Abstract
Neutrophils represent the most common granulocyte subtype present in blood. The short half-life of circulating neutrophils is regulated by spontaneous apoptosis, and tissue infiltrating neutrophils die by apoptosis and secondary necrosis. The mechanism of neutrophil apoptosis has been the subject of many studies; however, the mechanism of neutrophil secondary necrosis is less clear. Human cathelicidin cationic peptide 18, proteolytically processed to its active form, LL-37, is secreted by neutrophils and epithelial cells and shown to have effects in addition to bacterial lysis. We demonstrate here that LL-37 affects neutrophil lifespan by the pathway of secondary necrosis, rapidly converting annexin V-positive (AV+), propidium iodide-negative (PI−; apoptotic) cells into PI+ (necrotic) cells with the release of IL-8, IL-1R antagonist, ATP, and intact granules. The effects of LL-37 on apoptotic neutrophils are neither energy-dependent nor affected by pretreatment with G-CSF, GM-CSF, TNF-α, and LPS and are partially inhibited by human serum. Moreover, LL-37 decreases CXCR2 expression of AV−PI− (live) neutrophils, suggesting an effect on the neutrophil response to its chemotactic factors, including IL-8. Thus, the lifespan and inflammatory functions of neutrophils are directly affected by LL-37.
Keywords: apoptosis, CXCR2, IL-8, IL-1Ra
INTRODUCTION
Neutrophils represent the most common granulocyte subtype present in blood. On the positive side, they play an important role in antibacterial and antifungal defense mechanisms. However, on the negative side, neutrophils are implicated as a mediator of tissue-destructive events in inflammatory diseases ranging from rheumatoid arthritis and myocardial reperfusion injury to respiratory distress syndromes, blistering skin disorders, and ulcerative colitis [1, 2]. Neutrophils may also play a role in neoangiogenesis, as indicated by recent studies that demonstrate that tumor-infiltrating neutrophils activate angiogenesis in previously quiescent tissue vasculature during the early stages of tumorigenesis [3]. The short half-life of circulating neutrophils is regulated by spontaneous apoptosis as opposed to necrosis, and apoptotic neutrophils are phagocytosed by cells such as macrophages in a process that is associated with the release of anti-inflammatory mediators [4, 5]. However, tissue-infiltrating neutrohils may also undergo secondary necrosis as illustrated in a model of lung inflammation [2, 6,7,8]. Secondary necrosis of neutrophils is expected to release the pro- and anti-inflammatory contents of the neutrophils affecting the duration and intensity of an inflammatory response. In spite of its importance, the mechanism of neutrophil secondary necrosis is unclear.
Human cationic peptide (hCAP-18), the only human cathelicidin identified (CAMP located on chromosome 3p21.3), was first isolated in the specific granules of human neutrophils [9]. hCAP-18 is also produced by epithelial cells of the lung, intestine, and urogenital tract [10], and high concentrations of hCAP-18 have been detected in human seminal plasma [11] and in human plasma [12]. LL-37, the proteolytically (proteinase 3) active product of hCAP-18, is a multifunctional modulator of innate immune responses [13], involved in antibacterial function [14], protection of the urinary tract [10], stimulation of angiogenesis [15], cutaneous wound-healing [16], and chemoattraction of inflammatory and immune cells [17, 18]. The antibacterial function of LL-37 has been ascribed to its membrane pore-forming activity [19], and at higher concentrations (>13 μM), it is also cytotoxic for eukaryotic cells [20]. Notably increased concentrations of LL-37 in the airways have been found in inflammatory [21] and infectious lung disease [22], in which neutrophil secondary necrosis has been observed [6, 8]. Although LL-37 was first isolated in neutrophils, the direct effect of LL-37 on human neutrophils has not been well-studied in vitro.
Here, we investigate the in vitro regulation of human neutrophil lifespan and function by LL-37. We found that LL-37 directly affects neutrophil lifespan by the pathway of neutrophil secondary necrosis, rapidly converting annexin V-positive, propidium iodide-negative (AV+PI−) cells into PI+ (necrotic) cells with the release of IL-8, IL-1R antagonist (IL-1Ra), ATP, and intact granules. The effects of LL-37 on neutrophil secondary necrosis are not energy-dependent and are partially inhibited by human serum. Moreover, LL-37 also affects live neutrophils (AV−PI−) by decreasing neutrophil surface CXCR2 expression, the classic receptor for IL-8, growth-related oncogenes (GROs), neutrophil-activating peptide 2 (NAP-2), and epithelial-derived neutrophil-activating factor-78 (ENA-78), all of which are involved in neutrophil migration and secretion.
