Abstract
In many human cancers, the abundance of macrophages in the tumor microenvironment is correlated with poor prognosis. Experimental evidence for the causal relationship between macrophages and poor prognosis came from mouse models of breast cancer in which genetic ablation of macrophages resulted in attenuation of tumor progression and metastasis, and premature recruitment to hyperplastic lesions accelerated these processes. Malignancy is defined by the invasion of tumor cells into the stroma, a process that allows escape of these cells into the circulation and dissemination to distant sites. In this review, I argue that macrophages are recruited to the invasive front by expression of tumor-derived chemotactic factors and in response to the disruption of the basement membrane. At this invasive site, macrophages enhance tumor cell migration and invasion through their secretion of chemotactic and chemokinetic factors including epidermal growth factor (EGF). They promote angiogenesis by the synthesis of angiogenic factors including vascular endothelial growth factor (VEGF), and they remodel the extracellular matrix and in particular, regulate collagen fibrillogenesis. A combination of these factors provides a triple-whammy, as the more mobile and invasive tumor cells track along collagen fibers that are also anchored to blood vessels, which are fabricated at sites of invasion and through which macrophages potentiate tumor cell intravasation. All of these activities suggest that macrophage functions are significant targets for the generation of novel therapeutics that should improve the current cytotoxic armamentarium.
Keywords: metastasis, CSF-1, EGF, VEGF, chemotaxis
INTRODUCTION
Extensive studies over the last three decades have defined in tumor cells a large number of activating, oncogenic mutations and inactivating mutations in tumor suppressor genes that are responsible for the acquisition of the malignant phenotype. These studies upon adenocarcinomas have led to a profound understanding of how these epithelial cells acquire unlimited, replicative potential, become anchorage-independent while avoiding apoptosis, and acquire a motile and invasive phenotype [1]. However, more recently, it has become apparent that these mutations in themselves are not sufficient to give a fully malignant phenotype, as this is only manifested within a permissive microenvironment. Actually, this modulation of malignant cells by the microenvironment has been known for some time, as malignant teratocarcinoma cells were shown to be reverted to the normal developmental state by incorporation within an embryo, and also, potent oncogenic transformation can be suppressed by the normal embryonic environment unless there is a secondary insult such as wounding [2, 3].
The microenvironment is populated by many cells and is variable according to tumor type. For example, in breast cancer, there are the adipocytes of the mammary fat, fibroblasts, a wide range of hematopoietic cells that include leukocytes and lymphocytes, as well as newly formed blood vessels with their associated cells. It has been long-recognized that tumors require the establishment of a vasculature to progress above a certain size and to become malignant [4]. This vascularization has been considered to play a supportive role, but many of the other cells can play more instructive roles by increasing malignancy through the secretion of tumor-enhancing products. For example, in collaborative studies with my group, Scherer and colleagues [5, 6] have indicated that apipocytes secrete collagen VI, which is subsequently cleaved to give a C-terminal fragment that promotes tumor growth. Genetic ablation of collagen VI in a mouse model of breast cancer induced by the mammary epithelial-restricted expression of the polyoma middle T oncogene (PyMT) resulted in a reduced tumor burden and an inhibition of tumor progression. Others have shown that fibroblasts, especially those that are isolated from tumors, can enhance tumor cell growth [7, 8]. Similar results have been observed for hematopoietic cells in the tumor microenvironment such as mast cells and macrophages [9,10,11,12]. In a model of skin cancer caused by the expression of the adenovirus E6 oncoprotein in keratinocytes, genetic ablation of mast cells reduced the progression of benign tumors to malignancy, and this involved matrix metalloproteinase 9 (MMP9) synthesized by these cells [9, 13]. In addition, our experiments, that genetically ablated macrophages from the PyMT model of breast cancer, resulted in a slower rate of tumor progression and a substantial inhibition of metastasis, processes that involve, in part, the macrophage synthesis of epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) [11, 14, 15].
These few examples, selected out of many, indicate that cells within the microenvironment can significantly modulate the malignancy of tumors. Indeed, tumors can be viewed as aberrant organs in which the malignant cells sequester or recruit normal cells to promote their survival, growth, and invasion. This suggests a dynamic process with the cellular microenvironment changing in concert with the acquisition of oncogenic mutations. This review will focus on my studies about macrophages in tumors and how these cells participate in the development of a microenvironment that favors tumor cell invasion and thus, subsequent metastasis.
