Abstract
Clinical and experimental evidence demonstrates that ethanol exposure prior to injury alters local and systemic inflammatory responses, increasing morbidity and mortality. Moreover, the aberrant inflammatory responses can directly and indirectly lead to the poor prognosis after injury by altering leukocyte infiltration into the wound site and remote organs and by suppressing immunity leading to increased susceptibility to opportunistic infections. Recent studies from our laboratory have focused on inflammatory responses at the wound site and in other distal organs after exposure to acute ethanol and burn injury. This combined insult leads to increased mortality after dermal or intratracheal pseudomonas infection, relative to infected mice given ethanol or burn injury alone. The increased mortality in mice given ethanol and burn injury parallels elevated serum levels of proinflammatory cytokines, IL-6 and TNF-α, marked infiltration of leukocytes into the lung and gut, as well as immunosuppression at the sites of infection. Bacterial translocation from the gut is likely to be responsible, in part, for the aberrant accumulation of leukocytes in the lungs of ethanol-exposed, burn-injured mice. Additionally, other factors, such as expression of adhesion molecules, increased chemokine production, and leakiness of the vascular endothelium, may also be involved.
Keywords: neutrophils, T cells, cytokines, immunosuppression, lungs, gut
INTRODUCTION
Clinical and animal model studies demonstrate that burn injury leads to an enhanced systemic inflammatory response leading to multiple organ dysfunction syndrome (MODS) and multiple organ failure (MOF) [1, 2]. This dysregulated immune response in humans and mice can be characterized by higher levels of IL-6, IL-1β, TNF-α, and PGE2 and lower delayed-type hypersensitivity (DTH) response and lymphocyte proliferation [3,4,5]. The suppression of the immune response observed after burn is exaggerated with prior alcohol exposure. Approximately 100,000 burn-related hospital admissions occur annually in the United States [6], and half of the adult patients have alcohol in their system at the time of admission [7, 8]. Only 5–7% of drinkers in the United States are considered dependent, meaning that the majority of drinkers are occasional or binge users [6, 9]. Studies examining the burn patient population at Loyola Medical Center (Maywood, IL, USA) had similar findings that only a small percentage of patients with burn injury were dependent alcohol users, and the majority were acute or binge drinkers [10]. Patients with alcohol in their circulation at the time of injury suffer from increased infectious and/or surgical complications and mortality [8, 9, 11,12,13]. Burn patients who consumed alcohol spend more days on a ventilator, stay in the hospital longer, and cost significantly more to treat [14]. Numerous studies have demonstrated the detrimental effect of ethanol on immune function, including decreased lymphocyte activation following antigen stimulation, decreased neutrophil infiltration and phagocytic capability, and altered cytokine production by T cells and macrophages [15,16,17,18,19,20]. This increased immunosuppression after ethanol and burn injury can then result in increased susceptibility to bacterial infection [21,22,23].
SYSTEMIC IMMUNOSUPPRESSION AND IL-6
To begin to understand the mechanisms involved in the aberrant inflammatory response and the resultant immunosuppression following acute ethanol and burn injury, we developed a murine model of a single (acute) ethanol exposure and burn injury as described previously [21]. Briefly, mice were given a single dose of ethanol interperitoneally (i.p.) at 1.2 g/kg dose, which resulted in a blood ethanol level of approximately 150 mg/dL at 30 min. This route was selected to obtain more precise and reliable blood ethanol levels in a short time-frame (30 min) and cause minimal stress to the animal. Although oral gavage or feeding may be more physiologically relevant, they produce variable levels of ethanol. Gastric catheters are stressful to the animals and therefore, are also problematic. The mice were then anesthetized and their dorsum shaved and placed in a plastic template exposing 15% of the total body surface area [24] and subjected to a scald injury in 100°C water bath or a sham injury in room-temperature water. The scald injury resulted in an insensate, full-thickness burn injury of ∼15% total body surface area [25]. To try to model clinical conditions, the mice were then resuscitated with saline and allowed to recover on warming pads.
Using this murine model, we demonstrated that relative to either insult alone, the combination of ethanol and burn injury produced a significant suppression of the immune response [21]. Following a single exposure to ethanol and burn injury, we observed decreases in the DTH response compared with animals given burn injury alone. Splenocyte proliferation was also significantly decreased in mice given ethanol prior to burn injury. T cell suppression was also observed following ethanol and burn injury. At 48 h post-injury, significant decreases in Con A-stimulated T cell proliferation as well as the production of IL-2 were observed in splenic T cells from mice receiving burn injury. The suppression was even greater in animals receiving ethanol prior to the injury [26]. The decrease in Con A-stimulated proliferation appeared to be macrophage-dependent, as culture of nonadherent cells in the absence of adherent cells (macrophages) resulted in similar levels of cell proliferation [21]. No differences were observed in absolute cell numbers in the spleen or in percentages of T cells, B cells, and macrophages as determined by flow cytometry. The macrophage-induced suppression of cell-mediated immune response correlated with increases in mortality of ethanol-treated burn animals following topical inoculation with Pseudomonas aeruginosa (P. aeruginosa) immediately following the injury [21].
