished by differential expression of markers such as the β1-integrin, the Notch ligand Delta and Desmoglein3, but none of these appear to spatially identify a progenitor cell population in the mouse IFE (Jones and Simons, 2008). Similarly, the identity of the niche of IFE progenitor cells is unknown, although it is likely that dermal fibroblasts constitute part of the niche, as has been shown for the hair follicle stem cells located in the bulge (reviewed in Jones and Wagers, 2008).
In the epidermis, several downstream mediators, such as for example p63, the small GTPase Rac and c-Myc, have been implicated in the determination and/or maintenance of interfollicular epidermal progenitor cells and their proliferative potential (Arnold and Watt, 2001; Waikel et al, 2001; Koster et al, 2004; Benitah et al, 2005; Castilho et al, 2007; Senoo et al, 2007). However, the upstream extracellular signals and their receptors have remained largely elusive. In humans and mice, differential expression of α6- and β1-integrin, receptors for extracellular matrix, are hallmarks associated with differential proliferative potential of basal interfollicular cells (reviewed in Kaur, 2006). This suggests that cell–extracellular matrix adhesion may be one mechanism by which the environment can regulate progenitor cell maintenance.
Another potential mechanism is through the IGF growth factors and their relative insulin. IGF-II has recently been implicated in the regulation of clonogenicity of human embryonic stem cells through the activation of the IGF-1R (Bendall et al, 2007). Overexpression of IGF-II in either mouse colon or epidermis also increased the number of proliferative units without a change in cell size, or in colon, crypt area (Ward et al, 1994; Bennett et al, 2003). In addition, both insulin and IGFs negatively regulate the Foxo family of transcription factors (Taniguchi et al, 2006), which have been shown to have a central function in the regulation of stem cells (Arden, 2007). Consistently, conventional inactivation of IGF signalling components, such as IGF-1R, IGF-1 and/or IGF-II (Liu et al, 1993), insulin substrate I (IRSI; Sadagurski et al, 2007) or both of the downstream kinases AKT1 and AKT2 (Peng et al, 2003) in mice result in a hypomorphic epidermis, although the mechanisms remain unclear. Complete inactivation of the closest relative of IGF-1R, the insulin receptor (IR), did not reveal any obvious skin phenotype in mice even though proliferation and differentiation were altered (Wertheimer et al, 2001). As IR and IGF-1R are also key regulators of growth, apoptosis, differentiation and metabolism (Pollak et al, 2004; Taniguchi et al, 2006), the observed epidermal phenotype in the IGF-1R pathway knockout mice could be caused by cell-autonomous changes in any of these processes in keratinocytes, or, more indirectly, by alterations in the dermis that perturb dermal–epidermal communication.
Here, we show that cell-autonomous insulin and IGF-1 receptor signalling cooperatively regulate epidermal morphogenesis. Surprisingly, epidermal-specific loss of IR and/or IGF-1R did not affect epidermal differentiation or survival in vivo, whereas proliferation was only temporarily affected in epidermal development but only upon deletion of IGF-1R not IR. The data identify insulin and IGF-1 receptors as key upstream activators of the small GTPase Rac in the epidermis through which they regulate proliferative potential of keratinocytes in vitro and interfollicular morphogenesis in vivo.
Results
Cell-autonomous IR/IGF-1R signalling regulate epidermal morphogenesis
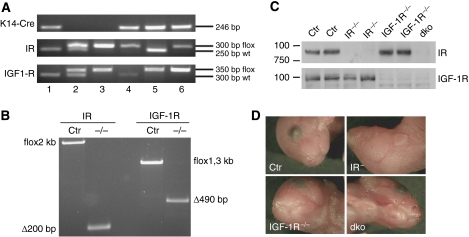
To examine the cell-autonomous role of insulin and IGF signalling in the epidermal compartment of skin, we specifically inactivated their receptors, either the IR (IRepi−/−), the IGF-1 receptor (IGF-1Repi−/−) or both receptors (double knockout or dkoepi), in the epidermis. This was achieved by crossing mice homozygous for the respective floxed alleles (Brüning et al, 1998) for these receptors with mice expressing the Cre recombinase under the K14 promotor (Hafner et al, 2004) and carrying one floxed allele for either the IR and/or IGF-1R (Figure 1A). This results in the deletion of the floxed region in IR or IGF-1R or both at the genomic level (Figure 1B) and the absence of protein expression (Figure 1C) in the epidermal compartment. Mice with epidermal loss of IR were viable and exhibited no macroscopically detectable defects either in the epidermis (Figure 1D) or in hair follicles, as was expected based on the total IR knockout mice (Wertheimer et al, 2001). In contrast, inactivation of either the IGF-1R or the combination of IGF-1R and IR (dkoepi) in the epidermis resulted in a fragile, translucent skin (Figure 1D), with a more severe appearance in the dkoepi. All dkoepi died perinatally, whereas around 55% of the IGF-1Repi−/− mice survived, but showed occasional hair loss.
Figure 1.
Epidermal inactivation of insulin receptor, IGF-1 receptor affects epidermal morphogenesis. (A) PCR analysis on genomic DNA isolated from tail biopsies showing the different genotypes of the mice: 1, K14-Cre; 2, IRfl/+;IGF-1Rfl/+; 3, IRfl/fl;IGF-1Rfl/fl; 4, K14-Cre; IRfl/fl;IGF-1R+/+; 5, K14-Cre; IR+/+;IGF-1Rfl/fl; 6,K14-Cre; IRfl/fl;IGF-1Rfl/fl. (B) PCR analysis on genomic DNA from split epidermis showing the efficiency of deletion of the floxed region in either the IR or the IGF-1R locus in the presence of keratin 14-driven Cre. (C) Western blot analysis on epidermal lysates using antibodies against either the IR receptor or the IGF-1R receptor. (D) Macroscopic appearance of control mice and mice with an epidermal deletion of IR, IGF-1R or both (dko).
