Abstract
Severe illness, type 2 cytokine production, and pulmonary eosinophilia are adverse immune responses resulting from respiratory syncytial virus (RSV) challenge of vvGs-immunized mice. We have shown IL-4 and IL-13 activity must be simultaneously inhibited to reduce disease severity. We now address the contributions of IL-5, eotaxin-1, and CD4+ and CD8+ T cells to the induction of disease-enhancing immune responses. Depletion of CD4+ T cells during immunization prevented IL-4, IL-13, and eotaxin-1 production, diminished eosinophilia, and reduced weight loss. Conversely, CD8+ T cell depletion did not decrease eosinophilia, weight loss, or type 2 cytokines but did dramatically reduce mucus production and increase eotaxin production. Anti-IL-5 administration at immunization or challenge significantly decreased pulmonary eosinophilia. Strikingly, there were not concomitant decreases in weight loss. Following RSV challenge eotaxin-1-deficient mice immunized with vvGs exhibited significantly less eosinophilia without decreased weight loss or type 2 cytokine production. We conclude CD4+ T cell production of IL-5 and induction of eotaxin-1 are required for vvGs-induced eosinophilia following RSV challenge, while CD8+ T cells appear to down-regulate eotaxin-1 and mucus production. In summary, we demonstrate that pulmonary eosinophilia 1) is a by-product of memory CD4+ T cell activation, 2) does not necessarily correlate with mucus production, and, most importantly, 3) is not required for the RSV G-induced illness in mice. These findings have important implications for the evaluation of candidate RSV vaccines.
Keywords: vaccine, pathogenesis, cytokines, chemokines, immunoregulation
INTRODUCTION
A challenge to the development of a safe and effective vaccine against respiratory syncytial virus (RSV) is the history of failed vaccine trials [1, 2] and the occurrence of vaccine-enhanced disease following live RSV challenge in animal models immunized with formalin-inactivated alum-precipitated RSV (FI-RSV) or vaccinia virus expressing RSV G glycoprotein (vvG) [3, 4]. This vaccine-enhanced disease is typified by greater illness, pulmonary eosinophilia, and production of type 2-associated cytokines and chemokines, particularly IL-4, IL-5, IL-13, and eotaxin-1. Similarly, type 2 cytokine production, eosinophils, and their degranulation products have been strongly correlated to disease severity in RSV-infected children during primary RSV infection [5,6,7,8,9]. These associations between RSV infection, eosinophil recruitment and activation, and disease severity in both human disease and animal models suggest that eosinophils may have a direct and causative role in severe RSV disease [10,11,12].
Type 2 T cells producing IL-4, IL-5, and IL-13 have been shown to mediate this severe disease and to produce pulmonary eosinophilia in mice immunized with FI-RSV or vvG [13, 14]. Transfer of G-specific CD4+ T cells to naive mice resulted in eosinophil recruitment and severe disease upon RSV infection. In contrast, in the absence of CD8+ T cells or IFN-γ, immunization with vvG resulted in increased pulmonary eosinophilia following RSV challenge [15, 16]. Thus, type 1 CD8+ T cells or natural killer cells, through production of IFN-γ, appear to serve a protective role by modulating induction of immune responses that predispose for eosinophil recruitment [15,16,17].
IL-5 plays a role in the proliferation, differentiation, and recruitment of B cells and eosinophils. In addition to roles in eosinophil proliferation, differentiation, and recruitment [18,19,20,21], IL-5 primes eosinophils for recruitment by other cytokines and chemokines, such as IL-8 [22], RANTES [22], and eotaxin-1 [20]. Thus, IL-5 is involved in eosinophil function at multiple stages, including development, chemotaxis, and activation. The effects of eotaxin on eosinophil recruitment [23, 24] and function [25, 26] are clearly defined. However, the regulation of eotaxin production is more ambiguous. Antigen exposure [27,28,29] and cytokines, including IL-1β, TNF-α, IFN-γ, IL-4, IL- 5, and serotonin [20, 26, 30,31,32,33] have been shown to induce or synergize with eotaxin. In contrast, other work demonstrates IL-5 and IFN-γ have no role or an inhibitory role in eotaxin production [32, 34, 35]. The existence of eotaxin-independent mechanisms of eosinophil recruitment was demonstrated when it was shown that mice deficient in eotaxin-1 or eotaxin-2 had significant, but incomplete, reductions in eosinophil recruitment [23]. These data demonstrate the multilayered complexity involved in regulating eosinophil activation, recruitment, and function.
Immunization with vaccinia virus expressing the secreted form of RSV G (vvGs) induces immune responses that predispose for increased production of IL-5, IL-13, and eotaxin-1, as well as severe pulmonary eosinophilia following subsequent RSV challenge [36, 37]. It has also been demonstrated that induction of G-specific responses does not require IL-4 or IL-13 [38, 39]. Using the model of vvGs immunization followed by RSV challenge, we now extend these data by investigating the contributions of CD4+ and CD8+ T cells, IL-5, and eotaxin-1 to pulmonary eosinophilia and its correlation with disease severity in vvGs-immunized RSV-challenged mice. While not a vaccine candidate itself, the use of vvGs provides a well-defined system in which the immune response to RSV G glycoprotein may be examined. Inhibition of IL-5 activity at the time of RSV challenge has been shown to decrease pulmonary eosinophilia in vvG-immunized mice [40]. Thus, we asked whether interference with IL-5 or whether the genetic absence of eotaxin-1 altered disease severity, pulmonary eosinophilia, or the production of type 2 cytokines in vvGs-immunized RSV-challenged mice. CD4+ and CD8+ T cells have been shown to perform distinct functions in RSV disease [14, 41]. The contributions of CD4+ and CD8+ T lymphocytes during induction of Gs-specific immune responses were, therefore, examined by depletion of these T cell subsets, alone or in combination, during vvGs immunization.
MATERIALS AND METHODS
Virus stocks
HEp-2 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in Eagle’s minimal essential media (EMEM) supplemented with 10% fetal calf serum, glutamine, and antibiotics (10% EMEM). A laboratory stock of RSV A2 strain was prepared, as described previously [36]. Stocks of vaccinia virus expressing either secreted RSV G (a gift of Gail W. Wertz, University of Alabama at Birmingham, designated vvGs) or β-galactosidase (a gift of Bernard Moss, National Institutes of Health, Bethesda, MD, USA; designated vac-lac) were generated as described [36]. All virus stocks were demonstrated to be free of mycoplasma contamination by PCR analysis.
Cell lines and monoclonal antibodies
Hybridoma cell lines GK1.5 and 2.43, producing rat monoclonal antibodies against murine CD4 and CD8 respectively, were gifts of Steven Martin (University of Tennessee, Knoxville, TN, USA). Anti-murine IL-5 monoclonal antibodies are produced by the hybridomas TRFK-4 and TRFK-5, which were gifts of Dr. Tim Mosmann. The HB151 hybridoma, specific for human HLA-DR5, (ATCC, Rockville, MD, USA) was used as an IgG2b isotype control for GK1.5 and 2.43. CRL1741 (IgG2a) and CRL1742 (IgG1), specific for p21 protein (v-ras and c-ras), were purchased from ATCC and used as isotype controls for the TRFK-4 and TRFK-5 antibodies, respectively. Ascites fluid was prepared in BALB/c nu/nu mice for all monoclonal antibodies, as described previously [41]. Concentrations of IgG in the ascites fluids were determined by sandwich ELISA.
Mice immunization and challenge
Wild-type BALB/c mice were purchased from Harlan Sprague Dawley (Indianapolis, IN, USA). Eotaxin-1-deficient mice were generated and characterized on a 129 SvEv genetic background [23]. Eotaxin-1-deficient and wild-type 129 SvEv mice were bred in house and colonies maintained in accordance with The Guide for the Care and Use of Laboratory Animals. The mice were primed with vaccinia virus and challenged with RSV 6 weeks later, as described previously [36]. With vaccinia virus detectable for at least 2 wk postimmunization [42], this 6-wk period allows 3-4 wk for resolution of antiviral immune responses induced by the vaccinia virus vectors. CD4+ T cells, CD8+ T cells, or both subsets were depleted at the time of immunization by administration of GK1.5, 2.43, or both GK1.5 and 2.43 ascites fluid. To deplete the T cells, 200 μg (in 200 μl PBS) of the appropriate antibody was injected intraperitoneally on days –1, 0, and 1 of priming. The requirements for IL-5 during induction of G-specific immune responses were examined by administration of antibodies at priming or at RSV challenge. The mice were treated with the isotype control or with anti-IL-5 on days –2, –1, 0, 1, and 2 around immunization and days –2, –1, 0, 1, and 2 around the time of challenge by injecting 200 μg (in 200 μl PBS) of the appropriate antibody intraperitoneally.