In patients, increased production of IL-6 has been correlated with poor prognosis after injury [4, 11, 27]. Consistent with this observation, in our mouse model, T cell suppression was directly associated with increased circulating levels of IL-6 as well as increased production of IL-6 in macrophage cultures. Furthermore, treatment of splenocyte cultures in vitro from burn and ethanol mice with mAb against IL-6 restored T cell proliferation [28]. In addition to alterations in cytokine production, hormone levels were affected after the combined insult of ethanol and burn injury [29, 30]. Acute ethanol and burn injury alone are known to increase levels of corticosteroids [31,32,33,34], which in turn, regulate IL-6 production [35, 36]. However, in our model of ethanol and burn injury, mice treated with the combined insult had significantly lower levels of corticosteroids than mice treated with burn injury alone [29]. In contrast, another model showed increased levels of corticosteroids in rats given ethanol and burn injury [30]. This difference may be a result of several factors, including using rats instead of mice, using an increased burn size (25% vs. 15%), and possibly the method of ethanol treatment (oral gavage vs. i.p.). However, despite these differences, decreased cell-mediated immunity was observed in rats treated with ethanol and burn injury similar to the mouse data [29, 30]. Interestingly, treatment of mice with exogenous corticosteroids after ethanol and burn injury restored DTH and proliferative responses in these animals. This restoration correlated with a decrease in circulating IL-6 levels [28, 29].
Additionally, treatment of animals in vivo with antibody against IL-6 prior to ethanol and burn injury resulted in increased DTH and splenoctye proliferation compared with animals receiving control antibody [37]. A second approach was undertaken to modulate IL-6 levels with the use of IL-4, which has anti-inflammatory properties. Lymphocytes from mice given ethanol and burn injury produced significantly less IL-4 compared with mice given either insult alone, suggesting a lack of IL-4 may promote the exaggerated inflammatory response in these mice [19]. Treatment of splenic cultures from burn ethanol mice with IL-4 resulted in decreased secretion of IL-6. Similarly, administration of mice with recombinant IL-4 prior to ethanol and burn injury completely restored DTH and splenocyte-proliferative responses as well as decreased circulating and macrophage-derived IL-6 in these mice [19]. Other treatments used to combat the excessive inflammation include hormone manipulation. Unlike other cytokines, IL-6 lacks an estrogen-response element; however, estrogen can still regulate IL-6 production through changes in activation of NF-κB [38]. Indeed, in our animal model, treatment of male mice with low doses of estrogen attenuated secretion of IL-6 and restored cellular immune responses, resulting in decreased mortality in male mice given bacterial infection after ethanol and burn injury [39].
To examine effects of dose-responsiveness to ethanol, the studies described above were repeated by giving a larger volume of ethanol that resulted in higher circulating levels of ethanol in mice (300 mg/dL). Although no differences in mortality were observed in animals with moderate levels of circulating ethanol (100 mg/dL) compared with animals given burn injury alone, animals given the higher dose had a significant increase in mortality [40]. Similar to previous studies, decreased DTH responses and splenocyte proliferation were observed in animals receiving either dose of ethanol and subsequent burn injury. As before, there were no changes in total numbers of splenocytes or in the percentages of T cells, B cells, or macrophages with either dose of ethanol. Increased production of IL-6 by macrophages was observed in animals given ethanol and burn injury, but significantly higher levels of circulating IL-6 were found in animals given the higher dose of ethanol prior to burn. Interestingly, moderate ethanol given 24 h prior to burn still showed a decrease in DTH, suggesting the effects of a single exposure to ethanol may lead to problems even after the ethanol has been cleared from the circulation [40].
IMMUNOSUPPRESSION AT THE SITE OF INJURY
The immunosuppression observed in the above studies was then analyzed in terms of effects on different organ systems. Looking at the skin after burn injury, mice given a subeschar infection of P. aeruginosa at 24 h after injury had a significantly increased rate of mortality with ethanol compared with vehicle-treated animals [41]. Increased mortality was also observed following topical infection after ethanol and burn injury [21]. Although mortality does occur following ethanol and burn injury without infection [40], mortality following bacterial infection is significantly greater. The increase in mortality after infection suggests an inability of the animal to clear the bacteria, which may be resultant from immunosuppression after ethanol and burn injury. Decreased bacterial clearance was observed in animal models of burn injury alone and in conjunction with ethanol exposure [42, 43]. Decreases in leukocyte migration and/or activation may be responsible for the lack of clearance in these animals. However, no differences in neutrophil infiltration or myeloperoxidase content were observed at the wound site. Consistent with this finding, the levels of KC, a neutrophil chemoattractant and murine homologue to IL-8, were actually elevated in the wound bed and surrounding tissue after burn and ethanol. The problem may therefore be a lack of effector cell activation, and indeed, a significant decrease in TNF-α production was observed in the skin at 24 h post-injury [41]. IL-6 was also examined; however, no differences were found between mice treated with ethanol prior to burn injury and mice treated with burn injury alone (E. J. Kovacs, unpublished observations).