MATERIALS AND METHODS
Reagents
Recombinant human TNF-α, G-CSF, GM-CSF, LPS (Escherichia coli 055:B5), ATP assay kit, and neutralized anti-IL-8 mAb were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rhodamine B-LL-37 (human) was from Phoenix Pharmaceuticals Inc. (Belmont, CA, USA). Neutralizing anti-IL-1Ra polyclonal antibody and an isotype control were from GeneTex Inc. (San Antonio, TX, USA). Anti-CXCR1-allophycoyanin (APC), anti-CXCR2-FITC, anti-CD16-PerCP-Cy5.5, anti-CCR3-PE, AV-FITC, AV-APC, AV-biotin, and PI were from BD Bioscences (Chicago, IL, USA), CFSE was from Molecular Probes (Eugene, OR, USA), FBS and human serum were from Irvine Scientific (Santa Ana, CA, USA), and a lactate dehydrogenase (LDH) cytotoxicity bioassay kit was from Promega (Madison, WI, USA). LL-37 was synthesized by N-(9-fluorenyl) methoxycarbonyl chemistry at the DNA/RNA/peptide synthesis lab at the City of Hope National Medical Center (Duarte, CA, USA). Peptides were purified by reverse-phase HPLC to at least 98% purity and were LPS-free as analyzed by Limulus amoebocyte lysate from Cambrex Bio Science (Walkersville, MD, USA). LL-37 was dissolved in endotoxin-free water from B. Braun Medical Inc. (Philadelphia, PA, USA) and stored at −20°C until further use. The concentration of the peptides in solution was determined by amino acid analysis. All reagents were tested to ensure that they were free of endotoxin and reconstituted in endotoxin-free water.
Cell preparation and CFSE labeling
This study was approved by the Institutional Human Subject’s Review Board (City of Hope National Medical Center). Neutrophils were isolated from citrated blood by dextran sedimentation of erythrocytes, followed by centrifugation over Ficoll-Paque Plus (GE Healthcare Biosciences, Pittsburgh, PA, USA) density gradient. Cell purities were determined by forward light-scatter/side light-scatter gating of cells stained with PerCP-Cy5.5-conjugated anti-CD16 mAb and APC-conjugated anti-CCR3 mAb using a flow cytometer (FACSCaliber, BD Biosciences). Neutrophils were defined as the CD16+CCR3− cell, and neutrophil purity was more than 95%. Neutrophils were suspended at 5 × 106 cells/mL in RPMI-1640 medium supplemented with 1% FCS (FCS contained <5 pg/100 mL LPS). After neutrophils were incubated for 18 h, AV+ neutrophils were positively sorted with AV-biotin and streptavidin-conjugated paramagnetic beads (>97% purity). Eighteen-hour-old neutrophils were also labeled with 5 μM CFSE, treated with 5 μM LL-37, and then stained with APC-AV.
Assessment of neutrophil apoptosis
Neutrophils (5×106 cells/mL) were incubated in the absence or presence of LL-37 (0.5–50 μM) at 37°C for 6 h, 12 h, 18 h, and 24 h in RPMI 1640, 1% FCS. After incubation, cells were stained with AV-FITC and PI and were analyzed by flow cytometry (FACSCaliber). Early apoptotic neutrophils were defined as the percentage of AV+ but PI− cells, live neutrophils as AV−PI− cells, and dead neutrophils as AV+PI+ cells. Results were expressed as a percentage of total detected cells. Total cell counts were performed by staining with trypan blue and counting viable cells in a hemocytometer. These numbers were used to correct the number of AV−PI− and AV+PI−cells by multiplying the percentages times the total number of viable cells.
Cytokine multiplex analysis
Fresh or 18-h-old AV+ sorted human blood neutrophils were plated at 5 × 106 cells/mL in RPMI 1640, 1% FCS, in 48-well plates. Fresh cells were then incubated in media for 18 h or 10 min in the presence of LL-37, and 18-h-aged AV+ cell were incubated for 10 min. Supernatant was analyzed using the Human Cytokine 10-Plex antibody bead kit from Biosource International Inc. (Camarillo, CA, USA). Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with an eight-parameter, curve-fitting algorithm applied for standard curve calculations [23].
LDH and ATP measurements
For quantification of cell cytolysis, release of the cytosolic enzyme LDH was measured using the fluorescence assay kit from Promega. Extracellularly released ATP in the culture supernatants of neutrophils was quantified by the sensitive firefly luciferase assay from Sigma-Aldrich. Measurements were conducted according to the manufacturer’s protocol.
Electron microscopy (EM)
AV+-sorted, 18-h-old neutrophils were treated with 0, 1, or 5 μM LL-37 and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2 (buffer A), for 1 h. After three washes in buffer A, the cells were postfixed with 1% osmium tetroxide in buffer A and then dehydrated in graded ethanol. The 100% ethanol solution was then replaced by propylene oxide, and the cells were embedded in Eponate. Thin sections were stained with uranyl acetate and lead citrate and examined with a FEI TECNAI G2 electron microscope.