MACROPHAGES AND THE TUMOR MICROENVIRONMENT
Macrophages populate the microenvironment of most if not all tumors. In some rare cases, particularly in breast cancers, they can represent >50% of the tumor mass [16]. Generally, macrophages are a smaller proportion of the tumor and are found in particular niches [11, 15,16,17,18]. Our studies of mouse and human mammary cancers have shown at least three separate populations. The majority of macrophages in both species is found in the stroma surrounding the tumor. At least in mouse models of breast cancer, they are enriched at the invasive front and also in avascular areas [11, 15, 17, 18]. Another population in both species is associated with necrotic areas in advanced tumors, where they tend to accumulate around the edge of the dying cells [19]. A third population is aligned long the abluminal side of the vessels [16]. Intravital imaging in mouse models suggests that this latter population patrols up and down these vessels, although the purpose of this behavior is as yet obscure [18].
Clinical studies have sought to identify correlations between macrophage density and prognosis. A summary in a recent review by Bingle et al. [20] using a meta-analysis showed that in >80% of the cases, an increased macrophage density was associated with poor prognosis, and the remaining 20% was split between having no effect and those with good prognosis. For example, in one revealing case in follicular lymphoma with a large cohort of patients with good clinical follow-up, unbiased microarray data indicated that a gene expression signature characteristic of macrophages was an independent predictor of poor outcome. This was confirmed with simple histological evaluation of macrophage density that in itself was an independent predictor of poor outcome [21, 22]. In breast cancers, where the most extensive studies have been performed, independent studies have shown a correlation of tumor-associated macrophages with poor prognosis, particularly if this is associated with microvascular density [23].
However, clinical studies suggest that the action of macrophages is not so straightforward, as there are cases where macrophages are associated with good outcome [20, 24, 25]. Indeed, in normal physiology, macrophages are generally thought of as being involved in immune reactions, where among other things, they are professional APCs and also are involved in cell and pathogen killing. Furthermore, they are quite capable of killing tumor cells and often do so in in vitro assays [26]. Recently, sophisticated microarray-derived gene expression data combined with cell-phenotyping studies in human colon cancer indicated that an immune environment characterized by an adaptive T cell-mediated immune response correlated with good prognosis in patients that had surgical resections, suggesting that in some cases, the immune system is biased toward tumor cell rejection [27, 28]. However, without surgical intervention, these tumors would have progressed and in all likelihood, would have killed the patients. These data strongly suggest an evolution of the immune cells in the microenvironment and in particular, macrophages, as the tumors progress to the state that they are trophic instead of hostile to tumors. Such a progression of the immune response that results in immune protection rather than rejection has been referred to as “immunoediting” [29] or “immunosculpting” [30]. This has been associated with the recruitment of T regulatory cells as well as with a subpopulation of myeloid cells that suppresses T cell responses [31,32,33]. Furthermore, following on from our studies about the role of trophic macrophages regulating the normal development of a variety of epithelial structures [34, 35], I had proposed that the macrophages in the tumor were educated by the local environment to perform tasks similar to the trophic, nonimmune roles that they perform to promote epithelial outgrowth and invasion during development [36, 37]. Mantovani and colleagues [38, 39] developed a similar view to that described above, suggesting that within the tumor microenvironment, the polarization of macrophages was from an activated, immunological type (referred to as M1) to an alternatively activated, inflammatory population (M2 macrophages) that potentiates tumor progression. These authors have presented evidence using DNA microarray technology and functional assays that this polarization to trophic macrophages occurs in the tumor microenvironment [40, 41].
MACROPHAGES ENHANCE TUMOR CELL INVASION
Our genetic studies, in which we ablated macrophages from the tumor microenvironment by removal of the major macrophage growth factor CSF-1 in mammary tumors of the PyMT model, showed two independent and significant effects whose consequence was a reduction of malignancy [11, 42]. The first effect was on the progression of benign tumors to malignant ones with many tumors in the macrophage-depleted animals growing to large size but maintaining their benign characteristics. The second effect was upon metastatic capacity of advanced carcinomas that was dramatically reduced in the relative absence of macrophages. Both of these phenotypes could be reversed in mutant animals by the transgenic expression of CSF-1 in the mammary epithelium that restored tumor macrophage populations but did not affect other macrophage populations in the body and failed to correct other phenotypes caused by the general macrophage deficiency in these mice. In addition, overexpression of CSF-1 in wild-type mice in a recapitulation of the situation in many human breast cancers [43] resulted in elevated numbers of macrophages and an acceleration of tumor progression and enhanced metastasis [11, 42].