INFLAMMATION IN THE GUT AFTER ETHANOL AND BURN
Maintenance of proper function in the gut, in barrier integrity and nutrient absorption, after injury is essential to prevent the promotion of bacterial translocation from the gut to other organs promoting MODS and MOF (reviewed in ref. [44]). Therefore, we are also looking at changes in the gut after ethanol and burn injury in our mouse model. Relative to either insult alone, the combination of ethanol and burn injury resulted in significant decreases in TNF-α in the ileum [45]. Increased IL-6 was observed in the gut at 24 h, which was concurrent with a shortening of villus height and decreased production of IL-10. Interestingly, immunohistochemical staining showed localization of IL-6 to almost all of the enterocytes in the ileum after burn and ethanol compared with relatively few IL-6-producing enterocytes in sham animals. No significant differences were observed in neutrophil accumulation in the ileum at 24 h [45].
Another animal model used by our colleague, Dr. Mashkoor Choudhry (University of Alabama at Birmingham), involves an acute ethanol exposure and subsequent burn injury in rats. This model was used to examine inflammation and barrier function in the gut [23]. Increased infection in burn patients is a major problem; however, the exact mechanism is unknown. Bacterial translocation from the gut leading to infection and sepsis is one possibility. In rats given ethanol and burn injury, bacterial translocation from the gut to the mesenteric lymph nodes was increased at 48 h post-injury compared with animals receiving either insult alone. Bacterial translocation can result from disruption of the physical barrier and/or from immune suppression. In these studies, there were no obvious differences in intestinal morphology between groups. However, increased suppression of T cells, as determined by decreased proliferation and decreased production of IFN-γ, was observed in the animals receiving ethanol prior to burn injury [23]. Interestingly, depletion of CD3+ T cells in normal animals resulted in increased bacterial translocation to the lymph nodes similar to levels observed in ethanol and burn-treated animals. Depletion of T cells in burn ethanol rats further exacerbated the bacterial accumulation in the lymph nodes, suggesting that T cell suppression may play a role in bacteria escaping the lumen of the gut. The suppression of T cell activity may be a result of a disruption in signaling by phosphatases following ethanol and burn injury [46]. Increased neutrophils were also observed in the gut after ethanol and burn [47]. These findings are in contrast to the mouse model described above; however, differences in the animal models including the use of oral gavage and a larger burn size in the rat experiments could account for the discrepancy. Ethanol given to rats prior to burn injury resulted in increased levels of IL-18, which could be a cause for the increased leukocyte recruitment and edema in these animals. Treatment of animals with the caspase-1 inhibitor, which prevents cleavage of pro-IL-18 into active IL-18, significantly attenuated the increase in IL-18, neutrophil accumulation, and intestinal edema in rats after burn and ethanol [47]. Treatment of animals with antibodies against CXC-chemokines decreased translocation of bacteria to the mesenteric lymph nodes [48].
Overgrowth of intestinal bacteria was also observed in the small intestine of rats treated with ethanol and burn injury in addition to the mesenteric lymph nodes. The bacterial translocation was most likely a result of increased intestinal permeability, as demonstrated by the leaking of radiolabeled sugars from the gut into the circulation [23, 49]. Ethanol was shown to disrupt the functional as well as structural integrity of the gut at high [50,51,52] and physiological doses [53]. Bacterial translocation may be a result of decreased enterocyte renewal and E-cadherin junction formation [54]. Furthermore, chronic alcohol was shown to weaken the immune defense system to enteric pathogens [55]. Significant decreases in the number of T cells and dendritic cells in the intestine were observed in the intestine after ethanol and burn [49], suggesting a reason, in combination with the disruption of the intestinal barrier, for the increased bacterial spread.