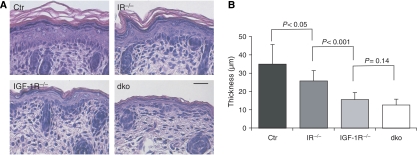
Histochemical analysis revealed a striking hypoplastic epidermis in the IGF-1Repi−/− mice (Figure 2A), showing that the previously observed hypomorphic epidermis of the total IGF-1R knockout mice (Liu et al, 1993) results from a direct signalling defect in the epidermis itself. Surprisingly, even though the IRepi−/− mice displayed no obvious macroscopic phenotype (Figure 1D), the IFE was significantly thinner than that of control mice (Figure 2B). Deletion of both IR and IGF-1R resulted in a further decrease (Figure 2), revealing that cell-autonomous insulin and IGF-1 signalling cooperatively regulate epidermal thickness. This hypomorphic phenotype was also observed in other stratifying epithelia that express K14, such as palate or tooth anlagen (Supplementary Figure 1A) and did not deteriorate further in surviving IGF-1Repi−/− mice (Supplementary Figure 1A) or IRepi−/− mice (not shown). Thus, insulin and IGF-1 receptor signalling cooperatively regulate the number of suprabasal cell layers and thereby interfollicular epidermal morphogenesis, with a more significant contribution of IGF-1R compared to IR signalling. As the IRepi−/− mice showed no obvious hair follicle defects and the dkoepi mice died within the first 2 days, we focused our study on the role of IR/IGF-1R in regulating interfollicular morphogenesis.
Figure 2.
Cooperative and cell-autonomous regulation of epidermal thickness by epidermal insulin and IGF-1 receptor signalling. (A) H&E staining of paraffin sections from newborn back skin. Scale bar is 50 μm. (B) Quantification of the thickness of the epidermis (without the stratum corneum) using H&E stained sections of back skin. N=7 for each genotype.
Normal differentiation in the absence of epidermal IR/IGF-1R
The appearance of a hypo- or hypermorphic epidermis is often associated with impaired differentiation. This was indeed reported to be abnormal in three-dimensional skin co-culture systems with keratinocytes and fibroblasts both deficient in IGF-1R (Sadagurski et al, 2006). Using different IFE markers, we thus examined whether epidermal loss of IR or IGF-1R impaired differentiation in vivo. In the IRepi−/−, IGF-1Repi−/− or dkoepi mice, appropriate keratin 10 and loricrin expression was still observed, with keratin 10 marking the suprabasal layers and loricrin marking granular layer. However, due to the reduction in spinous and granular layers in knockout mice, the domain was increasingly smaller (Supplementary Figure 2). The basal layer marker keratin 14 was also still confined to the basal layer in all mice examined (Supplementary Figure 2). Moreover, no obvious difference in integrin α6-staining, marking the epidermal basement membrane zone, was observed, suggesting normal polarization of basal keratinocytes and contact to the basement membrane (Supplementary Figure 2). These results show that the intrinsic differentiation program is not directly affected by the loss of epidermal insulin and/or IGF-1 signalling.
IR/IGF-1R signalling do not affect apoptosis in newborn epidermis
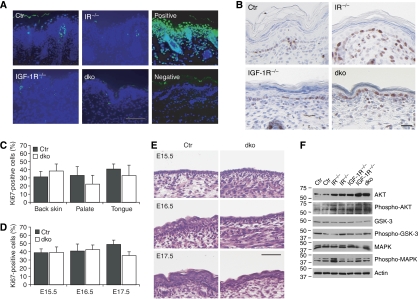
Both insulin and IGF-1 can promote cell survival by the activation of AKT (Pollak et al, 2004; Taniguchi et al, 2006). In vitro studies using keratinocytes negative for either IR or IGF-1R revealed changes in AKT signalling and increased apoptosis (Wertheimer et al, 2001; Sadagurski et al, 2006). However, no change in apoptotic activity was seen in the epidermis upon inactivation of either IR or IGF-1R alone or both as assessed by either TUNEL assays (Figure 3A) or cleaved caspase 3 protein levels (not shown). Although IGF-1 signalling can stimulate AKT in keratinocytes (Haase et al, 2003; Sadagurski et al, 2006), relatively little activated AKT was detected under steady-state conditions in isolated control newborn epidermis (Figure 3F) and this was similar or even slightly increased in the absence of IR, IGF-1R or both. Strikingly, an increase in the total levels of AKT was seen upon deletion of IR, IGF-1R or both, suggesting that epidermal keratinocytes attempt to compensate for the loss of IR and/or IGF-1R. Deletion of IR, IGF-1R or both also did not obviously alter phosphorylation of a downstream target of AKT, GSK3β (Figure 3F).
Figure 3.
Apoptosis and proliferation in the epidermis in the absence of IR/IGF-1R. (A) TUNEL staining (green) on sections isolated from back skin of newborn mice. Nuclei were counterstained using DAPI (blue). Scale bar is 100 μm. (B) Ki67 staining on sections of back skin isolated from newborn mice. Scale bar is 100 μm. (C) Quantification of Ki67 staining in the basal cells of IFE of control and dko mice in back skin, palate or tongue epithelium. N=5 and P>0.05 for each genotype. (D) Quantification of Ki67 staining of the epidermis in embryos. N=4 mice/group, P>0.05 for E15.5 and 16.5, P<0.01 for E17.5 (E) H&E staining on paraffin sections from E15.5, E16.5 and E17.5 embryos. Scale bar is 50 μm. (F) Western blot analysis for the indicated proteins on epidermal lysates of newborn mice.
IGF-1R but not IR regulates epidermal proliferation in vivo and in vitro
In the epidermis, overexpression of IGF-1 or IGF-II results in hyperproliferation (Bol et al, 1997; Bennett et al, 2003), whereas MAPK activation is altered in keratinocytes deficient for IGF-1R (Sadagurski et al, 2006), thus providing a potential explanation for the hypomorphic epidermis. Surprisingly, no obvious change could be detected in staining for the proliferation marker Ki67 between control, IRepi−/−, IGF-1Repi−/− and dkoepi newborn epidermis (Figure 3B and C). As an even stronger decrease in layers was seen in the stratifying epithelia of palate and tongue, which may thus more obviously reveal alterations in proliferation, we also examined Ki67 staining in these tissues. Again, no significant difference in Ki67-positive cells was observed in control versus dkoepi palate and tongue epithelium (Figure 3C).