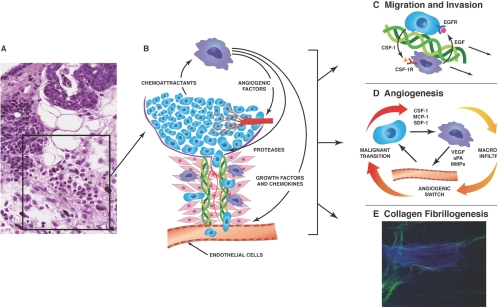
Observations in primary tumors of the PyMT mouse model showed that the leukocytic infiltrates occurred in tumors beginning at the malignant transition in the macrophage-replete mice and that such infiltrates were not found in the macrophage-deficient ones. These infiltrates were found at sites of basement-membrane breakdown and tumor invasion (Fig. 1A). These sites were populated with many macrophages but also, other hematopoietic cells such as neutrophils and mast cells. The invasion sites were also characterized by areas of extensive collagen fibrillogenesis and angiogenesis. This led to the model (Fig. 1B) that macrophages promote the migration of tumor cells out from the primary tumor thorough breaks in the basement membrane, creating a characteristic-invasive microenvironment that enhances tumor cell access to the vascular and lymphatic system and thereby, increased their metastatic capacity [37, 42, 47].
Fig. 1.
Model of the microenvironment at the tumor-invasive front. (A) Histological section of a malignant PyMT mammary tumor showing a site of invasion. This site is characterized by loss of integrity of the basement membrane, a strong leukocytic infiltrate with diverse immune cells including macrophages, neutrophils, and mast cells, angiogenesis, and deposition of extracellular matrix (ECM), particularly collagen I (ref. [11] with permission). (B) Schematic model of the area boxed in A showing the role of macrophages in the invasive process. Thus, macrophages are shown to promote angiogenesis and migration of tumor cells away from the tumor body on collagen fibers and their intravasation into blood and lymphatic vessels. (C) Model for the macrophage stimulation of tumor cell migration and invasion. In this model, there is a relayed chemotactic loop between macrophage-produced EGF that promotes the migration and invasion of tumor cells, which in turn produce CSF-1 to cause the migration of macrophages, and macrophages and tumor cells therefore migrate in lockstep along collagen fibers in an interdependent manner. These fibers tend to attach to vessels, where vessel-associated macrophages promote the intravasation of tumor cells into the circulation (modified from ref. [44]). (D) Model of macrophage promotion of angiogenesis. Macrophages modulate malignancy of tumors by regulating the angiogenic switch required for malignant progression. They also play a role in remodeling the newly formed, chaotic tumor vasculature into a functional network. Macrophages are recruited to the tumor by chemoattractants such as CSF-1, stromal cell-derived factor 1 (SDF-1; CCL-12), or CCL-2 (MCP-1), where they produce abundant factors that affect angiogenesis, such as growth factors, including VEGF, TNF-α, and proteases, for example, urokinase plasminogen activator (uPA) and MMP9 (modified from ref. [45]). (E) Macrophages promote collagen fibrillarogenesis. Shown is a multiphoton micrograph of a frozen section of a terminal end bud (TEB) in a developing mammary gland. Cells are stained with 4′,6-diamidino-2-phenylindole that highlights the bulbous TEB surrounded by a funnel of strands of fibrillar collagen I (pseudo-colored to green), which in turn connects to a collagen I sheaved blood vessel running vertically. Macrophages are labeled by expression of GFP (pseudo-colored to red) through expression from a CSF-1R promoter. They are abundant around the TEB and in many cases, are attached to the collagen fibers. Genetic ablation of the macrophages reduces the amount of collagen fibrillogenesis by ∼50% and also, a reduced rate of epithelial outgrowth through the fat pad. This image was part of data used to quantify the amount of collagen fibrillogenesis in ref. [46].
Metastatic capacity involves tumor cell migration, invasion through the stroma, and intravasation into the hematogenous or lymphatic circulation. By using a combination of a novel invasion assay developed by Condeelis and co-workers [48], together with intravital imaging of fluorescently labeled cells in tumors in vivo [49], my laboratory, together with my collaborators, showed a remarkable interaction between macrophages and tumor cells that involves reciprocal signaling between CSF-1 and EGF and the interdependency on both cell types for migration [14, 18].