PULMONARY INFLAMMATION AFTER ETHANOL AND BURN
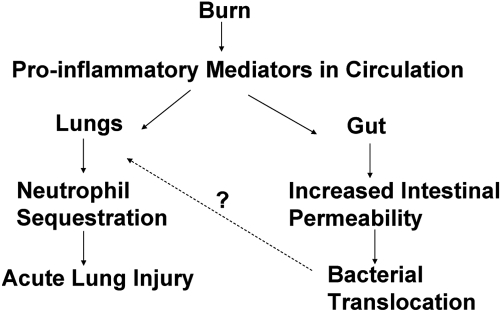
Prior to recent advances in antibiotics and standard care practices, infection in the wound bed was the major cause of morbidity and mortality in patients [56, 57]. Currently, the major post-injury complications result from sepsis and MOF [58], and one of the first organs to fail are the lungs. Consistently in humans, chronic ethanol consumption was linked with increased risk of acute respiratory distress syndrome [59]. Although exact mechanism by which ethanol increases pulmonary dysfunction after ethanol and burn injury is unknown, several possibilities include leakage of bacteria and endotoxin from the gut, the increased risk of contact with pathogens from the circulation and the airway, and the delicate architecture of the lung itself. Burn and other injuries were shown to cause intestinal bacterial translocation, resulting in bacteria and proinflammatory mediators (i.e., cytokines and endotoxin) being carried from the gut to distal organs, primarily the lung. Animal models have shown that these proinflammatory factors released from the gut after burn lead to neutrophil activation, endothelial cell activation and damage, and acute lung injury (reviewed in ref. [44]). These increases in transport of proinflammatory factors and bacteria from the gut may then lead to the observed acute lung injury (Fig. 1).
Fig. 1.
Schematic of events following burn, leading to acute lung injury. The increased proinflammatory mediators in the circulation may lead to decreased T cell activity and increased intestinal permeability with a subsequent translocation of bacteria or bacterial products to the lung. These inflammatory products may then produce aberrant inflammation in the lung, leading to tissue damage and subsequent organ failure.
Similar to what was observed in the gut and circulation, IL-6 was two-fold higher in the lungs of mice following ethanol and burn injury than animals subjected to sham injury or burn injury without ethanol, again suggesting an aberrant production of proinflammatory cytokines in these mice. This increase in IL-6 was concomitant with increased levels of KC and neutrophil infiltration at 24 h after injury in the lungs of burn and ethanol-treated mice (M. D. Bird, E. J. Kovacs, unpublished observations). Currently, it is unclear if the increased cytokines are in the lung tissue or in the passenger blood; however, experiments using saline-perfused lungs are underway to answer this question. Additionally, increased IL-18, ICAM-1, and chemokines were observed in the lungs of rats after ethanol and burn injury [60].
Neutrophil accumulation and edema were also present in the lungs at 24 h after injury, which is consistent with studies by our laboratory in mice [60]. As shown in the intestine, treatment of ethanol and burn rats with caspase-1 inhibitor ameliorated the inflammation and edema in the lungs of these animals. Interestingly, no differences were observed in IL-1β, which is also cleaved by caspase-1, production in the lung after ethanol, and burn injury [60]. This observation may be a result of IL-1β being expressed with different kinetics than IL-18 so that it is no longer present at 24 h post-injury. Treatment with mAb against IL-18 was beneficial, further suggesting a role for IL-18 in the excessive inflammation after ethanol and burn injury. Interestingly, anti-neutrophil antiserum prevented the infiltration of the phagocytes and associated tissue edema in the lungs but did not affect IL-18 production [60]. Similar results were also observed in the gut [61]. These studies suggest that ethanol prior to burn injury up-regulates the production of IL-18 in the gut and lungs, resulting in inflammation and barrier dysfunction.
In animal models, increased neutrophils were observed in the lungs of mice and rats following burn injury [62, 63]. Studies performed in our laboratory examined the inflammatory process in lungs over several time-points in mice given ethanol and burn injury. Histochemical analysis of H&E-stained lung sections at 24 h showed increased leukocyte emigration in the lungs of mice given ethanol and burn injury compared with mice given burn alone. These neutrophils were counted, and significantly higher numbers of infiltrating cells were observed at 2, 8, 12, and 24 h post-injury in the lungs of ethanol-treated mice. Levels of the leukocyte chemoattractant factor MIP-2 were also found to be elevated in lung homogenates at those same time-points. However, levels of KC were not observed to be significantly different in burn ethanol mice compared with mice subjected to burn injury alone. Interestingly, lung edema (as measured by lung wet weight) was significantly higher in mice given burn and ethanol, suggesting a possible increase in vascular permeability [64].
The increased neutrophil extravasation into the lung may not only be a result of the increased production of chemoattractant factors but also increases in adhesion molecules, such as ICAM-1. As stated above, increased levels of ICAM-1 were observed in the lungs after burn alone [65] and after ethanol and burn injury [60]. Additionally, increased expression of ICAM-1’s binding partner CD11b/CD18 was found on peripheral neutrophils [65]. Consistent with these observations, clinical studies found increased soluble ICAM-1 in the serum of burn patients [66] and that this increase in adhesion molecule expression correlated with poor outcome [67]. In vitro and in vivo work demonstrate that immune cell-adhesion molecule interactions are altered at physiological doses of ethanol [68], which may play a role in the increased innate cell infiltration and inflammation observed following ethanol and burn. Additionally, recent studies in our laboratory demonstrated that the increased chemokine and cytokine production and neutrophil infiltration observed in the lungs following ethanol and burn injury were completely ameliorated in the absence of ICAM-1, suggesting a role for this molecule in the aberrant pulmonary inflammation observed in our model (M. D. Bird, E. J. Kovacs, unpublished observations).