We next wanted to determine at which developmental stage loss of IR and IGF-1R affects interfollicular epidermal morphogenesis and whether this relates to temporary changes in proliferation. As the phenotype is most obvious in the dkoepi epidermis, we focused on these embryos. E15.5 dkoepi mice showed the expected 2–3 epidermal layers that are indistinguishable from control mice (Figure 3E), with no change in proliferation (Figure 3D). The first signs of a hypomorphic epidermis in the dkoepi mice became apparent at E16.5. Whereas control mice have formed a 4–6 layer epidermis at this stage, dkoepi epidermis remained a 3–4 layered epidermis more resembling the E15.5 mice (Figure 3E). Surprisingly, no changes in Ki67 (Figure 3D) or TUNEL staining (not shown) were found at E16.5, indicating that at this stage the inability to increase the number of suprabasal layers is not due to changes in proliferation or apoptosis. At E17.5, both the control and dkoepi mice showed epidermal stratification, even though the dkoepi epidermis remained hypomorphic. This coincided with impaired proliferation (Figure 3D) but not apoptosis (not shown). In contrast, E17.5 IRepi−/− embryos, which exhibit the mildest phenotype, showed no change in proliferation even though the epidermis was hypomorphic (Supplementary Figure 3A and B).
Defects in proliferation in vivo may be partially masked by mitogenic signals coming from the dermis. To directly examine the consequences of IR or IGF-1R signalling for proliferation, growth assays were performed with primary keratinocytes. Whereas IGF-1R keratinocytes did not grow in the absence of fibroblast feeders, no growth impairment was observed for the IR−/− keratinocytes in comparison to control keratinocytes isolated from littermates (Supplementary Figure 3C and D). Thus, cell-autonomous IGF-1R signalling regulates proliferation in keratinocytes but in vivo signals from the dermis most likely compensate at most time points.
Recently, it was demonstrated that inactivation of Mek1/2, upstream kinases of MAPK, in the epidermis almost completely abrogates MAPK activation in newborn mice. This, as observed in dkoepi mice, is associated with a hypomorphic epidermis and changes in proliferation only during embryogenesis (Scholl et al, 2007). However, we could not detect any differences in either total or active MAP kinase levels in the epidermis of control, IR-, IGF-1R- or IR/IGF-1R-negative epidermis (Figure 3F). This demonstrates that IR and IGF-1R are not the crucial activators of the MAPK pathway in murine epidermis, consistent with results in human keratinocytes (Haase et al, 2003). Taken together, the results show that IGF-1R but not IR regulates proliferation of keratinocytes, both in vivo and in vitro, and this most likely contributes to the hypoplastic epidermis in the IGF-1Repi−/− and dkoepi mice. However, more importantly, proliferative defects cannot solely be responsible as IR epidermis is hypomorphic without proliferative changes and the dkoepi hypomorphic epidermis is already obvious at E16.5 independent of proliferation.
IR and IGF-1R signalling regulate proliferative potential of primary keratinocytes
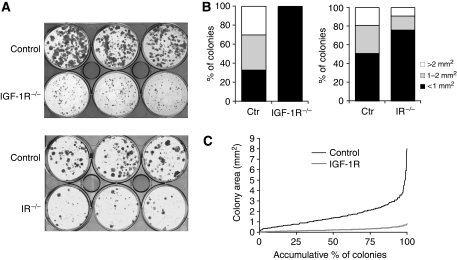
Insulin and IGF may directly regulate the proliferative potential of epidermal progenitor cells, similar to that observed for IGF-II using human embryonic stem cells (Bendall et al, 2007). Therefore, we assessed the colony-forming capacity of primary keratinocytes isolated from the control, IRepi−/− or IGF-1Repi−/− mice. A strong reduction in the size of the colonies, indicative of the number of cell divisions, was observed in IGF-1R−/− keratinocytes compared to control, with an almost complete loss of large-sized colonies (Figure 4A and B). A reduction in colony size was also observed in IR−/− keratinocytes compared to control (Figure 4A and B), although not as dramatic as the IGF-1R−/−. This is in line with the in vivo results showing that loss of the epidermal IR affects the thickness of the epidermis less severely than the loss of IGF-1R (Figure 2). When increasing colony size is plotted as a continuum against accumulative percentage of colonies, one could identify two different curves in control keratinocytes, one with a relative flat slope that represents 90% of all colonies and another where the steepness of the slope dramatically increases over the last 5–10% (Figure 4C). This indicates the presence of two different cell populations, one that represent over 90% of the colonies that have a similar, relatively low proliferative potential, and a second one, representing around 5–10% of the colonies with a much higher proliferative potential. This population likely represents epidermal progenitor cells. When comparing the curve for IGF-1R−/− cells the overall angle of the initial slope is less than in control, indicating that proliferative potential is reduced in all cells. In fact, for these cells the steepness of the curve remained unchanged, indicating that the population with high proliferative potential is almost completely absent in these cells (Figure 4C).
Figure 4.
Insulin/IGF-1R signalling affects the in vitro proliferative potential of keratinocytes. (A) Colony-forming assay using primary keratinocytes isolated from control, IRepi−/− or IGF-1Repi−/− mice. (B) Quantification of the colony-forming assays shown in (A). (C) Colonies of the control versus IGF-1R−/− keratinocytes plotted as increasing colony size against accumulating percentage of colonies.