The invasion assay measures the movement of cells into micro-needles that contain a matrigel plug inundated with specific growth factors and that are introduced into mammary tumors of mice under anesthesia. After a period of time, typically 4 h, the needles are removed and the cells that have moved into the plug, expelled, enumerated, and phenotyped [48]. In this chemotaxis assay, all soluble members of the EGF family of ligands and CSF-1 result in cell migration, and at least six other growth factors, including VEGF, platelet-derived growth factor, fibroblast growth factor, and CCL2 failed to recruit any cells [14]. Furthermore, significant numbers of cells are only collected from late-stage carcinomas, and few are collected at the early carcinoma or benign stages. Remarkably, tumor cells and macrophages move into the needles in lock step, and at least in mammary tumors, no other cell types are represented. Inhibition of EGF signaling using small molecule inhibitors of EGFR kinase activity or CSF-1 signaling by genetic mutation of the CSF-1 gene or anti-CSF-1R antibodies inhibited migration of both cell types. CSF-1 and EGFR expression was restricted to macrophages and tumor cells, respectively, and the ligands were produced by the reciprocal cell. Thus, this interdependent co-migration is an example of relayed chemotaxis that allows chemotactic gradients to be formed in large void volumes [50] with paracrine CSF-1 and EGF signaling between the two cell types (Fig. 1C). In this model, the migratory behavior of one cell type is entirely dependent on the other [14, 51].
The ability to observe cell behavior within tumors in vivo using multiphoton microscopy has also shown interactions between macrophages and tumor cells. In this case, the movement of the tumor cells away from the tumor mass occurred in >80% of cases next (within 20 μm) to macrophages [18]. Further, when intravasation events could be observed, they were found adjacent to clusters of macrophages that were aligned along the vessels. Inhibitor and neutralizing antibody studies suggest strong links between these tumor cell behaviors, as acute inhibitions of EGF or CSF-1 signaling resulted in a dramatic reduction in circulating tumor cells (a measure of intravasated cells), as measured by a micro-colony formation assay of blood obtained from the tumor-bearing mice by heart puncture. These data are consistent with our genetic studies in the Csf1op nullizygous mice that indicated a significant (∼80%) reduction in circulating tumor cells in these mice compared with their wild-type controls, although they carried large, late-stage carcinomas [18]. Together, these data are also consistent with the model that tumor cells and macrophages cross-talk through EGF and CSF-1 signaling and that this results in tumor cell migration toward macrophage-rich regions of vessels (Fig. 1B), sites where macrophages promote tumor cell intravasation into the circulation.
This EGF-CSF-1 signaling pathway emphasizes the intimate interaction between tumor cells and macrophages. However, it is unlikely that this is the only interaction between these two cell types in the microenvironment of breast cancers, and other pathways may be dominant in other tumor types. For example, as discussed below, VEGF is a powerful chemoattractant for monocytes [52] and macrophages [53], and this growth factor is synthesized by tumor cells in a wide range of human and in mouse cancers. Although it is unknown whether there is a reciprocal signal from VEGF-stimulated macrophages to tumor cells altering their invasive behavior, it does seem likely that such an interaction is important in biology of the tumor. Further, there is evidence of an interplay between macrophages and tumor cells acting over longer distances through the secreted products such as S100A8 and S100A9 [54]. These proteins are made by and act on macrophages to establish sites that act as preferred environments for metastatic tumor cell seeding, and they also enhance tumor cell invasion in in vitro assays [54]. Whether such interactions are involved in the behavior of tumor cells at the invasive front remains to be determined.
MACROPHAGES AND THE COLLAGENOUS ECM
Direct histological observation of the leukocytic infiltrates at points of basement membrane breakdown also indicated that these are areas of collagen fibrillogenesis (Fig. 1A). Fibrillar collagen, primarily collagen I, can be visualized in vivo directly by multiphoton microscopy through the quantum phenomenon of second harmonic resonance (SHR) that shows these structures in the blue channel. These SHR studies indicate that surrounding the tumor is a dense ring of fibrillar collagen abundantly populated by macrophages [18]. Importantly, this imaging also showed that tumor cells move upon collagen fibers ∼10 times faster than they do through the stroma [18, 55, 56]. Thus, tumor cells use these fibers as types of tramlines to rapidly move through the stroma away from the tumor mass. As collagen fibers often radiate out from anchorage points around vessels [46], this has the unfortunate consequence of tumor cells moving directly toward vessels, where at least in xenotransplant models of breast cancer, they have been shown to align by intravital imaging [49, 55].
The exact role of macrophages in collagen fibrillogenesis in tumors is unknown. However, during the development of the mammary gland, my laboratory has shown that macrophage depletion reduces the extent of collagen I fibers that align along the axis of outgrowing TEB [46]. This reduction of fibrillogenesis is independent on the collagen I synthesis that derives largely from fibroblasts found in the stroma adjacent to the TEB. It is, however, directly attributable to macrophage action, as restoration of macrophages to the TEB results in the correction of the collagen fibrillogenesis defect. In fact, macrophages are attached to the fibers and also locomote upon them (Fig. 1, B and E) [46]. The consequences of the reduced fibrillogenesis caused by macrophage depletion are a distortion in the shape of the TEB, and this is associated with a poorer rate of epithelial outgrowth through the fat pad [46]. Although it is unknown if similar dynamics occur in tumors, it is tempting to speculate that this fibroblast-macrophage-epithelial cell interplay at the invasive front influences the organization of a collagenous matrix. This stromal response may be the result of loss of basement membrane integrity, and this, in turn, would promote epithelial tumor cell migration and allow rapid migration of tumor cells toward vessels.