Live cell imaging
Eighteen-hour-aged neutrophils (2.5×106/mL) were suspended in AV-binding buffer, treated or not with LL-37 (5 μM), and imaged every 30 s using an Olympus 1X2-UCB inverted fluorescent microscope equipped with a Weatherstation precision control stage incubator and an Orca-ER Hammamatsu camera. Three sequentiual images were obtained: phase contrast, green channel (AV), and red channel (PI). Movies were processed in Final Cut Pro. Representative frames are shown in the figure.
Determination of receptor down-modulation by flow cytometry analysis
Neutrophils were treated with 5 μM LL-37 for 6 h, washed with PBS, blocked with 10% human serum, stained with anti-CXCR1-APC, anti-CXCR2-FITC, anti-CCR3-PE, and anti-CD16-PerCP-Cy5.5, and analyzed on a FACSCaliber using FlowJo software.
Statistical analysis
Assay results are expressed as means ± se, and unpaired t-tests were used for comparisons. All P values are two-sided. Data were analyzed with SPSS software (Release 10.0, SPSS, Chicago, IL, USA) and GraphPad Prism software, Version 5.0 (GraphPad Software, San Diego, CA, USA).
RESULTS
LL-37 decreases the numbers of early apoptotic neutrophils
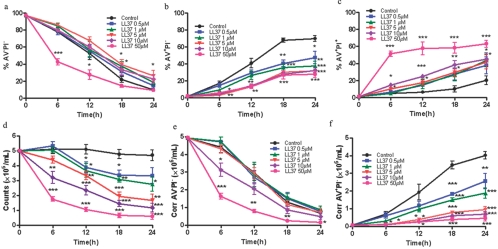
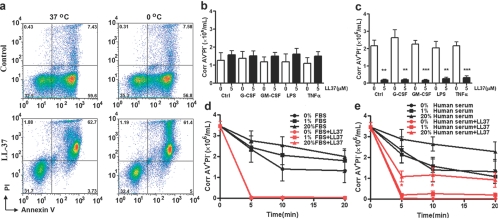
When fresh neutrophils treated with LL-37 are analyzed by flow cytometry, a dose-dependent increase in live (AV−PI−) and dead (AV+PI+) cells and a decrease in early apoptotic cells (AV+PI−) are seen over time (Fig., 1a–c). Although these data are similar to published reports that suggest LL-37 protects neutrophils from apoptosis [24, 25], we now show that this is a result of using flow cytometry as the sole method of quantitation of cells. As the apoptotic cells eventually necrose and disintegrate in culture, they are lost from this analysis. When the cells are visually observed (Supplementary Fig. 1) and counted in a hemocytometer (Fig. 1d), it is readily apparent that although the cell numbers for untreated cells barely decrease over 24 h, the number of LL-37-treated cells dramatically decreases over time in a dose-dependent manner. Thus, LL-37 removes cells from the flow cytomteric analysis, leading to an apparent increase in percentages of the remaining measureable populations, including live cells (AV−PI−), and neglects the drop in cell number as a result of cell loss. The most obvious population that is completely eliminated is the AV+PI− cells (see below). To account for a continuously decreasing number of cells in the analysis, it is necessary to correct the percentages seen in flow cytometry by accounting for the number of observable cells in the hemocytometer, which can distinguish dead cells from live and early apoptotic cells. Once this correction is made, a more accurate picture of the effect of LL-37 on live and apoptotic cells is obtained (Fig. e and f). Although toxicity for eukaryotic cells is clearly observed at doses of 13–25 μM for LL-37 and gradually increases at higher doses [20], it is noteworthy that at doses up to 5 μM, no change in corrected cell viability is seen over the 24-h treatment period. Only when doses exceed 5 μM do we observe a more rapid time course of loss of corrected cell viability (Fig. 1e). When the number of corrected apoptotic cells is plotted, a significant separation of curves is observed at all doses (Fig. 1f), demonstrating that the effect of LL-37 is on apoptotic but not on live cells.
Fig. 1.
LL-37 decreases the numbers of apoptotic neutrophils. Fresh neutrophils were treated with LL-37 (0–50 μM) and stained with AV and PI and analyzed by flow cytometry (a–c) or stained with trypan blue and viable cells counted in a hemocytometer, and the results are expressed as viable cells (d) and viable cells × the percent AV−PI− cells/(% AV−PI− cells+% AV+PI− cells) and reported as corrected AV−PI− (Corr AV−PI−) cells (e) and viable cells × % AV+PI− cells/(% AV−PI− cells+% AV+PI− cells) and reported as corrected AV+PI− (Corr AV+PI−) cells (f) versus time after treatment. Black (0 μM), blue (0.5 μM), green (1.0 μM), red (5.0 μM), purple (10.0 μM), and magenta (50.0 μM); n = 4; *, P < 0.05; **, P < 0.01; ***, P < 0.001, versus untreated controls (0 μM).