Regulation of progenitor markers by IR and IGF-1R
To examine whether alterations in proliferative potential were affecting epidermal progenitor cells, we used the progenitor cell marker keratin 15 (K15). Both western blot analysis (Figure 5A) and real-time PCR analysis (Figure 5B) showed a dramatic reduction in the overall K15 protein and mRNA levels in dkoepi epidermis compared with control. Indeed, other epidermal stem/progenitor cell markers, such as Igfbp-5 (Blanpain et al, 2004) were reduced in dko epidermis compared with control (Figure 5B), providing further evidence that insulin and IGF-1 receptor signalling regulate an epidermal progenitor cell compartment.
Figure 5.
Expression of epidermal progenitor cell markers. (A) Western blot analysis for keratin 15 on lysates isolated from epidermis of newborn mice. Same amount of lysates was run on a separate gel and probed for actin to control for loading. (B) Real-time PCR analysis of epidermis for the indicated markers. N=5 for both control and dko. (C) Keratin 15 (green) staining on back skin of newborn mice. Nuclei were counterstained using propidium iodide (red). Scale bar is 100 μm. Inset shows high magnification of basal cell layer. (D) Quantification of K15-positive basal IFE cells and HF in a 500 μM area of control, IRepi−/−, IGF-1Repi−/− and dko epidermis. N=4 independent mice per genotype with 5–6 sections per mouse. (E) FACS analysis of CD34 expression on ctr, IGF-1R−/− and dko primary keratinocytes. The average expression of four independent experiments is shown. See Supplementary Figure 4A for a representative profile.
A recent study suggested that the colonies formed in the clonogenic assay are derived from the HF stem cells (Langton et al, 2008). Although they used number, and not size, of colonies as a read-out, this suggests that IR/IGF-1R may exert their effect on proliferative potential by affecting HF stem cells. Unlike IFE progenitor cells, HF stem cells are well characterized at the molecular level (Morris et al, 2004; Tumbar et al, 2004). Indeed, K15 is commonly used as a marker for hair follicle stem cells, although in newborn mice K15 is also expressed in basal interfollicular keratinocytes (Liu et al, 2003). We therefore assessed the epidermal compartment in which the loss of K15 occurred. A strong decrease in K15 staining was observed in IR-negative IFE compared to hair follicles (Figure 5C and D). In the absence of IGF-1R or in the dkoepi mice, K15 was strongly reduced in both compartments (Figure 5C). However, in these two mutants the decrease in K15 was also more pronounced in the IFE compared to hair follicles (Figure 5D). This suggested that IR mainly affects the interfollicular compartment, whereas IGF-1R signalling affects both progenitor cell compartments. Nevertheless, several other HF-specific stem cell markers such as CD34, Tcf4 and CdKn1b (Blanpain et al, 2004; Morris et al, 2004), were not changed in dkoepi compared to control, implying that the number of HF stem cells remained similar (Figure 5B). In addition, using FACS analysis, similar levels of expression were found for CD34 on control, IGF-1R or dko primary keratinocytes, suggesting that also in vitro no obvious loss of HF stem cells occurs (Figure 5E).
Reduction in IFE label-retaining cells in IGF-1Repi−/− mice
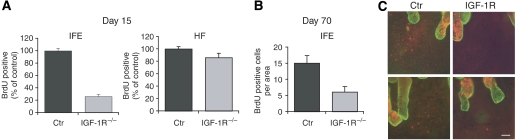
Progenitor cells are characterized by their ability to retain 5-bromo-2′-deoxyuridine (BrdU) for prolonged times after injection, both in the IFE and hair follicles. As dko mice die within the first 2 days, we assessed this property in IGF-1Repi−/− and control mice by injecting them for three consecutive days with BrdU and examining BrdU-positive cells after a chase of 15, 40 and 70 days. Compared to control a 80% reduction of BrdU-positive basal cells were found in the IFE of IGF-1Repi−/− mice after 15 days, whereas only a small but significant reduction was found in the number of hair follicles positive for BrdU (Figure 6A). The loss of label-retaining cells in the IFE, and subsequent epidermal thinning could also result from altered migration resulting in a shortened transition time. However, in vitro migratory properties of IGF-1R−/− keratinocytes were unchanged (not shown). Moreover, 24 h after labelling with BrdU, a method previously used to assess differences in transition time (Reichelt and Magin, 2002), did not reveal any difference either (Supplementary Figure 4B). The reduction in label-retaining cells was still observed after day 40, when the IFE has renewed at least once (Figure 6C). Indeed, after 70 days of chase a reduction of 60% in BrdU-positive cells was found in the IFE of IGF-1Repi−/− mice compared to control (Figure 6B).
Figure 6.
Loss of label-retaining cells in the IFE of IGF-1Repi−/− mice. (A) Quantification of BrdU-positive cells in 500 μm areas of sections of epidermis 15 days after BrdU labelling. N=3 mice per group; IFE: P<0.0005; HF: P<0.05. Counting of BrdU-positive cells in the IFE: all positive cells in a 500 μm area (5–6 sections per mice). Counting of BrdU-positive cells in HF: all BrdU-positive cells in a HF in a 500 μm area (5–6 sections per mice). (B) Quantification of BrdU-positive cells in 10 × 90 mm2 areas of IFE of whole mounts of ctr and IGF-1Repi−/− mice epidermis 70 days after BrdU labelling. N=2 mice per group. (C) BrdU staining (in red) of whole mounts of tail epidermis of ctr and IGF-1Repi−/− mice 40 days after BrdU labelling. Scale bar is 40 μm.
IR/IGF-1R are key regulators of the small GTPase Rac in the epidermis
We next sought to identify how IR and IGF-1R control proliferative potential of the IFE. Insulin and IGF-1R negatively affect the activity of Foxo transcription factors (Taniguchi et al, 2006; Arden, 2007), which have been implicated in stem cell regulation of the haematopoetic system (Tothova et al, 2007). A recent paper described a novel function of Foxo as a co-activator of RBJ (Kitamura et al, 2007). Given the critical role of Notch in driving basal keratinocytes towards differentiation (Blanpain et al, 2006), we decided to examine whether impaired epidermal IR/IGF-1R results in increased Notch activation, thereby promoting differentiation concomitant with a decrease in proliferative potential. However, we were unable to detect any difference in either RNA or protein levels of the Notch target Hes1, nor did we observe a change in Hes1 nuclear localization (Supplementary Figure 5). In addition, total amounts of Foxo1 RNA are decreased upon loss of IR and IGF-1R (Figure 5B), possibly as a consequence of reduced progenitor cells. Taken together, IR/IGF-1R ablation does not directly affect the Notch signal transduction pathway in the epidermis.