Cytokine and chemokine levels
Cytokine and chemokine protein levels in lung supernatants from day 4 plaque assays were quantitated by ELISA using cytokine-specific kits (R&D Systems, Minneapolis, MN, USA).
Bronchoalveolar lavage (BAL) eosinophils
Six or seven days following challenge, BAL was performed. BAL cell pellets were differentially stained with Diff-Quik (Fisher Scientific, Pittsburgh, PA, USA), and eosinophil numbers were counted as described previously [38].
Lung histopathology
Six or seven days after RSV challenge, mice were euthanized and the left lung was removed and placed in phosphate-buffered formalin (10% formalin). Thin sections were cut from paraffin-embedded lungs and stained with hematoxylin and eosin, with Wright Giemsa stain to assess tissue eosinophilia, or with Periodic Acid Schiff stain (PAS) to evaluate mucus production. As a semiquantitative analysis of induction of mucus, goblet cells were counted in H&E stained tissue sections, counting cells in small to medium airways cut in cross section. The goblet cell counts were calculated as the average of three airways in lung sections from each mouse and then reported as the mean and standard deviation for each treatment group.
Statistical analysis
Illness, weight loss, and viral titers data were maintained in a Paradox database and analyzed with SAS statistical software, as described previously [36]. Data for cytokine and chemokine levels and BAL eosinophilia were analyzed by ANOVA, comparing multiple groups using SigmaStat 3.0.1 software (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
RESULTS
Depletion of CD4+ T cells during RSV G immunization predisposes for less severe disease following RSV challenge
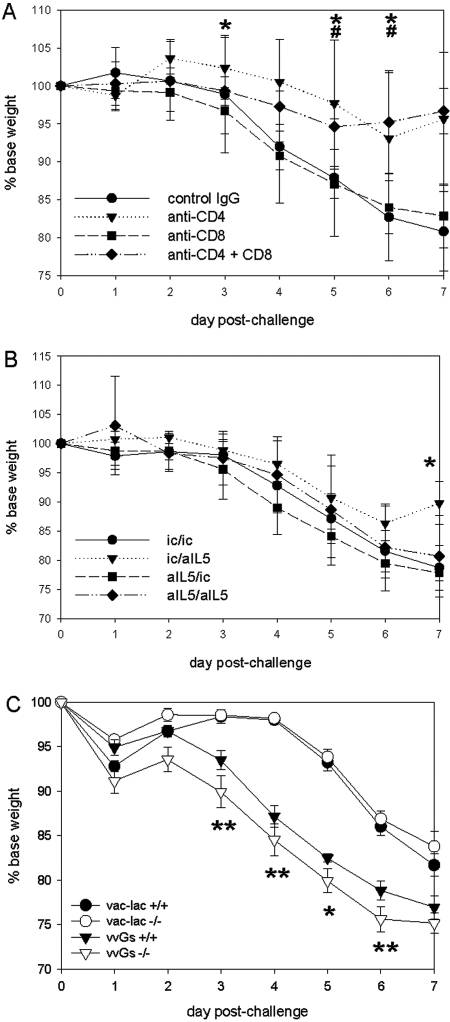
We evaluated the contribution of CD4+ and CD8+ T cells to RSV G-induced immunopathology by depletion of CD4+, CD8+, or both CD4+ and CD8+ T cells during vac-lac or vvGs immunization. By 5 wk postimmunization, the numbers of CD4+ and CD8+ T cells in the blood had returned to similar levels as isotype control-treated mice, as evaluated by flow cytometry (data not shown). At 6 wk postimmunization, the mice were challenged intranasally with live RSV. T-cell depletion had no statistically significant impact on illness in vac-lac-primed mice (data not shown). Following RSV challenge, illness (as evidenced by weight loss) was less severe in vvGs-primed mice depleted of CD4+ or both CD4+ and CD8+ T cells during priming (Fig. 1A). In vvGs-primed mice, differences were significant between isotype control-treated mice and CD4-depleted mice at days 3-6 after challenge (P<0.05), while weight loss was significantly decreased in mice depleted of both CD4+ and CD8+ T cells only on days 5 and 6. Thus, G-specific CD4+ T cells are necessary for illness in vvGs-immunized mice.
Fig. 1.
Determinants of illness in vvGs-immunized mice. The mice were immunized with vac-lac or vvGs by intradermal injection at the base of the tail 5 × 105 plaque-forming units (pfu) in 0.05 ml PBS of vvGs. Six weeks after immunization mice were challenged with 1 × 107 pfu (in 0.1 ml) live respiratory syncytial virus (RSV), and then the mice were weighed daily to assess illness. (A) Weight loss in mice that were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells at the time of vvGs immunization by intraperitoneal (IP) administration of specific antibodies on days –1, 0, and 1 of priming (0.2 mg per injection) is depicted. (B) Weight loss in mice depleted of IL-5 at immunization or at challenge by intraperitoneal injection of anti-IL5-specific antibodies at days –2, –1, 0, 1, and 2 of immunization or challenge (0.2 mg of antibody per injection) is illustrated. (C) Weight loss in eotaxin-1 deficient mice is shown. The weight loss is shown as percentage of baseline weight. The data represent mean ± sd for n = 15 mice from 3 separate experiments (A and B) and n = 5 from a single experiment (C). All data were maintained in a Paradox database and analyzed by ANOVA using SAS software. Differences were considered to be significant when P < 0.05. Significant differences are denoted by * (vvGs-immunized mice depleted of CD4 at priming (A) or IL-5 at challenge (B) had significant improvement in weight loss relative to isotype control-treated vvGs-immunized mice); #, (CD4+CD8-depleted vvGs-immunized mice had significant improvement in weight loss relative to isotype control-treated mice); and ** (vvGs-immunized mice significantly increased relative to vac-lac-immunized mice).
Interference with IL-5 activity does not reduce illness in vvGs-primed mice after RSV challenge
Mice were immunized with vvGs or FI-RSV and treated with anti-IL-5 antibodies, as described previously. Mice were weighed daily for 7 days after RSV challenge. Anti-IL-5 treatment had no significant impact on illness in vac-lac-primed mice (data not shown). In vvGs-primed mice treated with anti-IL-5 at the time of challenge only, recovery was accelerated, as evidenced by the significant increase in weight at day 7 relative to isotype control-treated vvGs-primed mice (Fig. 1B). No other significant differences were observed in weight loss following RSV challenge of anti-IL-5-treated vvGs-immunized mice. Thus, interference with IL-5 activity has minimal impact on weight loss following RSV challenge of vvGs-immunized mice.
The absence of eotaxin-1 does not significantly alter disease severity in RSV-challenged mice
As previously reported in BALB/c and C57BL/6 mice [36, 38], vvGs immunization of 129SvEv mice predisposed for more severe illness following RSV challenge with statistically significant differences observed at days 3-6 postchallenge (Fig. 1C, P<0.05 relative to either wild-type or eotaxin-1-deficient vac-lac-primed mice). Eotaxin-1-deficient mice immunized with vvGs and challenged with RSV exhibited a pattern of weight loss similar to that seen in vvGs-immunized wild-type 129 SvEv mice.
Production of IL-4, IL-13, IFN-γ, eotaxin-1, and MIP-1α upon challenge is reduced by depletion of CD4+ T cells during RSV G priming, while depletion of CD8+ T cells increases eotaxin-1 expression
Cytokine and chemokine levels in lung supernatants 4 days after RSV challenge were measured (Table 1). As previously reported [36, 38, 39], IL-4, IL-5, IL-13, eotaxin-1, MIP-1α, and MIP-1β levels are significantly increased in vvGs-immunized mice (P<0.05 relative to vac-lac-immunized mice), demonstrating that vvGs induces a memory T cell response that produces type 2 cytokines (Table 1). IL-4, IL-5, and eotaxin-1 levels were not significantly altered in vac-lac-primed mice by either CD4+ or CD8+ T cell depletion (Table 1). IL-5 and MIP-1β production were not significantly affected by either CD4+ or CD8+ T cell depletion in vvGs-immunized mice. However, in mice depleted of CD4+ T cells during vvGs immunization (alone or in combination with CD8+ T cells), IL-4, IL-13, IFN-γ, eotaxin-1, and MIP-1α levels were significantly reduced upon RSV challenge (P<0.05 relative to isotype control-treated mice). Conversely, CD8+ T cell depletion during vvGs immunization significantly increased production of eotaxin-1 following RSV challenge (P=0.037). Thus, CD4+ T cells play a major role in producing or inducing both type 1 and type 2 cytokines and eotaxin-1 in vvGs-immunized mice. In contrast, CD8+ T cells appear to diminish eotaxin-1 production. This observation provides the first potential mechanistic explanation for the ability of CD8+ T cell depletion and interference with IFN-γ to increase RSV-induced eosinophilia.