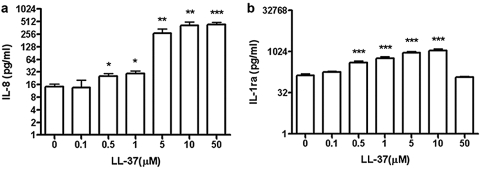
LL-37 increases secretion of IL-8 and IL-1Ra in human neutrophils
LL-37 has previously been reported to stimulate the secretion of IL-8 from monocyte and epithelial cells [13, 26], which in turn, would be expected to further enhance migration of neutrophils to the site of inflammation. Our data demonstrate that LL-37 induces the release of IL-8 from neutrophils in a dose-dependent manner (Fig. 2a). Furthermore, LL-37 stimulates the release of IL-1Ra (Fig. 2b), the natural antagonist to IL-1β, suggesting an anti-inflammatory role for LL-37-treated neutrophils. When a panel of 10 cytokines and chemokines was analyzed from LL-37-treated neutrophils from four different subjects, we found that only IL-8 and IL-1Ra were released in significant amounts (respectively, IL-1β, G-CSF, and GM-CSF are less than 20 pg/mL; IL-6, MIP-1α, IFN-γ, and monokine induced by IFN-γ are less than 5 pg/mL; and MIP-1β varied from subject to subject with the range 0–105 pg/mL). When LL-37-treated neutrophils were incubated with neutralizing antibodies to IL-8 or IL-1Ra, no change in live or apoptotic neutrophils was observed (Supplementary Fig. 2), demonstrating that the release of IL-8 or IL-1Ra was not responsible for the conversion of the apoptotic to secondary necrotic neutrophils.
Fig. 2.
LL-37 increases secretion of IL-8 and IL-1Ra. Fresh neutrophils were treated with LL-37 (0–50 μM) for 18 h, the supernatants collected, and the levels of IL-8 (a) and IL-1Ra (b) measured; n = 4; *, P < 0.05; **, P < 0.01; ***, P < 0.001, versus untreated controls (0 μM).
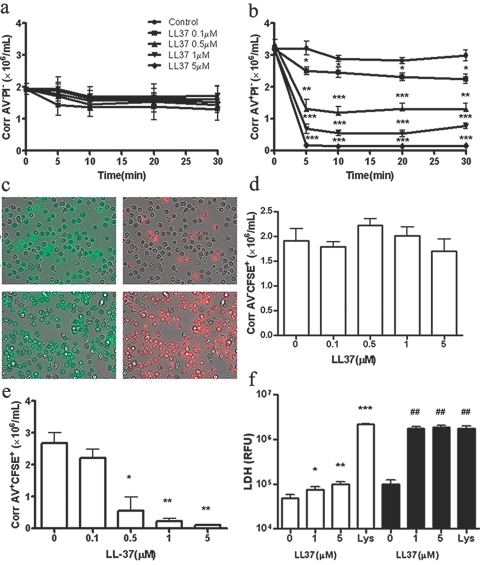
LL-37 rapidly converts neutrophils from AV+PI− to AV+PI+ with the release of cellular contents
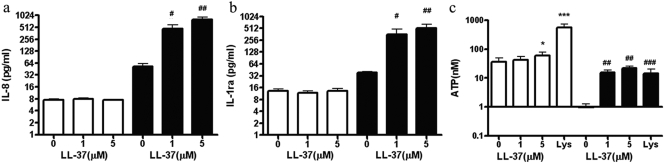
As the most potent effect of LL-37 is on apoptotic (AV+PI−) neutrophils, rather than on live (AV−PI−) neutrophils, we further investigated the pathway of neutrophil secondary necrosis [2]. Thus, we aged neutrophils in culture for 18 h, where up to 60% are AV+PI− and then treated them with LL-37. As before (Fig. 1), LL-37 had no effect on AV−PI− neutrophils (Fig. 3a), and AV+PI− neutrophils were rapidly converted to AV+PI+ neutrophils within 5 min (Fig. 3b). Time-lapse photography was also performed on the 18-h-old neutrophils incubated with FITC-AV and PI and treated with LL-37. The movies showed that AV−PI− cells do not become PI+, and AV+PI− cells rapidly become PI+ over the 10-min time course of the treatment (Supplementary Movies). Time frames at 10 min are shown for control and LL-37-treated cells (Fig. 3c). As these assays measure the influx of PI into cells, it was important to prove that LL-37 also caused the release of cellular contents. First, CFSE was used in our study. CFSE is a lipophilic molecule that is only minimally fluorescent until it is transported inside the cells, where esterase cleaves the acetyl groups, and the molecule becomes markedly fluorescent. The succinimidly ester group covalently binds to amino groups on intracellular macromolecules, anchoring the dye [27]. When CFSE-labeled, 18-h-old neutrophils were treated with LL-37 for 5 min, there was no CFSE release from live (AV−CFSE+) cells but a rapid, dose-dependent release from apoptotic (AV+CFSE+) cells (Fig. 3, d and e). Second, the 18-h-aged AV+ cells also released LDH (Fig. 3f), which is a marker of cell-membrane disintegration. Third, when the 18-h