Current studies in our laboratory are examining the impact of ethanol on pulmonary infection after burn injury. Bacterial pneumonia is one of the leading causes of death in burn patients [69]. A common pathogen in these nosocomial infections is P. aeruginosa. In our mouse model of acute ethanol and burn injury, intratracheal inoculation of P. aeruginosa resulted in a significantly higher mortality and numbers of bacteria in the lungs of mice given ethanol and subjected to burn injury compared with mice treated with burn or ethanol alone. Interestingly, high numbers of neutrophils were recovered from the lungs of the burn ethanol mice, suggesting that leukocyte chemotaxis is not impaired at this time-point, but effector function of these cells may be affected by the combined insult of ethanol and burn, resulting in decreased bacterial clearance in these animals [43]. Additionally, it is important to note that chemotaxis of neutrophils may be impaired at earlier time-points contributing to the lack of bacterial clearance observed. These studies may shed light on mechanisms involved in the increased infection rates in patients who consumed ethanol prior to the time of injury.
Disruption of barrier function in the lungs may lead to increased edema, increased leukocyte infiltration, and decreased lung function [43, 60, 64]. Damage to the microvascular endothelium plays a role in pathogenesis after burn injury [70]. Signaling cascades resulting from the aberrant inflammatory response may contribute to the disruption of the endothelial tight junctions, allowing for increased permeability. Studies performed by Tinsley and colleagues [71, 72] demonstrate that burn injury results in loosening of tight junctions between endothelial cells in the pulmonary microvasculature that is controlled by myosin light-chain kinase [71] and is protein kinase C-dependent [72]. Studies are currently underway in our laboratory to examine if ethanol treatment prior to burn injury yields a more marked disruption of endothelial cell barriers in the lung compared with animals given burn injury alone.
CONCLUSION
Ethanol use is an important factor in morbidity and mortality after burn injury as a result of an aberrant inflammatory response and distal organ damage. The exact mechanism by which acute ethanol exaggerates inflammation in the gut and lungs after injury has yet to be elucidated. However, it is apparent that vascular permeability and barrier dysfunction, bacterial translocation, increased leukocyte infiltration into tissues, and immune suppression of lymphocytes and other immune cells are likely to be involved in this process (Table 1). Therapeutics designed to prevent this excessive inflammation, through the use of mAb or through hormone manipulation, may in turn prevent secondary infection and subsequent morbidity and mortality or promote recovery in burn patients.
TABLE 1.
Effects of Ethanol and Burn Injury in Different Organ Systems
| Changes after ethanol + burn injury | References | |
|---|---|---|
| Systemic | ↑ IL-6 | [28, 40] |
| ↓ DTH | [21, 40] | |
| ↓ Cell proliferation | [21, 26, 40] | |
| Skin | ↑ KC | [41] |
| ↓ TNF-α | [41] | |
| ↓ Survival after topical bacterial challenge | [21, 39, 41] | |
| Gut | ↑ IL-6 | [45] |
| ↓ TNF-α | [45] | |
| ↑ IL-18 | [61] | |
| ↑ Bacterial translocation | [23] | |
| ↑ Neutrophil sequestration | [47] | |
| T cell suppression | [23] | |
| Lungs | ↑ IL-18 | [60] |
| ↑ ICAM-1 expression | [60] | |
| ↑ Neutrophil sequestration | [62, 63] | |
| ↓ Bacterial clearance | [43] | |
| ↓ Survival after intratracheal bacterial challenge | [43] |
Significant changes compared with sham animals or burn-alone animals.
Acknowledgments
This work was supported by National Institutes of Health R01 AA012034 and T32 AA013527, an Illinois Excellence in Academic Medicine Grant, and the Dr. Ralph and Marian C. Falk Medical Research Trust. The authors thank Dr. Joslyn Albright for thoughtful discussions and critical review of the manuscript. We also thank Luis Ramirez and Michelle Morgan for help with animal experiments.