TABLE 1.
Cytokine and Chemokine Levels After Challenge of Mice Depleted of CD4+ and CD8+ T Cells During vvGs Immunization
| Priming | Antibody | Cytokine/chemokine levelsa
|
||||||
|---|---|---|---|---|---|---|---|---|
| IL-4 | IL-5 | IL-13 | IFN-γ | Eotaxin | MIP-1α | MIP-1β | ||
| vac-lac | Isotype | 6.2 ± 3.1 | 3.3 ± 0.6 | 0.5 ± 0.2 | 105.2 ± 26.2 | 1.4 ± 0.1 | 163.3 ± 26.4 | 575.0 ± 133.6 |
| Anti-CD4 | 5.4 ± 3.8 | 1.7 ± 0.2 | 0.4 ± 0.1 | 73.2 ± 13.0 | 1.4 ± 0.2 | 264.4 ± 25.9 | 1616.1 ± 198.6 | |
| Anti-CD8 | 1.9 ± 0.8 | 4.7 ± 1.5 | 0.5 ± 0.1 | 385.2 ± 29.3 | 25.6 ± 3.2 | 70.5 ± 19.6b | 425.4 ± 67.1 | |
| Anti-CD4 + Anti-CD8 | 1.7 ± 1.1 | 7.1 ± 2.0 | 0.5 ± 0.1 | 39.6 ± 10.7 | 10.2 ± 4.3 | 54.5 ± 12.5b | 246.5 ± 58.0 | |
| vvGs | Isotype | 55.0 ± 12.9 | 61.4 ± 7.6 | 142.3 ± 54.4 | 432.4 ± 16.1 | 139.2 ± 37.2 | 1299.6 ± 205.0 | 2205.1 ± 423.1 |
| Anti-CD4 | 8.2 ± 2.5b | 49.7 ± 14.5 | 3.7 ± 0.9b | 73.2 ± 3.1b | 45.5 ± 8.8b | 445.8 ± 66.2b | 1216.2 ± 211.8 | |
| Anti-CD8 | 79.9 ± 7.7 | 47.5 ± 5.1 | 167.9 ± 25.4 | 385.2 ± 29.3 | 296.0 ± 41.8 | 1728.1 ± 184.2 | 2855.6 ± 96.3 | |
| Anti-CD4 + Anti-CD8 | 3.8 ± 1.3b | 42.1 ± 7.8 | 2.9 ± 1.3b | 39.6 ± 1.3b | 21.5 ± 5.8b | 858.5 ± 114.4 | 2185.1 ± 243.2 | |
Data represent the concentration (pg/ml) of cytokine or chemokine in the lung 4 days postchallenge in 15 mice from 3 separate experiments, except for MIP-1α and MIP-1β, where n = 6 from a single experiment. The limits of detection are 10 pg/ml for the IL-4, IL-5, and IL-13 kits and 50 pg/ml for the IFN-γ, eotaxin, MIP-1α, and MIP-1β kits.
Statistically significant (P<0.05) compared to mice of the same priming group treated with isotype control antibody. Mice were immunized with vac-lac or vvGs by intradermal injection at the base of the tail of 5 × 105 plaque-forming units (pfu) in 0.05 ml PBS of vvGs. At the time of priming, mice were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells by intraperitoneal (IP) administration of specific antibodies on days −1, 0, and 1 of priming (0.2 mg per injection). Six weeks after immunization, mice were challenged with 1 × 107 pfu (in 0.1 ml) live respiratory syncytial virus (RSV). Four days after RSV challenge, mice were euthanized, and the lungs were removed and frozen for subsequent analyses. The lung tissues were ground with mortar and pestle, and the suspension was clarified by centrifugation. The protein levels of cytokines and chemokines in these lung supernatants were then measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the kit protocol. The protein concentrations were calculated by linear regression of a positive control included in the kit and are shown in picograms per milliliter. Statistical analyses included Kruskal-Wallis ANOVA and Dunn’s t test using the SigmaStat software package. Differences were considered statistically significant when P < 0.05.
Anti-IL-5 treatment during challenge decreases IL-4, IL-13, and eotaxin-1 levels in vvGs-primed mice
Significantly lower levels of IL-5 were detected in lung supernatants from vvGs-primed mice treated with anti-IL-5 (Table 2, P<0.01, comparing mice treated with anti-IL-5 at challenge to isotype control-treated mice). Administration of anti-IL-5 at the time of immunization did not result in a statistically significant change in any other cytokine or chemokine measured following RSV challenge (Table 2). In contrast, neutralization of IL-5 only at challenge or at both the time of immunization and challenge significantly lowered IL-4, IL-13, and eotaxin-1 levels (Table 2, P<0.05). Thus, interference with IL-5 activity during immunization impacts cytokine production, reducing IL-4, IL-5, IL-13, and eotaxin-1 levels.
TABLE 2.
Cytokine and Chemokine Levels After Challenge of Anti-IL-5-Treated vvGs-Immunized Mice
| Priming | Antibodies at priming/challenge | Cytokine/chemokine levelsa
|
||||||
|---|---|---|---|---|---|---|---|---|
| IL-4 | IL-5 | IL-13 | IFN-γ | Eotaxin | MIP-1α | MIP-1β | ||
| vac-lac | ic/aIL5b | 24.2 ± 4.6 | 170.8 ± 19.5 | 4.7 ± 2.6 | 1696 ± 151 | 25.6 ± 8.2 | 580 ± 224 | 1063 ± 276 |
| aIL5/ic | 8.0 ± 2.0 | 142.3 ± 0.1 | NDb | 233 ± 49 | 30.3 ± 6.1 | 543 ± 111 | 1328 ± 289 | |
| vvGs | ic/ic | 86.4 ± 16.3 | 360.3 ± 93.5 | 146.2 ± 35.4 | 1730 ± 311 | 185.0 ± 26.4 | 2002 ± 278 | 1692 ± 304 |
| ic/aIL5 | 13.5 ± 3.0c | 184.3 ± 31.8c | 4.9 ± 3.4c | 1230 ± 188 | 55.6 ± 9.4c | 1469 ± 231 | 1613 ± 129 | |
| aIL5/ic | 74.5 ± 10.4 | 149.9 ± 37.1c | 153.2 ± 15.2 | 2167 ± 275 | 219.1 ± 24.7 | 1626 ± 264 | 1975 ± 292 | |
| aIL5/aIL5 | 23.9 ± 3.1c | 153.4 ± 10.2c | 14.8 ± 7.1c | 1676 ± 189 | 122.0 ± 20.0 | 2319 ± 274 | 2174 ± 341 | |
Data represent the concentration (pg/ml) of cytokine or chemokine in the lung 4 days postchallenge in 15 mice from 3 separate experiments except for MIP-1α and MIP-1β, where n = 6 from a single experiment. The limits of detection are 10 pg/ml for the IL-4, IL-5, and IL-13 kits and 50 pg/ml for the IFN-γ, eotaxin, MIP-1α, and MIP-1β kits.
ic, isotype control antibody; aIL5, anti-IL-5 antibodies; ND, not detectable.
Statistically significant (P<0.05) compared to mice of the same priming group treated with isotype control antibody at both priming and challenge. Mice were immunized with vac-lac or vvGs by intradermal injection at the base of the tail of 5 × 105 plaque-forming units (pfu) in 0.05 ml PBS of vvGs. At either the time of priming or at the time of RSV challenge, IL-5 was neutralized by intraperitoneal (IP) administration of specific antibodies on days −2, −1, 0, 1, and 2 of priming or of challenge (0.2 mg per injection). Six weeks after immunization mice were challenged with 1 × 107 pfu (in 0.1 ml) live RSV. Four days after RSV challenge, mice were euthanized, and the lungs were removed and frozen for subsequent analyses. The lung tissues were ground with mortar and pestle, and the suspension was clarified by centrifugation. The protein levels of cytokines and chemokines in these lung supernatants were then measured using commercially available ELISA kits (R&D Systems), according to the kit protocol. The protein concentrations were calculated by linear regression of a positive control included in the kit and are shown in picograms per milliliter. Statistical analyses included Kruskal-Wallis ANOVA and Dunn’s t test using the SigmaStat software package. a,b,c Differences were considered statistically significant when P < 0.05.