AV+ neutrophils were purified and treated with LL-37 for 10 min, they released IL-8 (Fig. 4a, solid bars) and IL-1Ra (Fig. 4b, solid bars) at similar levels as seen before (compare with Fig. 2), and in addition, they release significant amounts of ATP (Fig. 4c, solid bars). It is important to notice that in AV+ cells, no ATP is released until treatment with LL-37, whereas AV− cells continuously release ATP (Fig. 4c, open bars). When fresh neutrophils were treated with LL-37, no significant differences in release of IL-8 and IL-1Ra were observed at the same doses (Fig.4, a and b, open bars). The release of ATP, through a family of metabotropic (P2Y) and ionotropic (P2X) receptors, is expected to have physiological effects on the neighboring cells, including surrounding sensory neurons, macrophages, and mast cells [28]. In other studies, we show that IL-8 and IL-1Ra are also released to the same or greater extent for LPS treatment as for LL-37 (Supplementary Fig. 3), and it is in concordance with reports that neutrophils secrete large amounts of IL-8 and IL-1Ra [29]. These data demonstrate that the cell leakage is general and suggest that the disintegration of the apoptotic neutrophil membrane occurs after treatment with LL-37, releasing potent effectors such as ATP, IL-8, and IL-1Ra.
Fig. 3.
LL-37 rapidly converts neutrophils from AV+PI− to AV+PI+ with the release of cellular contents. Fresh neutrophils were aged in culture for 18 h (60% AV+PI−) and treated with LL-37 (0–5 μM), and the corrected number (see Fig. 1 for details of the calculation) of AV−PI− and AV+PI− cells was measured over time after treatment (a and b). Eighteen-hour-aged neutrophils were mixed with FITC-AV and PI, and time-lapse photography was performed on an inverted fluorescent microscope equipped with a 37°C incubator stage. Sequential images of phase contrasts and red and green channels were captured at a rate of three frames per minute over a period of 10 min after the addition of LL-37 (5 μM). The combined phase and green or red channels are shown for the untreated (c, upper) and treated cells (c, lower) after 10 min. Eighteen-hour-aged neutrophils were also labeled with CFSE, treated with LL-37 for 5 min, and stained with APC-labeled AV, and the corrected number of AV−CFSE+ (living; d) and AV+CFSE+ (apoptotic) cells (e) was measured. (a–e) n = 4; *, P < 0.05; **, P < 0.01; ***, P < 0.001, versus untreated controls. Fresh (open bars) or 18-h-aged AV+ (solid bars) neutrophils were incubated with LL-37 (0–5 μM) for 5 min, and the levels of LDH released into the medium were measured (f). Lys, Cell lysate; n = 4, *, P < 0.05; **, P < 0.01; ***, P < 0.001, versus untreated control of fresh neutrophils; ##, P < 0.01, versus untreated control of AV+ cell. RFU, Relative fluorescence units.
Fig. 4.
Release of IL-8, IL-1Ra, and ATP from LL-37-treated fresh and AV+ neutrophils. Fresh neutrophils (open bars) at concentration of 5 × 106/mL or 18-h-aged AV+ neutrophils (solid bars) at concentration of 5 × 106/mL were treated with 0, 1, or 5 μM LL-37. After treatment for 10 min, the levels of IL-8 (a) and IL-1Ra (b) are similar to those measured in Figure 2. (c) ATP is released into the supernatant after treatment for 5 min; n = 4; *, P < 0.05; ***, P < 0.001, versus untreated control of fresh neutrophils (open bars); #, P < 0.05; ##, P < 0.01; ###, P < 0.001, versus untreated control of AV+ cell (solid bars).