References
- Moore F A, Moore E E. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- Marshall J C. SIRS and MODS: what is their relevance to the science and practice of intensive care? Shock. 2000;14:586–589. [PubMed] [Google Scholar]
- Zhou D H, Munster A M, Winchurch R A. Inhibitory effects of interleukin 6 on immunity. Possible implications in burn patients. Arch Surg. 1992;127:65–68. doi: 10.1001/archsurg.1992.01420010079011. [DOI] [PubMed] [Google Scholar]
- Drost A C, Larsen B, Aulick L H. The effects of thermal injury on serum interleukin 1 activity in rats. Lymphokine Cytokine Res. 1993;12:181–185. [PubMed] [Google Scholar]
- Kowal-Vern A, Walenga J M, Sharp-Pucci M, Hoppensteadt D, Gamelli R L. Postburn edema and related changes in interleukin-2, leukocytes, platelet activation, endothelin-1, and C1 esterase inhibitor. J Burn Care Rehabil. 1997;18:99–103. doi: 10.1097/00004630-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Smith G S, Kraus J F. Alcohol and residential, recreational, and occupational injuries: a review of the epidemiologic evidence. Annu Rev Public Health. 1988;9:99–121. doi: 10.1146/annurev.pu.09.050188.000531. [DOI] [PubMed] [Google Scholar]
- Thal E R, Bost R O, Anderson R J. Effects of alcohol and other drugs on traumatized patients. Arch Surg. 1985;120:708–712. doi: 10.1001/archsurg.1985.01390300058010. [DOI] [PubMed] [Google Scholar]
- McGill V, Kowal-Vern A, Fisher S G, Kahn S, Gamelli R L. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- Howland J, Hingson R. Alcohol as a risk factor for injuries or death due to fires and burns: review of the literature. Public Health Rep. 1987;102:475–483. [PMC free article] [PubMed] [Google Scholar]
- Albright J M, Kovacs E J, Gamelli R L, Schermer C R. Implications of formal alcohol screening in burn patients. J Burn Care Res. 2008 doi: 10.1097/BCR.0b013e3181921f31. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal-Vern A, Walenga J M, Hoppensteadt D, Sharp-Pucci M, Gamelli R L. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. J Am Coll Surg. 1994;178:357–362. [PubMed] [Google Scholar]
- Grobmyer S R, Maniscalco S P, Purdue G F, Hunt J L. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil. 1996;17:532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Haum A, Perbix W, Hack H J, Stark G B, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns. 1995;21:194–199. doi: 10.1016/0305-4179(95)80008-c. [DOI] [PubMed] [Google Scholar]
- Silver G M, Albright J A, Schermer C R, Halerz M, Conrad P, Ackerman P D, Lau L, Emanuele M A, Kovacs E J, Gamelli R L. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008 doi: 10.1097/BCR.0b013e31818481bc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T R, Smith W, Eckardt M J. Murine model of ethanol-induced immunosuppression. Alcohol Clin Exp Res. 1990;14:546–550. doi: 10.1111/j.1530-0277.1990.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Meyer A A, Johnson M C, deSerres S, Peterson H D. Chronic ethanol exposure before injury produces greater immune dysfunction after thermal injury in rats. J Trauma. 1990;30:27–31. doi: 10.1097/00005373-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Cook R T. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Szabo G, Girouard L, Mandrekar P, Catalano D. Regulation of monocyte IL-12 production: augmentation by lymphocyte contact and acute ethanol treatment, inhibition by elevated intracellular cAMP. Int J Immunopharmacol. 1998;20:491–503. doi: 10.1016/s0192-0561(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Messingham K A, Faunce D E, Kovacs E J. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Sibley D A, Osna N, Kusynski C, Wilkie L, Jerrells T R. Alcohol consumption is associated with alterations in macrophage responses to interferon-γ and infection by Salmonella typhimurium. FEMS Immunol Med Microbiol. 2001;32:73–83. doi: 10.1111/j.1574-695X.2001.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Faunce D E, Gregory M S, Kovacs E J. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- Napolitano L M, Koruda M J, Zimmerman K, McCowan K, Chang J, Meyer A A. Chronic ethanol intake and burn injury: evidence for synergistic alteration in gut and immune integrity. J Trauma. 1995;38:198–207. doi: 10.1097/00005373-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Choudhry M A, Fazal N, Goto M, Gamelli R L, Sayeed M M. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- Spector W G, Walters M N, Willoughby D A. Venular and capillary permeability in thermal injury. J Pathol Bacteriol. 1965;90:635–640. doi: 10.1002/path.1700900233. [DOI] [PubMed] [Google Scholar]