Eotaxin-1 deficiency does not significantly alter the production of other cytokines following RSV challenge of vvGs-immunized mice
Apart from the absence of eotaxin in vvGs-immunized eotaxin-deficient mice, only modest changes were observed in cytokine levels of eotaxin-deficient mice after RSV challenge (Table 3). Reductions observed in IL-13 and IFN-γ production in both vac-lac- and vvGs-immunized mice were not statistically significant.
TABLE 3.
Cytokine and Chemokine Levels After Challenge of vvGs-Immunized Eotaxin-Deficient Mice
| Priming | Mouse strain | Cytokine/chemokine levelsa
|
||||||
|---|---|---|---|---|---|---|---|---|
| IL-4 | IL-5 | IL-13 | IFN-γ | Eotaxin | MIP-1α | MIP-1β | ||
| vac-lac | Wild-type CD1 | 9.8 ± 7.7 | 488.2 ± 88.8 | 60.8 ± 22.6 | 132.8 ± 35.9 | NDb | 119.3 ± 23.5 | 188.6 ± 15.4 |
| Eotaxin-deficient | NDb | 74.2 ± 12.3 | NDb | 62.2 ± 12.2 | NDb | 163.2 ± 53.5 | 147.3 ± 16.6 | |
| vvGs | Wild-type CD1 | 71.4 ± 6.9 | 222.7 ± 30.6 | 124.9 ± 24.8 | 2159.4 ± 88.5 | 298.7 ± 14.7 | 832.0 ± 171.9 | 727.9 ± 102.9 |
| Eotaxin-deficient | 36.5 ± 11.7 | 181.4 ± 27.7 | 84.9 ± 5.9 | 930.0 ± 54.7 | NDb | 438.3 ± 15.2 | 541.2 ± 12.4 | |
Data represent the concentration (pg/ml) of cytokine or chemokine in the lung 4 days postchallenge in 5 mice from a single experiment. The limits of detection are 10 pg/ml for the IL-4, IL-5, and IL-13 kits and 50 pg/ml for the IFN-γ, eotaxin, MIP-1α, and MIP-1β kits.
ND, not detectable. Wild-type or eotaxin-deficient mice were immunized with vac-lac or vvGs by intradermal injection at the base of the tail of 5 × 105 plaque-forming units (pfu) in 0.05 ml PBS of vvGs. Six weeks after immunization, mice were challenged with 1 × 107 pfu (in 0.1 ml) live RSV. Four days after RSV challenge, mice were euthanized, and the lungs were removed and frozen for subsequent analyses. The lung tissues were ground with a mortar and pestle, and the suspension was clarified by centrifugation. The protein levels of cytokines and chemokines in these lung supernatants were then measured using commercially available ELISA kits (R&D Systems), according to the kit protocol. The protein concentrations were calculated by linear regression of a positive control included in the kit and are shown in picograms per milliliter. Statistical analyses included Kruskal-Wallis ANOVA and Dunn’s t test using the SigmaStat software package.b Differences were considered statistically significant when P < 0.05.
In addition to eotaxin and the CCR3 ligands RANTES and MCP2-4 [43], CXCR3 ligands have been shown to bind CCR3 [44]. Therefore, levels of CCR3 and CXCR3 ligands were examined to verify CCR3 functionality and to determine whether production of these chemokines were increased as compensation for the global deficiency of eotaxin-1. We found no significant change in levels of RANTES, I-TAC, Mig, or IP-10 between wild-type and eotaxin-1-deficient mice of the same immunization group (data not shown).
Inflammation and pulmonary eosinophilia following challenge is reduced by CD4+ T cell depletion during RSV G immunization, while depletion of CD8+ T cells increases tissue eosinophilia but reduces mucus production
Work from multiple labs has demonstrated abundant pulmonary eosinophilia following RSV challenge of RSV G-immunized mice with low levels of neutrophil (PMN) recruitment [15, 36, 37, 45]. However, increased neutrophilia has been reported in several models, generally following RSV challenge of FI-RSV-immunized animals [3, 46, 47]. Similarly, eosinophils are commonly found in airways of children with RSV bronchiolitis [6, 48]. Furthermore, PMN recruitment has been reported in severe human disease [49, 50], but such increases are not always found [7]. We therefore examined eosinophil and PMN levels in bronchoalveolar lavage (BAL) of vvGs-immunized RSV-challenged mice. Depletion of CD4+ T cells during vvGs priming resulted in reduced recruitment of eosinophils to the airways following RSV challenge (Table 4, P<0.0001). In contrast, CD8+ T cell depletion increased pulmonary eosinophilia, although the difference in vvGs-primed mice did not reach statistical significance (P=0.08), while increases in vac-lac immunized mice were significant (P=0.01).
TABLE 4.
Presence of BAL Eosinophils and Neutrophils and Airway Goblet Cells
| Antibodya | Vac-lac
|
vvGs
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % cells in BAL
|
No. of cells in BAL (×104)
|
No. of goblet cells in small airways | % cells in BAL
|
No. of cells in BAL (×104)
|
No. of goblet cells in small airways | |||||
| Eos | PMN | Eos | PMN | Eos | PMN | Eos | PMN | |||
| ic | 4.7 ± 0.2 | 4.1 ± 0.7 | 1.2 ± 0.1 | 1.1 ± 0.2 | 8.1 ± 2.8 | 14.6 ± 0.6 | 4.2 ± 0.7 | 5.0 ± 0.4 | 1.4 ± 0.2 | 37.1 ± 6.8 |
| aCD4 | 4.3 ± 0.6 | 5.2 ± 0.6 | 1.5 ± 0.2 | 1.7 ± 0.2 | 9.8 ± 2.5 | 6.7 ± 0.6c | 5.2 ± 0.1c | 2.8 ± 0.2 | 2.1 ± 0.1 | 33.8 ± 7.2 |
| aCD8 | 20.0 ± 1.0c | 5.1 ± 0.4 | 1.8 ± 0.1c | 0.5 ± 0.04 | 15.3 ± 7.4 | 17.5 ± 1.6 | 4.7 ± 0.7 | 5.5 ± 1.1 | 1.5 ± 0.4 | 13.7 ± 4.1c |
| aCD4 + aCD8 | 8.6 ± 1.7 | 3.3 ± 0.9 | 0.9 ± 0.2 | 0.3 ± 0.1 | 11.1 ± 5.4 | 6.3 ± 0.8c | 2.8 ± 0.5 | 1.0 ± 0.2c | 0.4 ± 0.1 | 11.6 ± 3.7c |
| ic/ic | 1.8 ± 0.3 | 1.9 ± 0.5 | 1.0 ± 0.4 | 1.6 ± 0.5 | 8.1 ± 2.6 | 18.6 ± 1.8 | 1.5 ± 0.3 | 13.0 ± 1.0 | 3.0 ± 0.3 | 25.8 ± 6.5 |
| ic/aIL5 | NTb | NTb | NTb | NTb | NTb | 5.3 ± 0.5c | 2.1 ± 0.2 | 2.7 ± 0.4c | 1.7 ± 0.3 | 31.7 ± 7.3 |
| aIL5/ic | NTb | NTb | NTb | NTb | NTb | 6.3 ± 1.1c | 1.7 ± 0.2 | 4.6 ± 0.4c | 2.5 ± 0.2 | 28.6 ± 8.9 |
| aIL5/aIL5 | 2.2 ± 0.3 | 2.2 ± 0.3 | 1.4 ± 0.3 | 2.1 ± 0.2 | 10.7 ± 3.6 | 5.2 ± 0.9c | 2.2 ± 0.6 | 3.3 ± 0.5c | 1.9 ± 0.2 | 25.9 ± 2.8 |
| Eotaxin +/+ | 1.7 ± 0.3 | 2.0 ± 0.4 | 1.1 ± 0.3 | 1.4 ± 0.4 | 8.0 ± 2.0 | 12.1 ± 0.9 | 6.0 ± 0.9 | 14.5 ± 2.2 | 7.5 ± 1.6 | 23.9 ± 2.6 |
| Eotaxin −/− | 3.5 ± 0.3 | 6.1 ± 0.6 | 1.9 ± 0.2 | 3.3 ± 0.3 | 13.7 ± 3.8 | 3.4 ± 0.5c | 6.3 ± 0.3 | 2.2 ± 0.5c | 3.9 ± 0.4 | 32.5 ± 6.5 |
Antibody given is the same as defined in Tables 1and 2, and genetic background of mice is the same as defined in Table 3.