Another potential candidate by which IR and IGF-1R may determine proliferative potential of keratinocytes is by regulating the activity of the small GTPase Rac through the activation of PI3 kinase (Welch et al, 2003). Rac1 has recently been implicated in the maintenance of epidermal stem cells (Benitah et al, 2005; Castilho et al, 2007), but the upstream regulators in this process have not yet been identified. Analysis of Rac activity in IR- and IGF-1R-deficient epidermis revealed a dramatic 80% reduction in Rac activity compared to control, whereas total levels of Rac remained unaffected (Figure 7A and B). Importantly, Rac activation directly depended on insulin and IGF-1 stimulation in primary mouse keratinocytes (Figure 7C). As Rac activity can regulate c-Myc (Benitah et al, 2005), which itself negatively controls the number of progenitor cells in the IFE (Arnold and Watt, 2001; Waikel et al, 2001), we assessed whether c-Myc levels were altered in the absence of IR and IGF-1R. Both protein (Figure 7D and Supplementary Figure 6A) and RNA levels (Supplementary Figure 6B) of c-Myc were drastically increased in the epidermis of the IGF-1Repi−/− and dkoepi mice when compared to control. A similar increase in c-Myc levels was observed in primary dko keratinocytes (not shown).
Figure 7.
Insulin and IGF-1 signalling regulate epidermal Rac activity in vivo and in vitro. (A) Western blot analysis of Rac activity in the epidermis of newborn mice. A representative experiment is shown. (B) Quantification of Rac activity in the epidermis control and dko mice. N=9 for control and N=5 for dko mice; P<0.0001. (C) Insulin and IGF-1 directly activate the small GTPase Rac in mouse keratinocytes after serum starvation. Stimulation is in minutes. (D) Western blot analysis for c-Myc. Equal amounts of lysate were run on a separate gel and probed for actin.
IR/IGF-1R regulate proliferative potential through Rac in cultured keratinocytes
We next sought to examine whether IR/IGF-1R-dependent activation of Rac is indeed important for the regulation of proliferative potential of keratinocytes by IR/IGF-1R. We therefore transduced dko keratinocytes with either GFP or dominant active Rac (RacDA) lentivirus after which their colony-forming ability was assessed. The expression of RacDA increased colony size when compared to GFP (Figure 8A and B). Importantly, lentiviral expression of GFP in control cells did not impair but actually slightly enhanced colony-forming capacity (Supplementary Figure 7A). As RacDA may also directly affect the actin cytoskeleton, and thereby cell size, we wanted to rule out that the increase in colony size is not the result of increased cell size. The average number of cells per colony was significantly increased in RacDA compared to GFP expressing cells, thus showing that the larger colony size did not only result from an enhancement in cell size but instead resulted from increased proliferative potential (Figure 8C).
Figure 8.
Insulin/IGF-1R determine the proliferative potential of progenitor cells in vitro through the activation of the small GTPase Rac. (A) Colony-forming assay using dko primary keratinocytes transduced with either GFP lentivirus or RacDA lentivirus. (B) Quantification of size of colonies in colony formation assay. (C) Average number of cells in a colony. P<0.05.
Rac activation is necessary for epidermal morphogenesis in IGF-1Repi−/− mice
If Rac-dependent regulation of proliferative potential by IGF-1R/IR is relevant for in vivo epidermal morphogenesis, the prediction would be that in vivo expression of RacDA in the epidermis improves the hypomorphic epidermis induced by the loss of IR and/or IGF-1R. To directly test this hypothesis we generated mice with an epidermal deletion of IGF-1R in combination with K14 mediated basal expression of a Myc-tagged RacDA (IGF-1Repi−/−; RacDA, Supplementary Figure 7C) and compared the thickness of newborn epidermis and palate with those of IGF-1Repi−/− littermates. The expression of RacDA significantly ameliorated the hypomorphic epidermis caused by the absence of IGF-1R (Figure 9A and B), even though reversal is not complete. RacDA was even more efficient in rescuing the thinner palate in the IGF-1Repi−/− mice (Figure 9A and C). Thus, epidermal IR and IGF-1R are crucial regulators of the small GTPase Rac thereby determining proliferative potential and IFE morphogenesis.
Figure 9.
Rac activation is necessary for epidermal morphogenesis in the IGF-1Repi−/− mice. (A) H&E staining of back skin (scale bar is 50 μm) and palate (scale bar is 100 μm) of IGF-1Repi−/− and IGF-1Repi−/− RacDA mice. (B, C) Quantification of thickness of epidermis (B, without stratum corneum) and palate (C) in IGF-1Repi−/− and IGF-1Repi−/−; RacDA mice. N=5 mice per genotype. P=0.02.
Discussion
Our data reveal a novel and key function for insulin and IGF signalling in epidermal morphogenesis: their receptors cooperatively determine the number of interfollicular suprabasal layers and proliferative potential of keratinocytes by regulating the small GTPase Rac. This protein was previously shown to regulate stem cell maintenance of the epidermis and its appendages (Benitah et al, 2005; Castilho et al, 2007) but the identity of its upstream receptors was unknown. Our results show that epidermal Rac activation requires upstream signalling through insulin and IGF-1 receptors and that this activation contributes to epidermal morphogenesis in vivo and clonogenic capacity of epidermal keratinocytes in vitro. As insulin/IGF pathways also regulate metabolic activity in the epidermis (Wertheimer et al, 2001), this allows keratinocytes to couple energy capacity directly to proliferative potential of progenitor cells, thus assuring that self-renewal of the epidermis is appropriately fuelled.