MACROPHAGES AND ANGIOGENESIS
The invasive fronts of tumors are also sites of angiogenesis. Of course, it is well known that growing tumors above a certain size require the establishment of a vasculature to provide nutrition and for the removal of toxic wastes. Indeed, there is a significant body of evidence that there is a dramatic increase in density of vessels during the benign-to-malignant transition. This led to the hypothesis that the angiogenic switch is required for this transition [4, 57]. In support of this hypothesis, our genetic depletion of macrophage from primary mammary tumors in the PyMT model inhibited this angiogenic switch and delayed tumor progression to malignancy. Importantly, premature recruitment of macrophages to benign tumors resulted in a dramatic increase in vessel density in a process reminiscent of the angiogenic switch. This premature angiogenic switch accelerated tumor progression to malignancy and increased metastasis in the PyMT model of breast cancer [15, 45].
Macrophage depletion resulted in an ∼40% reduction in vessel density, even in late-stage carcinomas. This effect suggests that macrophages are not only involved in the initiation of the formation of the vasculature in malignant areas of the tumor but also in the remodeling of the vasculature once formed [15, 45]. Although it hasn’t been studied explicitly, these data also suggest that macrophages may regulate the angiogenesis observed at the invasive front.
These data obtained using genetic depletion of CSF-1-regulated macrophages are reinforced by experiments using transplantable models of cancer in mice treated with antisense oligonucleotides or antibodies against CSF-1 or CSF-1R. These treatments resulted in slower tumor growth, characterized by reduced angiogenesis [58, 59]. In addition, in a transgenic mouse model of cervical cancer, inhibition of the MMP9 in macrophages inhibited release of matrix-bound VEGF, and the consequent reduction of angiogenesis was correlated with a slower rate of tumor progression [60]. In an interesting study, treatment of tumor-bearing mice with a vaccine against legumain and asparaginyl-endopeptidase that is overexpressed in tumor-associated macrophages (TAMs) resulted in a robust T cell response against this protein and TAM depletion from the tumors [61]. This caused a reduction in expression of proangiogenic molecules, such as MMP-9 and VEGF, which was associated with an inhibition of tumor angiogenesis, growth, and metastases in xenograft tumor models [62]. Other monocyte populations that express tyrosine kinase with Ig and EGF homology domain-2 (Tie-2; Tie-2-expressing monocytes) have been shown to be proangiogenic in a variety of tumor models, and their ablation using a suicide gene approach inhibited tumor angiogenesis and growth [63, 64].
All of these studies reinforce the idea that members of the monocyte-macrophage lineage play major roles in tumor angiogenesis. Macrophages produce VEGF-A in human breast cancers as well as in several transgenic mouse models of cancer [65]. In the PyMT model of breast cancer, stromal cells contain VEGF-A, and this is lost in the Csf1op nullizygous mice, suggesting production or response in these cells [15]. Using a tetracycline-induced transgenic model, whereby VEGF-A is overexpressed in the mammary epithelium under the control of the mouse mammary tumor virus promoter at a time straddling the period when the PyMT model makes the transition to malignancy, we showed that there is a dramatic recruitment of macrophages coincident with the induction of vascularization in the mammary tumors [53]. Macrophages were recruited, even in the Csflop nullizygous mice, indicating that VEGF-A is a potent chemoattractant and differentiation factor for mononuclear phagocytes, independent of CSF-1. In these normally macrophage-depleted Csf1op/op mice, the VEGF overexpression resulted in angiogenesis, and the normally retarded tumor progression accelerated to wild-type rates. The tumors in these Csf1op/op mice often showed signs of increased invasiveness, adopting a medusa-like structure of highly proliferating cells [53].
This tetracycline-induced VEGF binary transgenic model described above [53] is similar to that developed by Keshet and co-workers [66], who used it to express VEGF-A in the lung and liver. They demonstrated that this VEGF-A expression resulted in the recruitment of a bone marrow-derived myeloid population to these tissues and angiogenesis. They showed that the recruited myeloid cells, when isolated, produced angiogenic factors, as assessed by an aortic ring vascular-sprouting assay. Their data show that VEGF recruits populations of myeloid cells (in our experiments, they are bona fide macrophages) to normal and malignant tissue. It suggests that these cells are, at least in part, responsible for the angiogenesis in the target tissues.