NT, not tested. Mice were immunized, antibody treated, and RSV challenged as described in the Materials and Methods and in the legends for Tables 123. Six or seven days after RSV challenge, the mice were euthanized, and bronchoalveolar lavage (BAL) was performed by washing the lungs with 0.5 ml PBS through an incision in the trachea. The BAL cells were pelleted and differentially stained, and the total number of live cells in the BAL was determined by Trypan blue exclusion and counting. The relative percentages and numbers of eosinophils and neutrophils were determined by counting at least 300 cells per sample. Following BAL, the left lung was inflated with formalin, fixed by submersion in formalin, then paraffin-embedded. Hematoxylin-and-eosin and Periodic Acid Schiff stained thin sections (5 μm) were prepared for each lung. The numbers of goblet cells were identified by cytologic appearance and were determined by counting cells in 3 small to medium airways. The counts were performed in airways that were cut in cross section, were located at distinct and well-separated locations in the lung, and were representative of the airways throughout the lung. Statistical analyses included Kruskal-Wallis ANOVA and Dunn’s t test using the SigmaStat software package.
Differences were considered statistically significant when P < 0.05.
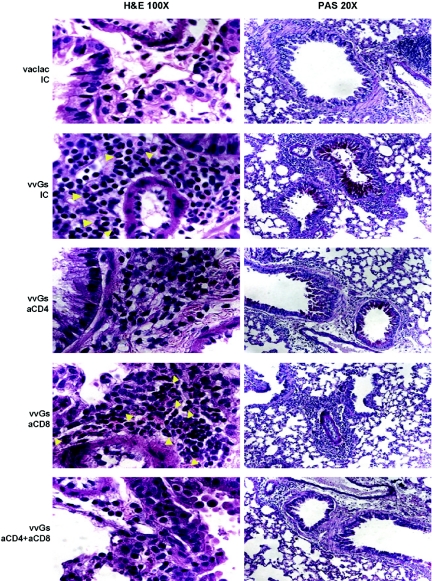
Depletion of CD4+ T cells during vvGs immunization, either alone or in conjunction with CD8+ T cells, reduced both the severity of pulmonary inflammation and the degree of eosinophilia following RSV challenge (Fig. 2). In contrast, depletion of CD8+ T cells during vvGs immunization did not reduce the degree of inflammation as evidenced by pronounced peribronchiolar infiltration, which was comparable to that observed in isotype control-treated vvGs-primed mice, but did increase the extent of eosinophilia in the lung tissue (Fig. 2).
Fig. 2.
Depletion of CD4+ T cells reduces eosinophilia, while depletion of CD8+ T cells increases eosinophil recruitment and reduces mucus production. Seven days after RSV challenge, mice were euthanized, BAL was performed, and lungs were removed and formalin-fixed. Thin sections (5-μM thickness) were stained with hematoxylin and eosin or with Period-Acid Schiff. Yellow arrows indicate representative eosinophils. Images from one representative mouse (of 15 across 3 separate experiments) for each group is shown.
Although CD4 depletion reduced pulmonary eosinophilia and the production of type 2-associated cytokines and chemokines, the extent of PAS staining (Fig. 2) and the number of goblet cells in the small and medium airways (Table 4) were comparable between vvGs-immunized mice treated with isotype control antibody or with anti-CD4. Surprisingly, depletion of CD8+ T cells had dramatic effects on mucus production with 60% of mice having no PAS-positive cells in either small or large airways and the remaining 40% of mice having fewer numbers of airway goblet cells, demonstrating PAS staining (Fig. 2). Similarly, fewer goblet cells were observed in the airways of mice treated with anti-CD8 alone or anti-CD8 in combination with anti-CD4 (Table 4). Depletion of CD4+ and CD8+ T cells did not alter the extent or composition of the pulmonary infiltrates in any vac-lac-immunized mouse (data not shown). Thus, while CD4+ T cells appear to regulate type 2 cytokine production, pulmonary eosinophilia, and illness in vvGs-immunized mice, these immune mediators are not sufficient or necessary for the production of mucus. Rather, factors produced by CD8+ T cells appear to limit eosinophil recruitment and, paradoxically, to be necessary to promote mucus production.
Anti-IL-5 treatment of vvGs-immunized mice reduces pulmonary eosinophilia following RSV challenge
Significant eosinophilia resulted after challenge of vvGs-immunized mice treated with isotype control antibodies at both priming and challenge (Table 4). However, anti-IL-5 antibody administration at either the time of immunization or at the time of challenge reduced eosinophilia (P<0.001 comparing vvGs-immunized anti-IL-5-treated mice to isotype control-treated control mice). Although BAL eosinophilia in vvGs-immunized mice was reduced by administration of anti-IL-5 antibody, the degree of eosinophilia was still significantly greater than in vac-lac- and mock-primed mice (P=0.045), suggesting minimal recruitment of eosinophils may occur independently of IL-5 or that neutralization of IL-5 was incomplete.
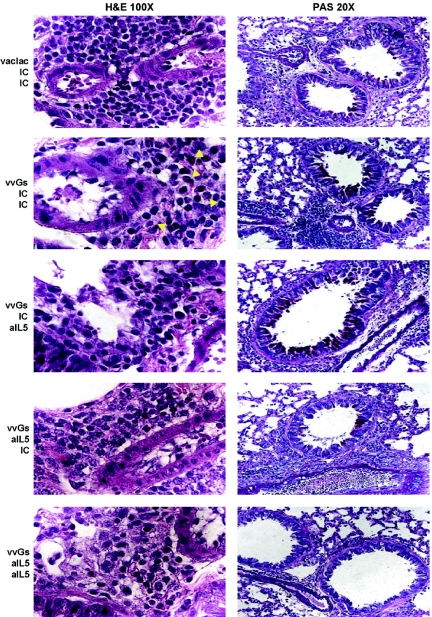
In vvGs-immunized mice treated with isotype control antibody at both immunization and challenge, peribronchiolar inflammation composed of lymphocytes and eosinophils is observed (Fig. 3). Reflecting the findings of reduced eosinophils in the large airways as evaluated by BAL, in mice treated with anti-IL-5 antibody at immunization only, at RSV challenge only, or at both time points, there is a conspicuous absence of eosinophils in the lung tissue. In contrast to the observations in anti-CD4-treated mice, this reduction in pulmonary eosinophilia was not accompanied by reduced inflammation around the bronchovascular bundles, where comparable numbers of mononuclear cells were observed in all vvGs-immunized mice.
Fig. 3.
Neutralization of IL-5 at priming or at challenge reduces eosinophilia. Seven days after RSV challenge, mice were euthanized, and lung sections were prepared as in Figure 2. Yellow arrows indicate representative eosinophils. Images from one representative mouse (of 15 across 3 separate experiments) for each group is shown.
Anti-IL-5 antibody during RSV challenge increased both the number of PAS-positive airways and the number of PAS-positive cells in each airway (Fig. 3). In contrast, inhibition of IL-5 function during immunization reduced mucus production following RSV challenge, as evaluated by PAS staining, although corresponding reductions in the number of goblet cells were not observed (Table 4). Thus, inhibition of IL-5 function at either the time of immunization or at the time of RSV challenge reduces pulmonary eosinophilia but does not alter the magnitude of pulmonary inflammation.
Eotaxin-1-deficient mice have significantly less BAL and pulmonary eosinophilia than wild-type vvGs-immunized mice but exhibit increased mucus production following RSV challenge
Immunization of wild-type 129 SvEv mice, an outbred genetic background, with vvGs resulted in significant pulmonary eosinophilia in the large airways following RSV challenge (Table 4, P<0.001 comparing wild-type vac-lac- and vvGs-primed mice), although at lower levels than seen in vvGs-immunized BALB/c mice (Table 4). However, in the genetic absence of eotaxin-1, BAL eosinophilia was significantly reduced (Table 4, P=0.003 comparing vvGs-immunized wild-type and eotaxin-1-deficient mice).