Morphological changes of 18-h-aged AV+ neutrophils treated with LL-37
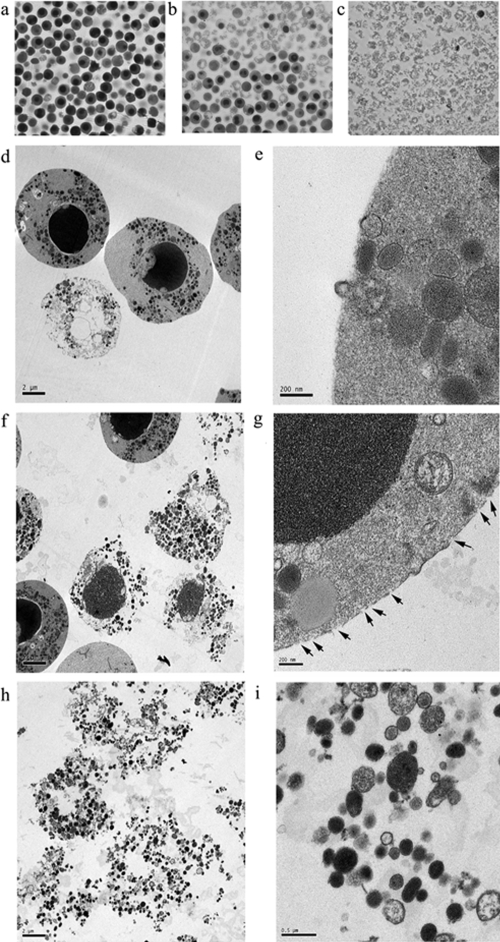
Visual evidence of the disintegration of LL-37-treated AV+ neutrophils was obtained by phase contrast microscopy and EM. When AV+ cells were purified from 18-h-old neutrophils and examined by phase contrast microscopy after centrifugation, the cells were round and bright without treatment with LL-37 (Fig. 5a), separated into two layers after treatment with 1 μM LL-37 for 5 min (Fig. 5b), and only intact granules (with the exception of an occasional intact eosinophil) were seen after treatment with 5 μM LL-37 for 5 min (Fig. 5c). EM analysis of cells taken from Figure 5a (AV+, LL-37-untreated) revealed cells with round nulcei and condensed chromatin, cells with condensed chromatin and broken nuclear membranes, and cells without any nuclei (Fig. 5d). A higher magnified view of these cells reveals intact plamsa membranes explaining why they are PI− (Fig. 5e). EM analysis of cells taken from Figure 5b (AV+, LL-37 1 μM-treated) reveals signifcant changes in morphology, including loss of the plasma membrane on some of the cells and the appearance of abundant granules (Fig. 5f). A higher magnified view of the cells with apparently intact plasma membranes reveals scattered holes in the plasma membrane (Fig. 5g), explaining why they are PI+. EM analysis of cells taken from Figure 5c (AV+, treated with 5 μM LL-37) reveals essentially only abundant granules with no evidence of nuclei (Fig. 5h). A higher magnified view indicates that the granules (varying in size and density) have intact membranes and are likely functional (Fig. 5i). These morphological results suggest that LL-37 rapidly converts AV+ neutrophils into a mass of intact granules, which is characteristic for the pathway of secondary necrosis [2]. In contrast, we did not find the same morphological changes for 18-h-old live neutrophils sorted by gating on the AV−PI− group (data not shown).
Fig. 5.
Morphology of sorted AV+ neutrophils. After neutrophils were aged for 18 h in culture, AV+ neutrophils were sorted and treated with LL-37 (0, 1, and 5 μM) and centrifuged and layers removed for phase contrast (a–c) and EM (d–i) analysis. (a) Untreated controls have round nuclei and little cell debris. (b) Treatment with 1 μM LL-37 shows intact cells and cell debris. (c) Treatment with 5 μM LL-37 shows only cell debris (except for the occasional eosinophil that copurifies with neutrophils). EM of cells taken from a (untreated control) shows intact cells with condensed chromatin (d, ×1100) and cells with intact plasma membranes (e, ×15,000). EM of cells taken from b (1 μM LL-37) shows many cells with broken plasma membranes (f, ×1100) and disintegrating cells with or without nuclei releasing their granular contents (g, ×11,000; arrows indicate the pores on the membrane of an intact cell). EM of cells taken from c (5 μM LL-37) shows broken cells (h, ×1100) with loosely grouped granules that appear intact (i, ×6500).
The effect of LL-37 on apoptotic neutrophils is neither energy-dependent nor affected by pretreatment with G-CSF, GM-CSF, TNF-α, and LPS and is partially inhibited by human serum
Previous studies demonstrated that human serum but not FBS was able to inhibit the effects of LL-37 on apoptosis of airway epithelial cells [30]. Therefore, it was important for us to investigate if human serum or other factors including temperature, cytokines, and LPS could inhibit the function of LL-37 on apoptotic neutrophils. First, we demonstrated that the specificity for AV+ neutrophils was the same whether the cells (aged 18 h) were treated at 37°C or at 0°C (Fig. 6a), suggesting that its effects are not energy-dependent. Second, preincubation of the 18-h-old neutrophils with G-CSF (30 ng/mL), GM-CSF (50 ng/mL), LPS (10 ng/mL), or TNF-α (20 ng/mL), important factors for regulating neutrophil lifespan, for 1 h had no effect on the conversion of AV+PI− cells into AV+PI+ cells by LL-37 (Fig. 6, b and c). Third, the addition of 5 μM LL-37 caused an immediate drop in the number of AV+PI− neutrophils in the absence or presence of FBS (Fig. 6d) but was inhibited significantly by 20% human serum (Fig. 6e). These results suggest that the main site of LL-37 function on apoptotic neutrophils is in tissues that are low in human serum, rather than in the bloodstream. This observation may explain why neutrophil secondary necrosis is observed in tissues rather than in blood.