Thus at least one function of macrophages in tumors is to deliver VEGF-A (or MMP9 to release matrix-bound VEGF) and/or other angiogenic factors temporarily and spatially to constitutively promote angiogenesis. Furthermore, the localization of macrophages at the invasive front marked by angiogenesis suggests that these macrophage functions would further promote tumor cell dissemination via the formation of more vessels that would be targets for tumor cell intravasation.
A MODEL FOR TUMOR CELL INVASION AND DISSEMINATION
Pathologists regard the invasion of carcinomas through the basement membrane as being indicative of malignancy and therefore a sign of poor prognosis. In breast cancer, it is believed that escape from the constraints of the basement membrane, subsequent or consequent on the loss of myoepithelial cells [17], allows malignant tumor cells to grow without constraints, become vascularized, and disseminate to distant sites through the hematogenous and lymphatic systems. Many hematopoietic cells, fibroblasts, and endothelial cells populate these invasive areas. They are also regions of focal angiogenesis, deposition of ECM, particularly collagen fibrillogenesis, and perhaps even of immune privilege. My observations suggest that these sites are areas where the local environment is altered in a manner that facilitates tumor cell escape, migration, invasion, and intravasation.
Macrophages are important to all of these processes (Fig. 1A–E). For example, the experimental data described above indicate that macrophages promote tumor cell invasion by producing EGF ligands that stimulate tumor cell motility, especially along collagen fibers that macrophages help to fabricate (Fig. 1, B, C, and E). Macrophages also induce the formation of new vessels (Fig. 1D), which are sheaved in collagen, and thus, the migration of tumor cells tends to be focused toward these newly formed vessels, thereby targeting tumor cells to sites of intravasation. The latter process in turn is enhanced by macrophages that align upon the outside of vessels, which are elaborated under the influence of macrophages within the malignant areas and provide portals of escape for the tumor cells. All of these macrophage-promoted activities of increased angiogenesis as well as enhanced tumor cell migration, invasion, and intravasation will result in increased metastatic capacity of the tumor. Furthermore, cells of the mononuclear phagocytic lineage can also differentiate into myeloid-derived suppressor cells that act to inhibit T cells that ordinarily would kill malignant-mutated tumor cells [32, 67, 68]. In light of all of these activities, it is not surprising that macrophage depletion in mouse models of breast cancer results in a reduced metastatic capacity, even in models where the mammary epithelial cells are transformed by a potent oncogene such as the PyMT oncoprotein.
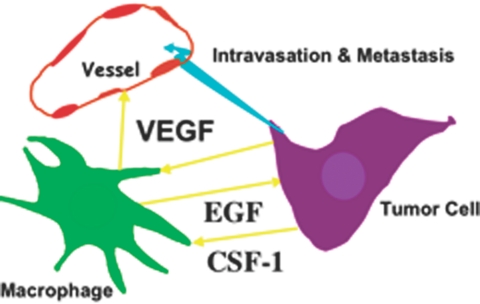
Many of these metastasis-promoting activities of macrophages are mediated through three transmembrane receptor tyrosine kinases, those responding to VEGF, EGF, and CSF-1 ligands (Fig. 2). VEGFR1 and CSF-1R are expressed on macrophages, and ErbB1 (EGFR) is often on tumor cells. It is informative that overexpression of all of these three growth factors in breast cancer is associated with poor prognoses [23, 43, 65, 69,70,71,72]. Furthermore, in human breast cancers, macrophages express VEGF (along with tumor cells), and all of the EGF is derived from these cells [65], while CSF-1 is predominantly expressed in tumor cells at the invasive front [43]. This suggests that combined inhibition of these pathways would have substantial therapeutic benefit in reducing mortality by reducing metastatic spread.
Fig. 2.
Growth factors mediate paracrine interactions among macrophages, tumor cells, and blood vessels. Shown schematically are interactions among macrophages, tumor cells, and blood vessels. Macrophages produce EGF that stimulates migration and invasion of tumor cells as well as their intravasation once the tumor cells reach the blood vessels. In turn, tumor cells produce CSF-1 that causes the migration of macrophages. Inhibition of CSF-1 or EGF signaling blocks migration of both cell types synchronously, showing a relayed chemotaxis operating between these two cell types that is required for their migration in the tumor. Macrophages also produce VEGF that allows the spatial and temporal expression of this vascular growth factor at sites of angiogenesis. Tumor-produced VEGF also acts as a potent chemoattractant to macrophages that in turn expresses other angiogenic factors and also further promotes tumor cell invasion. In both cases, CSF-1 can stimulate VEGF and EGF synthesis by macrophages. Therefore, macrophages provide a triple whammy at the invasive front: they promote tumor cell migration and invasion, intravasation as well as increasing the number of blood vessels as targets for intravasation. This results in increased escape of cells from the primary tumor (turquoise arrow).