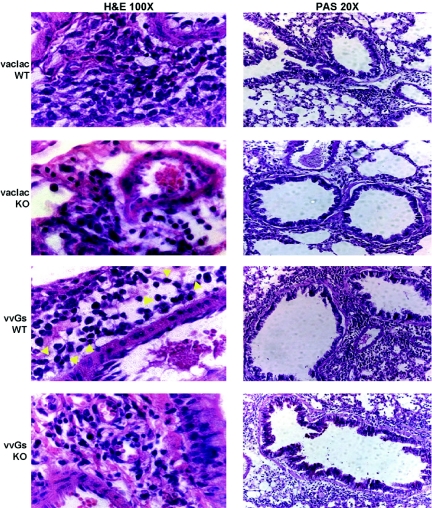
As seen in inbred strains of mice, vac-lac immunization and RSV challenge of wild-type 129 SvEv mice resulted in recruitment of modest numbers of mononuclear cells to the lung tissue, while vvGs immunization predisposed for increased peribronchiolar inflammation with a clear eosinophilic component following RSV challenge (Fig. 4). As in BAL, tissue eosinophilia was significantly reduced in vvGs-immunized eotaxin-1-deficient mice, but the extent of inflammatory cuffing around the bronchovascular bundles is similar between wild-type and eotaxin-1-deficient mice. Thus, in contrast to some studies of ovalbumin-induced eosinophilia [23, 51], genetic disruption of eotaxin-1 results in significant reductions in both BAL and tissue eosinophilia in vvGs-immunized RSV-challenged mice.
Fig. 4.
Genetic deficiency of eotaxin-1 eliminates eosinophilia in vvGs-immunized, RSV-challenged mice. Six days after RSV challenge, mice were euthanized, and lung sections were prepared as in Figure 2. Yellow arrows indicate representative eosinophils. Images from one representative mouse (of 5) for each group is shown.
In this 129 SvEv genetic background, vvGs immunization induces minimal mucus production following RSV challenge (Fig. 4) in contrast to the pattern observed in BALB/c mice (Fig. 2 and 3). However, in the absence of eotaxin-1, a distinct increase in the number of PAS-positive mucus-producing cells in vvGs-immunized mice occurred. This increase in PAS staining was paralleled by an increased number of goblet cells in the small and medium airways (Table 4). Therefore, while reducing RSV G-induced eosinophil recruitment, the absence of eotaxin-1 favored the induction of immune responses that promote mucus production in RSV-infected mice.
Production of CXCR2 ligands KC and MIP-2 are elevated in CD8-depleted vvGs-immunized mice
CXCR2 has been shown to play a role in mucus production in the respiratory epithelium [52, 53]. Treatment with anti-CXCR2 antibody or CXCR2 deficiency reduced mucus and airway hyperreactivity during RSV infection of mice [52]. Similarly, administration of CXCR2 antagonists reduced globet cell hyperplasia at low doses [53]. We therefore examined the production of the CXCR2 ligands KC and MIP-2 in these lung supernatants to determine whether the changes in mucus production in these mice correlated with alterations in production of CXCR2 ligands. We found no significant change in KC or MIP-2 levels in any treatment group in the anti-IL-5-treated or eotaxin-deficient mice (data not shown). Similarly, in vvGs-immunized mice depleted of CD4+ T cells alone or of both CD4+ and CD8+ T cells, little change was seen (Table 5). In contrast, production of both KC and MIP-2 was elevated in CD8-depleted mice (Table 5). While these increases were not statistically significant relative to isotype control-treated mice, the changes were significant when compared with the CD4-depleted mice, which may suggest a role for CD4+ T cells in promoting production of these chemokines. The lack of an increase in eotaxin-deficient mice when elevated mucus production is seen, and the increased production in mice depleted of CD8+ T cells that exhibit marked reduction in mucus are surprising, given the ability of CXCR2 inhibition to decrease mucus [52, 53]. These data suggest RSV G glycoprotein induces goblet cell hyperplasia by an alternative pathway not regulated by CXCR2 ligands.
TABLE 5.
Levels of CXCR2 Ligands After Challenge of Mice Depleted of CD4+ and CD8+ T Cells During vvGs Immunization
| Priming | Antibody | Chemokine levelsa
|
|
|---|---|---|---|
| KC | MIP-2 | ||
| vvGs | Isotype | 238.3 ± 97.7 | 10.7 ± 8.9 |
| Anti-CD4 | 214.6 ± 131.1 | 8.9 ± 8.4 | |
| Anti-CD8 | 554.7 ± 361.6b | 21.9 ± 6.9b,c | |
| Anti-CD4 + Anti-CD8 | 158.1 ± 103.1 | 6.9 ± 5.8 | |
Data represent the concentration (pg/ml) of cytokine or chemokine in the lung 4 days postchallenge in 9 mice from 2 separate experiments. The limits of detection are 2 pg/ml.
Statistically significant (P<0.05) compared to mice treated with anti-CD4 antibody only.
Statistically significant (P<0.05) compared to mice treated with both anti-CD4 and anti-CD8 antibody. Cytokine and chemokine levels after RSV challenge of mice depleted of CD4+ and CD8+ T cells during vvGs immunization. Mice were immunized with vac-lac or vvGs by intradermal injection at the base of the tail of 5 × 105 plaque-forming units (pfu) in 0.05 ml PBS of vvGs. At the time of priming, mice were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells by intraperitoneal (IP) administration of specific antibodies on days −1, 0, and 1 of priming (0.2 mg per injection). Six weeks after immunization, mice were challenged with 1 × 107 pfu (in 0.1 ml) live RSV. Four days after RSV challenge, mice were euthanized, and the lungs were removed and frozen for subsequent analyses. The lung tissues were ground with a mortar and pestle, and the suspension was clarified by centrifugation. The protein levels of cytokines and chemokines in these lung supernatants were then measured using commercially available ELISA kits (R&D Systems) according to the kit protocol. The protein concentrations were calculated by linear regression of a positive control included in the kit and are shown in picograms per milliliter. Statistical analyses included Kruskal-Wallis ANOVA and Dunn’s t test using the SigmaStat software package. b,c Differences were considered statistically significant when P < 0.05.
DISCUSSION
Pulmonary eosinophilia has been strongly associated with severe RSV disease in both RSV-infected children and in animal models of RSV pathogenesis. Thus, in both humans and animal models, pulmonary eosinophilia in severe RSV disease has been hypothesized to play a direct role in potentiating RSV disease [10,11,12, 48, 54]. Previous work in animal models has demonstrated that FI-RSV-induced vaccine-enhanced disease is reduced by depletion of CD4+ T cells [13] and by interference with IL-4 or IL-13 activity [38, 39, 55, 56]. Additionally, it was shown that transfer of RSV G-specific CD4+ T cells resulted in eosinophilia and illness upon RSV infection [14], that CD8+ T cells reduce eosinophil recruitment in vvG-primed mice [15, 16], and that vvGs-induced disease is decreased in the absence of functional IL-4 and IL-13 [38, 39]. Here, we examine the relative roles of T cells, IL-5, and eotaxin-1 in RSV G-induced illness by selected interventions that specifically diminish eosinophil and/or T-cell responses during vvGs immunization and RSV murine model.
It has been shown that, in the absence of IL-5 and eotaxin-1, eosinophil activation and recruitment are greatly reduced in allergen-sensitized animals [51, 57,58,59]. We now demonstrate that memory CD4+ T cells elicited during vvGs immunization are necessary for pulmonary eosinophilia following RSV challenge, as shown in transfer studies [14] and by depletion studies in FI-RSV-immunized mice [13]. While CD4+ T cells are required for RSV-induced weight loss, we demonstrate for the first time that IL-5 and eotaxin-1 are not (Figs. 234 and Table 6). Similar changes in eosinophil recruitment were observed in both the BAL and parenchyma. These data are the first demonstration in a model of RSV vaccine-enhanced disease to show discordance between the induction of pulmonary eosinophilia and more severe illness. In addition, we show discordance between the induction of airway mucus production and lung pathology and eosinophilia.
TABLE 6.