Previously, it has been shown that IR regulates differentiation (Wertheimer et al, 2001), whereas IGF-1R signalling contributes to proliferation, apoptosis and differentiation in keratinocytes (Sadagurski et al, 2005). However, those studies were mostly carried out in vitro using keratinocytes isolated from either IR or IGF-1R knockout mice and in the absence of feeders. We confirm that IGF-1R regulates proliferation but our studies did not reveal any obvious impairment in epidermal differentiation or apoptosis in vivo in the absence of IR and IGF-1R. This indicates that although epidermal IR and IGF-1R contribute to these processes, signals from the dermis can compensate thus resulting in normal apoptosis, differentiation, and mostly normal proliferation.
To our knowledge, this is the first in vivo evidence that IR and IGF-1R signalling are coupled to the regulation of Rac. Although earlier studies demonstrated that IGF-1 signalling regulates Rac activity in vitro (for example, Meyer et al, 2005; Sugimoto et al, 2006), most of these were carried out in the context of migration and cytoskeletal alterations and none coupled this activation to proliferative potential of cells. Importantly, under normal physiological conditions IR and IGF-1R are key activators of Rac in the epidermis, as in the absence of both receptors overall Rac activity was reduced by around 80% (Figure 7B). This strong dependence on IR/IGF-1R activity is somewhat surprising in the light of the many other pathways implicated in the regulation of Rac (Etienne-Manneville and Hall, 2002). These may be more important in directing epidermal Rac activity in situations that perturb skin homoeostasis, for example, during wound repair.
By utilizing epidermal-specific knockout mice, Rac1, the most abundant member of the Rac subfamily of Rho GTPases in the epidermis, has previously been implicated in epidermal stem cell maintenance (Benitah et al, 2005; Castilho et al, 2007), even though the data are somewhat conflicting. Similar to the IGF-1epi−/− mice, Benitah et al (2005) and Benitah and Watt (2007) showed that the hair follicle compartment and the IFE are affected, whereas two other studies reported only defects in the hair follicle (Chrostek et al, 2006; Castilho et al, 2007). At present, the reason for these differential observations is unclear. The IR/IGF-1R epidermal mutant mice do not completely phenocopy the IFE phenotype of Racepi−/− mice. These latter mice (Benitah et al, 2005) initially showed a phase of hyperproliferation in the dkoepi mice followed by loss of the IFE. Instead, our mice showed an inability to increase the number of suprabasal layers during epidermal morphogenesis and, although the phenotype was sustained in adult IRepi−/− and IGF-1Repi−/− (Supplementary Figure 1B), no further loss of IFE occurred. This may be due to residual Rac activity (around 20%; Figure 7B) left in the dkoepi mice.
Basal expression of RacDA is able to only partially reverse the in vitro loss of proliferative potential and the hypomorphic epidermis induced by the loss of IR/IGF-1R. This may be due to low expression levels of c-Myc-tagged RacDA, which can be detected by western blot (Supplementary Figure 7C) but not by IF. Many groups have noted that high levels of RacDA expression are deleterious for cells. Alternatively, other downstream pathways contribute or RacDA activity is required in suprabasal cells, additionally to the basal layer.
Regulation of proliferation likely forms part of the mechanism by which epidermal IGF-1R but not IR regulates epidermal morphogenesis. This is consistent with results that show that overexpression of IGF-I or IGF-II results in epidermal hyperproliferation (Ward et al, 1984; DiGiovanni et al, 2000). Nevertheless, proliferative defects cannot solely explain the hypomorphic epidermis observed in all mutant mice for at least two reasons. First, no difference in proliferation can be observed in the IRepi−/− mice, and IR−/− keratinocytes show no growth defect when grown in vitro. Second, the observed difference in proliferation at E17.5 did not coincide with the first signs of the phenotype at E16.5 in the dkoepi−/− mice. In contrast, loss of proliferative potential was observed in IR−/−, IGF-1R−/− and dko keratinocytes (Figure 4A and B), thus correlating with the epidermal phenotype observed in the different mice. In line with this, overexpression of IGF-II expands the number of proliferative units in the epidermis (Bennett et al, 2003).
At present, we cannot exclude the possibility that IR/IGF-1R mediate their effect by regulating IFE progenitors, hair follicle stem cells or both. Indeed, the first signs of hypoplasia occur at E16.5 when the primary hair placodes have already formed (E14.5). Epidermal IGF-1 signalling may also have an effect on HF stem cells as IGF-1Repi−/− mice that survive showed hair follicle defects. However, this requires further characterization of these defects. On the other hand, several different observations suggest that IR/IGF-1R do not affect hair follicle stem cells. First, epidermal deletion of IR did not result in any obvious changes to hair follicle morphology and cycling. Moreover, key markers for HF stem cells (for example, CD34, Tcf4) were unaltered in dko in vivo and the percentage of CD34-positive cells was not significantly different in IR−/−, IGF-1R−/− or dko keratinocytes (Figure 5). Finally, the reduction in label-retaining cells was more substantial in the IFE than in hair follicles in the IGF-1Repi−/− mice. Taken together, the results suggest that IR only regulates the interfollicular compartment, whereas IGF-1R regulates both.
The mechanism by which IR/IGF-1R regulate proliferative potential of epidermal progenitor cells is less clear. IR/IGF-1R most likely do not regulate stem cell maintenance as the IFE phenotype is sustained but does not deteriorate over time in the adult IGF-1Repi−/− mice (Supplementary Figure 1B). Potentially, epidermal insulin and IGF signalling may regulate the activation of quiescent progenitor cells or set a threshold to the maximum number of cell divisions that progenitor cells can undergo. Alternatively, IR/IGF-1R signalling may act earlier by determining the number of (IFE) progenitor cells during epidermal morphogenesis. The observed epidermis loss of K15 (Figure 5) in all three mutant mice combined with a reduction in label-retaining cells in IGF-1R-deficient epidermis (Figure 6 and Supplementary Figure 4B) suggest the latter. However, confirmation of this model requires more detailed characterization of the interfollicular epidermal progenitor cell, which, at present, is only poorly defined (Kaur, 2006). In addition, virtually nothing is known regarding when and how progenitor cells are recruited and/or defined in the IFE. How then may a reduction in proliferative potential result in a hypomorphic epidermis during embryogenesis, even though proliferation or migration is not obviously impaired? Perhaps tissues have a ‘long-term' sense of the maximum number of divisions that their progenitor cells can undergo and directly couple this to the number of suprabasal layers that are formed to assure that stem cells can sustain the lifelong renewal that is necessary to maintain the epidermis.