- Faunce D E, Llanas J N, Patel P J, Gregory M S, Duffner L A, Kovacs E J. Neutrophil chemokine production in the skin following scald injury. Burns. 1999;25:403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- Choudhry M A, Messingham K A, Namak S, Colantoni A, Fontanilla C V, Duffner L A, Sayeed M M, Kovacs E J. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Biffl W L, Moore E E, Moore F A, Peterson V M. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunce D E, Gregory M S, Kovacs E J. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–140. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Faunce D E, Gregory M S, Kovacs E J. Glucocorticoids protect against suppression of T cell responses in a murine model of acute ethanol exposure and thermal injury by regulating IL-6. J Leukoc Biol. 1998;64:724–732. doi: 10.1002/jlb.64.6.724. [DOI] [PubMed] [Google Scholar]
- Li X, Rana S N, Kovacs E J, Gamelli R L, Chaudry I H, Choudhry M A. Corticosterone suppresses mesenteric lymph node T cells by inhibiting p38/ERK pathway and promotes bacterial translocation after alcohol and burn injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R37–R44. doi: 10.1152/ajpregu.00782.2004. [DOI] [PubMed] [Google Scholar]
- Laszlo F A, Varga C, Pavo I, Gardi J, Vecsernyes M, Galfi M, Morschl E, Laszlo F, Makara G B. Vasopressin pressor receptor-mediated activation of HPA axis by acute ethanol stress in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R458–R465. doi: 10.1152/ajpregu.2001.280.2.R458. [DOI] [PubMed] [Google Scholar]
- Lang C H, Frost R A, Kumar V, Vary T C. Impaired myocardial protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4F. Am J Physiol Endocrinol Metab. 2000;279:E1029–E1038. doi: 10.1152/ajpendo.2000.279.5.E1029. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Nishi Y, Sato E F, Ishii M, Hamada T, Inoue M. Thermal injury induces thymocyte apoptosis in the rat. J Trauma. 1998;44:143–148. doi: 10.1097/00005373-199801000-00019. [DOI] [PubMed] [Google Scholar]
- Fukuzuka K, Edwards C K, III, Clare-Salzler M, Copeland E M, III, Moldawer L L, Mozingo D W. Glucocorticoid-induced, caspase-dependent organ apoptosis early after burn injury. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1005–R1018. doi: 10.1152/ajpregu.2000.278.4.R1005. [DOI] [PubMed] [Google Scholar]
- Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990;20:2439–2443. doi: 10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine K E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κ B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla C V, Faunce D E, Gregory M S, Messingham K A, Durbin E A, Duffner L A, Kovacs E J. Anti-interleukin-6 antibody treatment restores cell-mediated immune function in mice with acute ethanol exposure before burn trauma. Alcohol Clin Exp Res. 2000;24:1392–1399. [PubMed] [Google Scholar]
- Deshpande R, Khalili H, Pergolizzi R G, Michael S D, Chang M D. Estradiol down-regulates LPS-induced cytokine production and NFκB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Messingham K A, Heinrich S A, Kovacs E J. Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. J Leukoc Biol. 2001;70:887–895. [PubMed] [Google Scholar]
- Messingham K A, Fontanilla C V, Colantoni A, Duffner L A, Kovacs E J. Cellular immunity after ethanol exposure and burn injury: dose and time dependence. Alcohol. 2000;22:35–44. doi: 10.1016/s0741-8329(00)00100-2. [DOI] [PubMed] [Google Scholar]
- Faunce D E, Garner J L, Llanas J N, Patel P J, Gregory M S, Duffner L A, Gamelli R L, Kovacs E J. Effect of acute ethanol exposure on the dermal inflammatory response after burn injury. Alcohol Clin Exp Res. 2003;27:1199–1206. doi: 10.1097/01.ALC.0000075833.92139.35. [DOI] [PubMed] [Google Scholar]
- Wilkinson R A, Fishman J A. Effect of thermal injury with Pseudomonas aeruginosa infection on pulmonary and systemic bacterial clearance. J Trauma. 1999;47:912–917. doi: 10.1097/00005373-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Murdoch E L, Brown H G, Gamelli R L, Kovacs E J. Effects of ethanol on pulmonary inflammation in post-burn intratracheal infection. J Burn Care Res. 2008;29:323–330. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- Magnotti L J, Deitch E A. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- Scalfani M T, Chan D M, Murdoch E L, Kovacs E J, White F A. Acute ethanol exposure combined with burn injury enhances IL-6 levels in the murine ileum. Alcohol Clin Exp Res. 2007;31:1731–1737. doi: 10.1111/j.1530-0277.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- Li X, Schwacha M G, Chaudry I H, Choudhry M A. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;79:453–462. doi: 10.1189/jlb.0705369. [DOI] [PubMed] [Google Scholar]
- Rana S N, Li X, Chaudry I H, Bland K I, Choudhry M A. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- Fazal N, Shamim M, Khan S S, Gamelli R L, Sayeed M M. Neutrophil depletion in rats reduces burn-injury induced intestinal bacterial translocation. Crit Care Med. 2000;28:1550–1555. doi: 10.1097/00003246-200005000-00048. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M J, Clark C, Goto M, Kovacs E J, Gamelli R L, Sayeed M M, Choudhry M A. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290–296. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Banan A, Smith G S, Rieckenberg C L, Kokoska E R, Miller T A. Protection against ethanol injury by prostaglandin in a human intestinal cell line: role of microtubules. Am J Physiol. 1998;274:G111–G121. doi: 10.1152/ajpgi.1998.274.1.G111. [DOI] [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields J Z, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Ma T Y, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- Kishikawa H, Miura S, Nishida J, Nakano M, Hirano E, Sudo N, Morishita T, Ishii H. Ethanol-induced CXC-chemokine synthesis and barrier dysfunction in intestinal epithelial cells. Alcohol Clin Exp Res. 2005;29:2116–2122. doi: 10.1097/01.alc.0000192299.63463.50. [DOI] [PubMed] [Google Scholar]