Acknowledgments
This research presented in this review was supported by grants from the National Institutes of Health P30 CA 13330, P01 100324, and RO1 CA 131270. J. W. P. is the Betty and Sheldon E. Feinberg Faculty Scholar in Cancer Research. I acknowledge my collaborators within my laboratory and among members of the Program-Project PO1 100324 for the experimental data and discussion that formed the basis for this hypothesis about the invasive front. The multiphoton image shown in Figure 1E was generated in my laboratory by Dr. Wendy Ingman. Thanks to Tatyana Harris for preparing Figure 1.
References
- Hanahan D, Weinberg R A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Green M, Boudreau N, Bissell M J. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–4341. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Combs T P, Shah S J, Gouon-Evans V, Pollard J W, Albanese C, Flanagan L, Tenniswood M P, Guha C, Lisanti M P, Pestell R G, Scherer P E. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Espina V, Williams T W, Lin Y, Berry D, Jelicks L A, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell R G, Sasisekharan R, Trock B J, Lippman M, Calvert V S, Petricoin E F, III, Liotta L, Dadachova E, Pestell R G, Lisanti M P, Bonaldo P, Scherer P E. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick N A, Chytil A, Plieth D, Gorska A E, Dumont N, Shappell S, Washington M K, Neilson E G, Moses H L. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez P Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L M, Raymond W W, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey G H, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser K E, Coussens L M. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–137. doi: 10.1159/000092969. [DOI] [PubMed] [Google Scholar]
- Lin E Y, Nguyen A V, Russell R G, Pollard J W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest. 1994;71:5–16. [PubMed] [Google Scholar]
- Coussens L M, Tinkle C L, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin E Y, Wang Y, Pixley F, Stanley E R, Graf T, Pollard J W, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Lin E Y, Li J F, Gnatovskiy L, Deng Y, Zhu L, Grzesik D A, Qian H, Xue X N, Pollard J W. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- Lewis C E, Pollard J W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Lin E Y, Jones J G, Li P, Zhu L, Whitney K D, Muller W J, Pollard J W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J B, Wang Y, Lin E Y, Li J F, Goswami S, Stanley E R, Segall J E, Pollard J W, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis C E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- Bingle L, Brown N J, Lewis C E. The role of tumor-associated macrophages in tumor progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Farinha P, Masoudi H, Skinnider B F, Shumansky K, Spinelli J J, Gill K, Klasa R, Voss N, Connors J M, Gascoyne R D. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- Dave S S, Wright G, Tan B, Rosenwald A, Gascoyne R D, Chan W C, Fisher R I, Braziel R M, Rimsza L M, Grogan T M. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- Leek R D, Harris A L. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- Kim D W, Min H S, Lee K H, Kim Y J, Oh D Y, Jeon Y K, Lee S H, Im S A, Chung D H, Kim Y T, Kim T Y, Bang Y J, Sung S W, Kim J H, Heo D S. High tumor islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected nonsmall cell lung cancer. Br J Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Bij, G. J., Bogels, M., Oosterling, S. J., Kroon, J., Schuckmann, D. T., de Vries, H. E., Meijer, S., Beelen, R. H., van Egmond, M. (2008) Tumor infiltrating macrophages reduce development of peritoneal colorectal carcinoma metastases. Cancer Lett., Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jadus M R, Irwin M C, Irwin M R, Horansky R D, Sekhon S, Pepper K A, Kohn D B, Wepsic H T. Macrophages can recognize and kill tumor cells bearing the membrane isoform of macrophage colony-stimulating factor. Blood. 1996;87:5232–5241. [PubMed] [Google Scholar]
- Galon J, Fridman W H, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc P H, Trajanoski Z, Fridman W H, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Dunn G P, Old L J, Schreiber R D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Reiman J M, Kmieciak M, Manjili M H, Knutson K L. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–287. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G P, Ikeda H, Bruce A T, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White J M, Sheehan K C, Schreiber R D. Interferon-γ and cancer immunoediting. Immunol Res. 2005;32:231–246. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska A E, Chytil A, Aakre M, Carbone D P, Matrisian L M, Richmond A, Lin P C, Moses H L. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S K, Yang L, Sinha P, Clements V K, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J W, Stanley E R. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic (op) Adv Develop Biochem. 1996;4:153–193. [Google Scholar]