Our findings suggest that insulin/IGF-1 may be a key component of the progenitor cell niche. Human embryonic stem cells create their own niche by generating fibroblasts, which then secrete IGF-II thereby regulating the proliferative potential of ES cells (Bendall et al, 2007). At present, it is not known which cells or compartments form the IFE niche. Similar to ES cells, epidermal progenitor cells might form their own niche as IGFs are not only produced by dermal cells but also by suprabasal keratinocytes (Edmondson et al, 2003). These cells may thereby directly communicate their requirements for renewal. Together with recent observations in human embryonic stem cells, our results indicate that insulin and IGF signalling through control of Rac activity may function as crucial set points for the proliferative capacity of stem/progenitor cells in general.
Materials and methods
Mice
The generation and genotyping of the IR floxed mice and the K14-Cre mice have been described (Brüning et al, 1998; Hafner et al, 2004). Generation of IGF-1R floxed mice and the crossings to generate IGF-1Repi−/−;K14-RacDA mice are described in the Supplementary data. IGFf-1R floxed mice and the K14-Cre mice are in Bl6, the IR floxed mice and the dkoepi−/− mice are Bl6 mixed Bl6 in the fourth to fifth generation.
Primary keratinocytes and colony-forming assays
Primary keratinocytes were isolated from the skin of newborn mice and passaged until P3 as described (Pasparakis et al, 2002). For colony-forming assays, 4000 cells were plated in triplicate in a six-well plate and cultured for 2–3 weeks in the presence of feeders. Feeders were changed two times a week. Because of mixed background, only keratinocytes isolated from littermates were compared. Cells were fixed with 1% PFA for 15 min and subsequently stained for 1 h with 0.05% crystal violet in PBS. For each knockout line, experiments were repeated for a minimum of three times with independently isolated keratinocytes. For RacDA rescue experiments, IGF-1R−/− or dko cells were transduced with lentivirus as described in Supplementary data. Three independent transduction experiments in combination with colony-forming assays were performed.
FACS analysis and antibodies
Keratinocytes were trypsinized, resuspended in PBS/EDTA and fixed for 2 h in 70% EtOH. After washing, cells were permeabilized with 0.01% saponin in 0.5% BSA/PBS and 0.5–1 × 106 cells were incubated with the primary FITC-labelled CD34 antibody for 1 h, washed and analysed.
BrdU label-retaining experiments
Short-term BrdU-labelling experiments were performed to visualize cells transiting in the epidermis. Postnatal mice were injected intraperitoneally with BrdU at 100 mg/kg bodyweight and killed following a 24-h chase period. To compare populations of quiescent label-retaining cells, 10-day-old mice were injected with BrdU at 50 mg/kg bodyweight every 24 h three times. Following a 15-, 40- or 70-day chase period, the mice were killed, and dorsal skin (day 15) and tail skin whole mounts (day 40 and 70) were isolated for immunostaining and analysis.
Isolation of epidermis and whole mount tail skin
Epidermis was separated from the dermis by floating skin biopsies, epidermis side up, in a 0.5 M ammonium thiocyanate (NH4SCN) in phosphate buffer, pH 6.8 (0.1 M Na2HPO4, 0.1 M KH2PO4) for 20 min on ice. Epidermis was either snap frozen in liquid nitrogen or processed for RNA isolation or protein lysates. Whole mounts of mouse tail epidermis were prepared as previously described (Braun and Watt, 2004). Briefly, whole skin was removed from the tail of mice and incubated for 3 h at 37°C in 5 mM EDTA/PBS. The epidermis was separated from the dermis and fixed in 4% formalin for 2 h at room temperature.
Immunohistochemistry
Immunohistochemistry was performed on paraffin sections, cryosections (6 μm) or whole mounts, which were fixed with 4% PFA or methanol and processed as described in Supplementary data. Photos were taken with a NIKON Eclipse E800 microscope equipped with a NIKON DMZ1200 camera or a LEICA confocal microscope.
Antibodies, Rac activation assays and western blot analysis
Antibodies used are listed in the Supplementary data. Rac activation assays and western blot were carried out as described previously using freshly isolated skin biopsies, epidermis or keratinocytes (Tunggal et al, 2005).
Gene expression analysis
Gene expression was analysed using quantitative RT–PCR on RNA as described in Supplementary data.
Supplementary Material
Supplementary Figures
Supplementary Figure Legends
Supplementary Material
Acknowledgments
We thank Karla Seifert and Monika Petersson for experimental help and suggestions. We thank Thomas Krieg and Catherin Niemann for discussion and critical reading of the paper. We appreciate the help of Hamid Kaskhar, Benjamin Yazdanpah (Institute for Medical Microbiology, Immunology and Hygiene, Cologne), Alexander Peifer and Andreas Hofmann (Institute of Pharmacology and Toxicology, University of Bonn) in setting up the lentiviral system and Professor C Ronald Kahn (Harvard Medical School, Boston) for providing us with the IR floxed mice. This study was supported by SFB589, DFG NI 689/3-1 and Köln Fortune to CMN and Br1492/7 to JCB.
Contributions of the authors HI, HS, IH, JCB and CMN designed the experiments. HI, HS, LK, AS, JS and CMN carried out the experiments. CM generated the RacDA lentivirus. LK and TW generated the IGF-1R floxed mice. MT and IH generated the K14-RacL61 mice. CW assisted with the evaluation of histochemistry and immunohistochemistry. CMN wrote the paper.