- Al-Ghoul W M, Khan M, Fazal N, Sayeed M M. Mechanisms of postburn intestinal barrier dysfunction in the rat: roles of epithelial cell renewal, E-cadherin, and neutrophil extravasation. Crit Care Med. 2004;32:1730–1739. doi: 10.1097/01.ccm.0000132896.62368.01. [DOI] [PubMed] [Google Scholar]
- Padgett E L, Sibley D A, Jerrells T R. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulations in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–298. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Tompkins R G, Burke J F. Burn therapy 1985: acute management. Intensive Care Med. 1986;12:289–295. doi: 10.1007/BF00261738. [DOI] [PubMed] [Google Scholar]
- Tompkins R G, Remensnyder J P, Burke J F, Tompkins D M, Hilton J F, Schoenfeld D A, Behringer G E, Bondoc C C, Briggs S E, Quinby W C., Jr Significant reductions in mortality for children with burn injuries through the use of prompt eschar excision. Ann Surg. 1988;208:577–585. doi: 10.1097/00000658-198811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomkin J S. Neutrophil disorders in burn injury: complement, cytokines, and organ injury. J Trauma. 1990;30:S80–S85. doi: 10.1097/00005373-199012001-00019. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore F A, Moore E E, Parsons P E. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- Li X, Kovacs E J, Schwacha M G, Chaudry I H, Choudhry M A. Acute alcohol intoxication increases interleukin-18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- Li X, Schwacha M G, Chaudry I H, Choudhry M A. Acute alcohol intoxiction potentiates neutrophil-mediated intestinal tissue damage after burn injury. Shock. 2007;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester M, Carter E A, Tompkins R G, Gelfand J A, Dinarello C A, Burke J F, Clark B D. Thermal injury induces very early production of interleukin-1 α in the rat by mechanisms other than endotoxemia. Surgery. 1994;115:588–596. [PubMed] [Google Scholar]
- Stengle J, Meyers R, Pyle J, Dries D J. Neutrophil recruitment after remote scald injury. J Burn Care Rehabil. 1996;17:14–18. doi: 10.1097/00004630-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Patel P J, Faunce D E, Gregory M S, Duffner L A, Kovacs E J. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am J Respir Cell Mol Biol. 1999;20:1229–1237. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- Jin R B, Zhu P F, Wang Z G, Liu D W, Zhou J H. Changes of pulmonary intercellular adhesion molecule-1 and CD11b/CD18 in peripheral polymorphonuclear neutrophils and their significance at the early stage of burns. Chin J Traumatol. 2003;6:156–159. [PubMed] [Google Scholar]
- Ahmed S el-D, el-Shahat A S, Saad S O. Assessment of certain neutrophil receptors, opsonophagocytosis and soluble intercellular adhesion molecule-1 (ICAM-1) following thermal injury. Burns. 1999;25:395–401. doi: 10.1016/s0305-4179(98)00164-8. [DOI] [PubMed] [Google Scholar]
- Nakae H, Endo S, Yamada Y, Inada K. Bound and soluble adhesion molecule and cytokine levels in patients with severe burns. Burns. 2000;26:139–144. doi: 10.1016/s0305-4179(99)00118-7. [DOI] [PubMed] [Google Scholar]
- Saeed R W, Varma S, Peng T, Tracey K J, Sherry B, Metz C N. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J Immunol. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- Berger M M, Eggimann P, Heyland D K, Chiolero R L, Revelly J P, Day A, Raffoul W, Shenkin A. Reduction of nosocomial pneumonia after major burns by trace element supplementation: aggregation of two randomized trials. Crit Care. 2006;10:R153. doi: 10.1186/cc5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Xu W, Ustinova E, Wu M, Childs E, Hunter F, Yuan S. Myosin light chain kinase-dependent microvascular hyperpermeability in thermal injury. Shock. 2003;20:363–368. doi: 10.1097/01.shk.0000079425.0000.db. [DOI] [PubMed] [Google Scholar]
- Tinsley J H, Teasdale N R, Yuan S Y. Myosin light chain phosphorylation and pulmonary endothelial cell hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol. 2004;286:L841–L847. doi: 10.1152/ajplung.00341.2003. [DOI] [PubMed] [Google Scholar]
- Tinsley J H, Breslin J W, Teasdale N R, Yuan S Y. PKC-dependent, burn-induced adherens junction reorganization and barrier dysfunction in pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L217–L223. doi: 10.1152/ajplung.00248.2004. [DOI] [PubMed] [Google Scholar]