Summary of Combined Results
| Intervention | Illness | BAL Eos | Tissue Eos | Mucus | IL-13 | IFN-γ | Eotaxin | MIP-1α/β | CXCR2 ligands |
|---|---|---|---|---|---|---|---|---|---|
| anti-CD4 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | — |
| anti-CD8 | — | — | ↑ | ↓↓ | — | — | ↑ | — | ↑ |
| anti-CD4 + anti-CD8 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | — |
| anti-IL-5 at priming | — | ↓ | ↓ | ↓ | — | — | — | — | — |
| anti-IL-5 at challenge | — | ↓ | ↓ | — | ↓ | — | ↓ | — | — |
| anti-IL-5 at both | — | ↓ | ↓ | ↓ | ↓ | — | ↓ | — | — |
| eotaxin-1-deficient | — | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | — | — |
The data from all previous figures and tables have been condensed into a non-numerical representation of the results to facilitate comparisons across each experimental endpoint and each experimental treatment. This depiction is designed to more readily elucidate trends across the data and allow potential mechanistic connections to be made.
Our data are in conflict with an earlier report demonstrating that anti-eotaxin-1 antibody given during RSV challenge of vvG-immunized mice reduced CD4+ T cell and eosinophil recruitment, decreased IL-5 levels, and, in one of two experiments, diminished weight loss [60]. These differences may be explained by the impact of global eotaxin-1 deficiency at both immunization and challenge in comparison with eotaxin-1 neutralization at the time of challenge only or potentially by other unknown developmental adaptations in eotaxin-1-deficient mice. While the intervention described in this report will impact both the induction and effector stages of RSV G-specific T cell responses, neutralization at challenge will impact only the effector stage of the immune response established by vvG immunization.
When the production of cytokines and chemokines is examined in these various treatment groups, two distinct patterns emerge (as summarized in Table 6). In the first pattern, type 2 cytokine production, tissue eosinophilia, and mucus production are altered by multiple interventions, including CD4+ T cell depletion, neutralization of IL-5, or eotaxin-1 deficiency. In contrast, the second pattern of response demonstrates that illness, IFN-γ levels, and the production of MIP-1α or MIP-1β were reduced only by depletion of CD4+ T cells with no impact apparent following interference with IL-5 or eotaxin-1 activity.
These data suggest that distinct subsets of CD4+ T cells are induced during vvG immunization. vvGs priming induces a subset(s) of memory CD4+ T cells that, upon reactivation during RSV challenge, produce type 1 and 2 cytokines, induce mucus production, and recruit eosinophils to the lung tissue and large airways. IL-5 and eotaxin-1 induced by vvGs are required for the generation of these T cell responses. In addition, a second subset(s) of CD4+ T cells are induced by IL-5- and eotaxin-1-independent pathways that are associated with weight loss, but not eosinophil recruitment. MIP-1α and MIP-1β production associated with the IL-5-independent CD4+ T cells most closely parallels the resulting illness.
While generation of an oligoclonal Vβ-restricted CD4+ T cell response to vvG-immunization has been described [61], this report describes the first data that suggests this response may be further separated into T cell subsets with distinct effector functions. These results suggest that inhibition of eosinophil activation during vvGs immunization, by either IL-5 neutralization or eotaxin-1 deficiency, alters the function or presence of some CD4+ T cell subpopulation(s). This may potentially be explained by the demonstration that two signals are required for eosinophil activation [62].
It must be noted that the mice used in these studies were deficient in eotaxin-1 only. Since murine eotaxin-2 has been shown to have a more restricted tissue distribution and is not induced as readily as eotaxin-1 [63, 64], it is possible that murine eotaxin-2 would not be produced at sufficient levels in the skin or draining lymph nodes following vvGs immunization to compensate for the loss of eotaxin-1 production. This loss of eotaxin-1, either via direct effects on CCR3+ CD4+ T cells or by indirect effects through recruited eosinophils, may subsequently alter activation or differentiation of CD4+ T cells that then result in eosinophilia during RSV challenge.
In the studies described in this paper, we influenced BAL and tissue eosinophilia in the prime-challenge setting through a variety of approaches. Importantly, we found that eosinophilia did not correlate with weight loss, mucus production, or pathology, indicating that it is not a determinant of illness. Rather, illness is most strongly correlated with CD4+ T cells, suggesting that eosinophilia is a by-product of a subset of CD4+ T cells involved in the inflammatory response. A corollary of this is that the CD4-mediated illness is caused by factors other than eosinophils or their granular components. The only cytokine or chemokine measured in these experiments that consistently correlated with illness was MIP-1α, presumably produced by CD4+ T cells or indirectly influenced by the presence of CD4+ T cells primed by vvGs immunization. Other unmeasured factors associated with the vaccine-induced CD4+ T cells may also be involved. Interestingly, MIP-1α is also a correlate of illness in a murine model of pneumovirus of mice (PVM) [65].
Another finding of this study was the increased eosinophilia when CD8+ T cells were depleted. While described previously as a consequence of IFN-γ deficiency [15, 16], we demonstrate for the first time an association with elevated eotaxin-1 levels (Table 1). The precise pathway for this regulation remains to be elucidated. Paradoxically, mucus production was dramatically reduced when CD8+ T cells were depleted and increased in the absence of eotaxin-1 (Table 6). Although links between CD8+ T cells, eotaxin-1, and mucus hypersecretion have not been described, our data suggest these processes may be connected and deserve additional investigation. Given the demonstrated role for CXCR2 in mucus hypersecretion in models of airway inflammation [52, 53], we postulated that vvGs-immunized eotaxin-1-deficient mice might have elevated levels of CXCR2 ligands and CD8-depleted vvGs-primed mice might have reduced levels of CXCR2 ligands. However, the converse was observed, so alternative hypotheses will need to be explored.
Using a murine model of vvGs immunization and subsequent RSV challenge, we have made detailed investigations into the pathogenesis of RSV, particularly the contributions of various cytokines, chemokines, and cell populations to pulmonary eosinophilia and illness. We conclude that pulmonary eosinophilia is not a surrogate marker for mucus production and is not a correlate for illness in this model. While a Th2-biased immune response is commonly associated with a constellation of immunological and clinical findings, such as eosinophilia and mucus production, each aspect of disease expression has an independent set of determinants. These findings further refine the parameters that should be evaluated in the preclinical assessment of candidate RSV vaccines.
Acknowledgments
We gratefully acknowledge Dr. Srinivas Rao in assistance in learning to accurately identify goblet cells in the mouse airway and Dr. Joan Durbin for insightful discussions concerning mouse airway physiology and mucus production as it particularly relates to the RSV model.