References
- Arden KC (2007) FoxOs in tumor suppression and stem cell maintenance. Cell 128: 235–237 [DOI] [PubMed] [Google Scholar]
- Arnold I, Watt FM (2001) c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol 11: 558–568 [DOI] [PubMed] [Google Scholar]
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, Lajoie G, Bhatia M (2007) IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448: 1015–1021 [DOI] [PubMed] [Google Scholar]
- Benitah SA, Frye M, Glogauer M, Watt FM (2005) Stem cell depletion through epidermal deletion of Rac1. Science 309: 933–935 [DOI] [PubMed] [Google Scholar]
- Benitah SA, Watt FM (2007) Epidermal deletion of Rac1 causes stem cell depletion, irrespective of whether deletion occurs during embryogenesis or adulthood. J Invest Dermatol 127: 1555–1557 [DOI] [PubMed] [Google Scholar]
- Bennett WR, Crew TE, Slack JM, Ward A (2003) Structural-proliferative units and organ growth: effects of insulin-like growth factor 2 on the growth of colon and skin. Development 130: 1079–1088 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E (2006) Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev 20: 3022–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol DK, Kiguchi K, Gimenez-Conti I, Rupp T, DiGiovanni J (1997) Overexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic mice. Oncogene 14: 1725–1734 [DOI] [PubMed] [Google Scholar]
- Braun KM, Watt FM (2004) Epidermal label-retaining cells: background and recent applications. J Investig Dermatol Symp Proc 9: 196–201 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569 [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Patel V, Millar SE, Zheng Y, Molinolo A, Gutkind JS (2007) Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene 26: 5078–5085 [DOI] [PubMed] [Google Scholar]
- Chrostek A, Wu X, Quondamatteo F, Hu R, Sanecka A, Niemann C, Langbein L, Haase I, Brakebusch C (2006) Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol Cell Biol 26: 6957–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH (2007) A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189 [DOI] [PubMed] [Google Scholar]
- DiGiovanni J, Bol DK, Wilker E, Beltran L, Carbajal S, Moats S, Ramirez A, Jorcano J, Kiguchi K (2000) Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res 60: 1561–1570 [PubMed] [Google Scholar]
- Edmondson SR, Thumiger SP, Werther GA, Wraight CJ (2003) Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev 24: 737–764 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Haase I, Evans R, Pofahl R, Watt FM (2003) Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci 116: 3227–3238 [DOI] [PubMed] [Google Scholar]
- Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkotter O, Shephard P, Kudlow JE, Smola H, Haase I, Schippers A, Krieg T, Muller W (2004) Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38: 176–181 [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354 [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ (2008) No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 9: 11–21 [DOI] [PubMed] [Google Scholar]
- Jones P, Simons BD (2008) Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat Rev Mol Cell Biol 9: 82–88 [DOI] [PubMed] [Google Scholar]
- Kaur P (2006) Interfollicular epidermal stem cells: identification, challenges, potential. J Invest Dermatol 126: 1450–1458 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, DePinho RA, Kitajewski J, Accili D (2007) A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 117: 2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR (2004) p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 18: 126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton AK, Herrick SE, Headon DJ (2008) An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 128: 1311–1318 [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA (2005) Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9: 855–861 [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75: 59–72 [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G (2003) Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol 121: 963–968 [DOI] [PubMed] [Google Scholar]
- Meyer G, Kim B, van Golen C, Feldman EL (2005) Cofilin activity during insulin-like growth factor I-stimulated neuroblastoma cell motility. Cell Mol Life Sci 62: 461–470 [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, Krieg T, Rajewsky K, Haase I (2002) TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417: 861–866 [DOI] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N (2003) Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17: 1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4: 505–518 [DOI] [PubMed] [Google Scholar]
- Reichelt J, Magin TM (2002) Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci 115: 2639–2650 [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Nofech-Mozes S, Weingarten G, White MF, Kadowaki T, Wertheimer E (2007) Insulin receptor substrate 1 (IRS-1) plays a unique role in normal epidermal physiology. J Cell Physiol 213: 519–527 [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Weingarten G, Rhodes CJ, White MF, Wertheimer E (2005) Insulin receptor substrate 2 plays diverse cell-specific roles in the regulation of glucose transport. J Biol Chem 280: 14536–14544 [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Yakar S, Weingarten G, Holzenberger M, Rhodes CJ, Breitkreutz D, Leroith D, Wertheimer E (2006) Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol Cell Biol 26: 2675–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Barragan DI, Harada K, Bissonauth V, Charron J, Khavari PA (2007) Mek1/2 MAPK kinases are essential for mammalian development, homeostasis, and Raf-induced hyperplasia. Dev Cell 12: 615–629 [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F (2007) p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129: 523–536 [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Takuwa N, Yoshioka K, Takuwa Y (2006) Rho-dependent, Rho kinase-independent inhibitory regulation of Rac and cell migration by LPA1 receptor in Gi-inactivated CHO cells. Exp Cell Res 312: 1899–1908 [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96 [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339 [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM (2005) E-Cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 24: 1146–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR (2001) Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet 28: 165–168 [DOI] [PubMed] [Google Scholar]
- Ward A, Bates P, Fisher R, Richardson L, Graham CF (1994) Disproportionate growth in mice with Igf-2 transgenes. Proc Natl Acad Sci USA 91: 10365–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Lo Celso C, Silva-Vargas V (2006) Epidermal stem cells: an update. Curr Opin Genet Dev 16: 518–524 [DOI] [PubMed] [Google Scholar]
- Welch HC, Coadwell WJ, Stephens LR, Hawkins PT (2003) Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett 546: 93–97 [DOI] [PubMed] [Google Scholar]
- Wertheimer E, Spravchikov N, Trebicz M, Gartsbein M, Accili D, Avinoah I, Nofeh-Moses S, Sizyakov G, Tennenbaum T (2001) The regulation of skin proliferation and differentiation in the IR null mouse: implications for skin complications of diabetes. Endocrinology 142: 1234–1241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Figure Legends
Supplementary Material