Fig. 6.
Effect of temperature, human serum, and pretreatment of cytokines or LPS on the loss of AV+ cells. Eighteen-hour-aged neutrophils were treated with 0 or 5 μM LL-37 for 5 min. (a) At 37°C or at 4°C, stained with FITC-AV and PI and analyzed by flow cytomtery. Note the absence of AV+ cells after treatment with LL-37. Eighteen-hour-aged neutrophils were treated with 0 or 5 μM LL-37 for 5 min. (b) Corrected AV−PI− neutrophil counts. (c) Corrected AV+PI− neutrophil counts (see Fig. 1 for details of the calculation). Pretreatments at 1 h as shown on x-axis. (a–c) n = 3; **, P < 0.01; ***, P < 0.001, versus untreated control. When the 18-h-aged neutrophils were incubated in different amounts of FBS (0–20%) in the presence or absence of LL-37, there was no inhibition of the conversion of AV+PI− to AV+PI+ cells (d). However, human serum (0–20%) afforded a modest degree of inhibition (e). (d and e) n = 3; *, P < 0.05, versus 0% human serum + LL-37 5 μM.
LL-37 treatment down-regulates surface expression of CXCR2 on live neutrophils
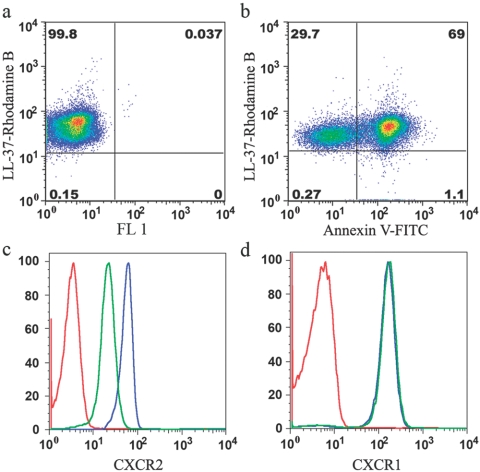
Although LL-37 has potent effects on AV+ neutrophils, we were interested in investigating whether LL-37 binds to apoptotic neutrophils alone or apoptotic and live neutrophils. Our data demonstrate that Rhodamine B-labeled LL-37 (10 pM) bound equally well to AV− and AV+ neutrophils (Fig. 7, a and b). Furthermore, staining of cell surface markers, including CXCR1, CXCR2, CXCR3, CCR1, CCR2, CCR3, CCR5, CCR7, TLR4, CD14, formyl peptide receptor-like 1, CD88, CD125, CD32, CD64, CD15, CD66, CD11b, CD18, and CD29, revealed that AV− cells treated with LL-37 down-regulate CXCR2 only. The results for CXCR2 and CXCR1 (Fig. 7, c and d), the receptors of IL-8, demonstrate that LL-37 is specific for CXCR2 over CXCR1. As CXCR2 signaling pathways are essential for neutrophil migration in response to IL-8, GROα, NAP-2, ENA-78, and granulocyte chemotactic protein-2 [31], it is possible that LL-37 has a local role in arresting the migration of neutrophils that have arrived at the site of inflammation by the down-regulation of CXCR2.
Fig. 7.
LL-37 binds to apoptotic and live neutrophils and down-regulates surface expression of CXCR2 but not CXCR1 on live neutrophils. Eighteen-hour-aged neutrophils were stained with 10 pM LL-37 Rhodamine B in the presence or absence of AV (a and b).These results show that LL-37 can bind to live (AV−) and apoptotic (AV+) neutrophils. Fresh neutrophils were treated with 0 or 5 μM LL-37 for 6 h (>90% AV−PI− cells) and the cell surface expression of CXCR1 and CXCR2 measured by flow cytomtery. (c) CXCR2. (d) CXCR1. Red (isotype control), blue (no LL-37), green (+LL-37). One representative of six independent experiments is shown. FL 1, Fluorescence 1.