References
- Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Prince G A, Jenson A B, Hemming V G, Murphy B R, Walsh E E, Horswood R L, Chanock R M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B S, Henderson G S, Tang Y-W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- Tachibana A, Kimura H, Kato M, Nako Y, Kozawa K, Morikawa A. Respiratory syncytial virus enhances the expression of CD11b molecules and the generation of superoxide anion by human eosinophils primed with platelet-activating factor. Intervirology. 2002;45:43–51. doi: 10.1159/000050086. [DOI] [PubMed] [Google Scholar]
- Dimova-Yaneva D, Russell D, Main M, Brooker R J, Helms P J. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34:555–558. doi: 10.1111/j.1365-2222.2004.1918.x. [DOI] [PubMed] [Google Scholar]
- Noah T L, Ivins S S, Murphy P M, Kazachkova I, Moats-Staats B, Henderson F W. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin Immunol. 2002;104:86–95. doi: 10.1006/clim.2002.5248. [DOI] [PubMed] [Google Scholar]
- Shirakawa I, Deichmann K A, Izuhara K, Mao X Q, Adra C N, Hopkin J M. Atopy and asthma: genetic variants of IL-4 and IL-13 signalling. Immunol Today. 2000;21:60–64. doi: 10.1016/s0167-5699(99)01492-9. [DOI] [PubMed] [Google Scholar]
- Ehlenfield D R, Cameron K, Welliver R C. Eosinophilia at the time of respiratory syncytial virus bronchiolitis predicts childhood reactive airway disease. Pediatrics. 2000;105:79–83. doi: 10.1542/peds.105.1.79. [DOI] [PubMed] [Google Scholar]
- McNamara P S, Smyth R L. The pathogenesis of respiratory syncytial virus disease in childhood. Br Med Bull. 2002;61:13–28. doi: 10.1093/bmb/61.1.13. [DOI] [PubMed] [Google Scholar]
- Rosenberg H F, Domachowske J B. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- Pifferi M, Ragazzo V, Caramella D, Baldini G. Eosinophil cationic protein in infants with respiratory syncytial virus bronchiolitis: predictive value for subsequent development of persistent wheezing. Pediatr Pulmonol. 2001;31:419–424. doi: 10.1002/ppul.1069. [DOI] [PubMed] [Google Scholar]
- Tang Y-W, Graham B S. T cell source of Type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J Clin Invest. 1997;99:2183–2191. doi: 10.1172/JCI119391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan W H, Kozlowska W J, Openshaw P J M. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Braciale T J. Virus-specific, CD8+ T lymphocytes down regulate Th2 type cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Baldwin C J, O'Garra A, Openshaw P J M. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- Hussell T, Openshaw P J M. Intracellular IFN-γ expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–2601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Kohler G, Young I G. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- Resnick M B, Weller P F. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8:349–355. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- Rothenberg M E, Ownbey R, Mehlhop P D, Loiselle P M, van de Rijn M, Bonventre J V, Oettgen H C, Leder P, Luster A D. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- Mould A W, Matthaei K I, Young I G, Foster P S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer R C, Welmers B A C, Raaijmakers J A M, Zanen P, Lammers J-W J, Koenderman L. RANTES- and interleukin-8-induced responses in normal human eosinophils: effects of priming with interleukin-5. Blood. 1994;83:3697–3704. [PubMed] [Google Scholar]
- Rothenberg M E, MacLean J A, Pearlman E, Luster A D, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo J A, Jia G Q, Aguirre V, Friend D, Coyle A J, Jenkins N A, Lin G S, Katz H, Lichtman A, Copeland N. Mouse eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4:1–4. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Herbst A, Weller P F. Eotaxins: contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol. 2001;24:653–657. doi: 10.1165/ajrcmb.24.6.f209. [DOI] [PubMed] [Google Scholar]
- Badewa A P, Hudson C E, Heiman A S. Regulatory effects of eotaxin, eotaxin-2, and eotaxin-3 on eosinophil degranulation and superoxide anion generation. Exp Biol Med. 2002;227:645–651. doi: 10.1177/153537020222700814. [DOI] [PubMed] [Google Scholar]
- MacLean J A, Ownbey R, Luster A D. T cell-dependent regulation of eotaxin in antigen-induced pulmonary eosinophilia. J Exp Med. 1996;184:1461–1469. doi: 10.1084/jem.184.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan S P, Mishra A, Brandt E B, Royalty M P, Pope S M, Zimmerman N, Foster P S, Rothenberg M E. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Jose P J, Rankin S M. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J Immunol. 2002;168:1911–1918. doi: 10.4049/jimmunol.168.4.1911. [DOI] [PubMed] [Google Scholar]
- Rothenberg M E, Luster A D, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Hogan S P, Mahalingam S, Pope S M, Zimmerman N, Fulkerson P, Dent L A, Young I G, Matthaei K I, Rothenberg M E. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–943. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U, Lezcano-Meza D, Bartels J, Montes-Vizuet A R, Schroder J M, Teran L M. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and Gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3, and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol. 2003;131:264–271. doi: 10.1159/000072138. [DOI] [PubMed] [Google Scholar]
- Boehme S A, Lio F M, Sikora L, Pandit T S, Lavrador K, Rao S P, Sriramarao P. Serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol. 2004;173:3599–3603. doi: 10.4049/jimmunol.173.6.3599. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Yamada N, Fujitsu Y, Kumagai N, Nishida T. Inhibition of eotaxin expression in human corneal fibroblasts by interferon-γ. Int Arch Allergy Immunol. 2002;129:138–144. doi: 10.1159/000065880. [DOI] [PubMed] [Google Scholar]
- Matsukura S, Kokubu F, Kuga H, Kawaguchi M, Ieki K, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Adachi M. Differential regulation of eotaxin expression by IFN-γ in airway epithelial cells. J Allergy Clin Immunol. 2003;111:1337–1344. doi: 10.1067/mai.2003.1513. [DOI] [PubMed] [Google Scholar]
- Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bembridge G P, Garcia-Beato R, Lopez J A, Melero J A, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J Immunol. 1998;161:2473–2480. [PubMed] [Google Scholar]
- Johnson T R, Graham B S. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol. 1999;73:8485–8495. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T R, Parker R A, Johnson J E, Graham B S. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J Immunol. 2003;170:2037–2045. doi: 10.4049/jimmunol.170.4.2037. [DOI] [PubMed] [Google Scholar]
- Hancock G E, Speelman D J, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B S, Bunton L A, Wright P F, Karzon D T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T R, Fischer J E, Graham B S. Construction and characterization of recombinant vaccinia viruses co-expressing a respiratory syncytial virus protein and a cytokine. J Gen Virol. 2001;82:2107–2116. doi: 10.1099/0022-1317-82-9-2107. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243.1–243.11. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P, Pellegrino A, Gong J-H, Mattioli I, Loetscher M, Bardi G, Baggiolini M, Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10 are natural antagonists for CCR3. J Biol Chem. 2001;276:2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- Hussell T, Georgiou A, Sparer T E, Matthews S, Pala P, Openshaw P J M. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161:6215–6222. [PubMed] [Google Scholar]
- Neuzil K M, Johnson J E, Tang Y-W, Prieels J-P, Slaoui M, Gar N, Graham B S. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine. 1997;15:525–532. doi: 10.1016/s0264-410x(97)00218-1. [DOI] [PubMed] [Google Scholar]
- Taylor G, Thomas L H, Stott E J. Effect of vaccination on cell populations in lung washes from calves after infection with respiratory syncytial virus. Res Vet Sci. 1989;47:231–235. [PubMed] [Google Scholar]
- Garofalo R, Kimpen J L L, Welliver R C, Ogra P L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- Johnson J E, Gonzales R A, Olson S J, Wright P F, Graham B S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- McNamara P S, Ritson P, Selby A, Hart C A, Smyth R L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope S M, Brandt E B, Mishra A, Hogan S P, Zimmerman N, Matthaei K I, Foster P S, Rothenberg M E. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- Miller A L, Strieter R M, Gruber A D, Ho S B, Lukacs N W. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- Stevenson C S, Coote K, Webster R, Johnston H, Atherton H C, Nicholls A, Sugar R, Jackson A, Press N J, Brown Z. Characterization of cigarette smoke-induced inflammatory and mucus hypersecretory changes in rat lung and the role of CXCR2 ligands in mediating this effect. Am J Physiol Lung Cell Mol Physiol. 2005;288:L514–L522. doi: 10.1152/ajplung.00317.2004. [DOI] [PubMed] [Google Scholar]
- Sigurs N, Bjarnason R, Sigurbergsson F. Eosinophil cationic protein in nasal secretion and in serum and myeloperoxidase in serum in respiratory syncytial virus bronchiolitis: relation to asthma and atopy. Acta Paediatr. 1994;83:1151–1155. doi: 10.1111/j.1651-2227.1994.tb18269.x. [DOI] [PubMed] [Google Scholar]
- Tang Y-W, Graham B S. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest. 1994;94:1953–1958. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Giese N A, Kulkarni A B, Firestone C-Y, Morse I, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Palmer K, Lotvall J, Milan S, Lei X-F, Matthaei K I, Gauldie J, Inman M D, Jordana M, Xing Z. Circulating, but not local lung, IL-5 is required for the development of antigen-induced airways eosinophilia. J Clin Invest. 1998;102:1132–1141. doi: 10.1172/JCI2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope S M, Zimmerman N, Stringer K F, Karow M L, Rothenberg M E. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- Eum S Y, Maghni K, Tolloczko B, Eidelman D H, Martin J G. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:L576–L584. doi: 10.1152/ajplung.00380.2003. [DOI] [PubMed] [Google Scholar]
- Matthews S P, Tregoning J S, Coyle A J, Hussell T, Openshaw P J. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J Virol. 2005;79:2050–2057. doi: 10.1128/JVI.79.4.2050-2057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga S M, Wang X, Welsh R M, Braciale T J. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- Rothenberg M E. Eotaxin: an essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Hogan S P, Mishra A, Brandt E B, Bodette T R, Pope S M, Finkelman F D, Rothenberg M E. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2005;165:5839–5846. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- Branwell M E, Tolley N S, Williams T J, Mitchell T J. Regulation of human eotaxin-3/CCL26 expression: modulation by cytokines and glucocorticoids. Cytokine. 2002;17:317–323. doi: 10.1006/cyto.2002.1021. [DOI] [PubMed] [Google Scholar]
- Domachowske J B, Bonville C A, Dyer K D, Easton A J, Rosenberg H F. Pulmonary eosinophilia and production of MIP-1α are prominent responses to infection with pneumonia virus of mice. Cell Immunol. 2000;200:98–104. doi: 10.1006/cimm.2000.1620. [DOI] [PubMed] [Google Scholar]