- Cecchini M G, Dominguez M G, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard J W, Stanley R E. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Lin E Y, Pollard J W. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J W. Tumor educated macrophages promote tumor progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Sica A, Rubino L, Mancino A, Larghi P, Porta C, Rimoldi M, Solinas G, Locati M, Allavena P, Mantovani A. Targeting tumor-associated macrophages. Expert Opin Ther Targets. 2007;11:1219–1229. doi: 10.1517/14728222.11.9.1219. [DOI] [PubMed] [Google Scholar]
- Saccani A, Schioppa T, Porta C, Biswas S K, Nebuloni M, Vago L, Bottazzi B, Colombo M P, Mantovani A, Sica A. p50 nuclear factor-κB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Marchesi F, Porta C, Sica A, Allavena P. Inflammation and cancer: breast cancer as a prototype. Breast. 2007;16:S27–S33. doi: 10.1016/j.breast.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Lin E Y, Gouon-Evans V, Nguyen A V, Pollard J W. The macrophage growth factor, CSF-1, in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- Scholl S M, Pallud C, Beuvon F, Hacene K, Stanley E R, Rohrschneider L R, Tang R, Pouillart P, Lidereau R. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J Natl Cancer Inst. 1994;86:120–126. doi: 10.1093/jnci/86.2.120. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard J W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Lin E Y, Pollard J W. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 46.Ingman, W., Wyckoff, J., Gouon-Evans, V., Condeelis, J. S., Pollard, J. (2006) Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn., In press. [DOI] [PubMed] [Google Scholar]
- Lin E Y, Pollard J W. Role of infiltrated leukocytes in tumor growth and spread. Br J Cancer. 2004;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J B, Segall J E, Condeelis J S. The collection of the motile population of cells from a living tumor. Cancer Res. 2000;60:5401–5404. [PubMed] [Google Scholar]
- Condeelis J, Segall J E. Intravital imaging of cell movement in tumors. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- Sawai S, Thomason P A, Cox E C. An autoregulatory circuit for long-range self-organization in Dictyostelium cell populations. Nature. 2005;433:323–326. doi: 10.1038/nature03228. [DOI] [PubMed] [Google Scholar]
- Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall J E. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12:143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- Barleon B, Sozzani S, Zhou D, Weich H A, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- Lin E Y, Li J, Bricard G, Wang W, Deng Y, Sellers R, Porcelli S A, Pollard J W. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Molecular Oncology. 2007;1:288–302. doi: 10.1016/j.molonc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumor-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Wang W, Wyckoff J B, Frohlich V C, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger E P, Singer R H, White J G, Segall J E, Condeelis J S. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. [PubMed] [Google Scholar]
- Ingman W, Wyckoff J, Xue C, Lin E Y, Wang W, Goswami S, Pollard J W, Condeelis J, Segall J E. Imaging invasions and metastasis in vivo. Wells A D, editor. Amsterdam: Kluwer Academic; 2005:55–72. [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schafer R, Stanley E R, Abraham D. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res. 2004;64:5378–5384. doi: 10.1158/0008-5472.CAN-04-0961. [DOI] [PubMed] [Google Scholar]
- Paulus P, Stanley E R, Schafer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349–4356. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee S H, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld R A, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen S, Zhou H, Hu H D, Cheng T, Markowitz D, Reisfeld R A, Xiang R, Luo Y. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–515. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri M A, Galli R, Sergi L S, Politi L S, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Lewis C E, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- Leek R D, Hunt N C, Landers R J, Lewis C E, Royds J A, Harris A L. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan P Y, Li Q, Sato A I, Levy D E, Bromberg J, Divino C M, Chen S H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Scholl S M, Crocker P, Tang R, Pouillart P, Pollard J W. Is colony stimulating factor-1 a key mediator in breast cancer invasion and metastasis? Mol Carcinog. 1993;7:207–211. doi: 10.1002/mc.2940070402. [DOI] [PubMed] [Google Scholar]
- Tang R, Beuvon F, Ojeda M, Mosseri V, Pouillart P, Scholl S. M-CSF (monocyte colony stimulating factor) and M-CSF receptor expression by breast tumor cells: M-CSF mediated recruitment of tumor infiltrating monocytes? J Cell Biochem. 1992;50:350–356. doi: 10.1002/jcb.240500403. [DOI] [PubMed] [Google Scholar]
- Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. 2000;5:37–44. doi: 10.1634/theoncologist.5-suppl_1-37. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–431. [PubMed] [Google Scholar]