DISCUSSION
Whereas neutrophils have a short half-life in the circulation (8–12 h), their numbers can increase several fold once they enter inflamed tissues. If apoptosis—the usual physiologic fate of neutrophils—has been engaged, neutrophil secretory activity is shut down; the cells remain intact and are phagocytosed by macrophages using recognition mechanisms that fail to elicit a proinflammatory response [32]. However, in inflamed tissues where LL-37 is present, apoptotic (AV+PI−) neutrophils will be converted rapidly to secondary necrotic (AV+PI+) neutrophils with the release of their cellular contents, including ATP, IL-8, IL-1Ra, and intact granules as shown here. In fact, evidence of neutrophil secondary necrosis, as shown by EM during lung inflammation [6, 8], appears identical to the EM images we showed for treatment of neutrophils with LL-37 in vitro. Although the authors did not consider the possibility that LL-37 was responsible for the secondary necrosis seen in their model, it was reported that LL-37 was detected in bronchoalveolar lavage fluid (BALF) from infants with lung infections at 20 μg/mL, well within the range of our in vitro studies [22]. The release of ATP, IL-8, IL-1Ra, and intact granules from apoptotic neutrophils after exposure to LL-37 suggests a major role for LL-37 in regulation of inflammation responses, for example, the release of ATP affecting neighboring neurons, macrophages, and masts cells [28], the release of IL-8 recruiting more neutrophils [31], and the release of IL-1Ra opposing IL-1 activity [33]. Although it is well known that neutrophil apoptosis at the inflammatory site is affected by factors such as G-CSF, GM-CSF, LPS, and TNF-α [34], none of these agents counteracts the effect of LL-37 on apoptotic neutrophils. The effect of LL-37 on neutrophil secondary necrosis is fast and energy-independent, as it occurs to the same extent at 37°C and 0°C within 5 min. Thus, LL-37 plays a dominant role in controlling neutrophil lifespan. In addition, the fact that LL-37 function is partially protected by 20% human serum agrees with the report that hCAP-18 binds to lipoproteins, such as very low-density lipoprotein (VLDL), LDL, and high-density lipoprotein [12], and suggests that the main effect of LL-37 function is in tissues, which are low in human serum, rather than in the bloodstream.
The importance of cathelicidins for effective host defense against infection by bacterial lysis has been thought to be their major function [14]. The mechanism of action of LL-37 in causing bacterial lysis is a result of its membrane pore-forming ability [19]. However, its ability to form membrane pores in mammalian cells has not been well-studied. Based on our studies, it is possible that LL-37 causes membrane pores only in apoptotic mammalian cells. This point requires further study. In human disease, LL-37 in the biopsy of atopic lesions was decreased significantly in patients with atopic dermatitis [35], and the neutrophils from patients with morbus Kostmann, a severe congenital neutropenia, are deficient in CAP-18/LL-37 [36], where this deficiency is accompanied by the occurrence of infections and periodontal disease. In other studies, LL-37 has also been reported to stimulate angiogenesis [15], cutaneous wound-healing [16], and chemoattraction of inflammatory and immune cells [17, 18]. Our data show that lower doses of LL-37 (≤5 μM) only affect apoptotic neutrophils, and higher doses of LL-37 (≥10 μM) cause toxicity, even on live neutrophils. A major question then is what is the physiological concentration of LL-37 in human tissue? LL-37 can be detected at concentrations of approximately 5 μg/mL in BALF of healthy infants and is up-regulated to 20 μg/mL from infants with lung infections [22]. Plasma has been reported to contain hCAP-18 bound to lipoproteins at a concentration of 1.2 μg/mL [12], suggesting that the precursor to LL-37 is constantly released into the bloodstream. Likewise, the hCAP-18 concentration in seminal plasma is in the range of 41.8–142.8 μg/mL [11], and LL-37 is present in psoriatic skin plaques at a median concentration of 304 μM [35]. The EC50 of LL-37 for the killing of 50% of a bacterial challenge was 4 μM [37]. The toxicity for eukaryotic cells is clearly observed at 13–25 μM LL-37 and gradually increases at higher concentrations [20]. Thus, it is suggested that the physiological concentration of LL-37 in human tissue is less than 10 μM, and it is important to measure the concentration of LL-37 in tissue to predict its effect on bacteria and recruited neutrophils.
It was previously reported that LL-37 inhibits neutrophil apoptosis [24, 25]. Our data show that these conclusions were based on using flow cytometry data alone. Although flow cytometry is good at calculating the percentage of AV−PI−, AV+PI−, and AV+PI+ cells at a given time-point, it ignores the loss of cells as a result of cell death. Although the hemocytometer can accurately count viable cells, it cannot distinguish AV−PI− from AV+PI− cells. Thus, the combination of the two methods overcomes the deficiencies of each method and allows one to calculate the cell number-corrected percentages of neutrophil apoptosis over time. Our results from the combined analysis show that LL-37 doses up to 5 μM cause no change in corrected, live cell (AV−PI−) numbers and demonstrate a signficant separation of curves in corrected apoptotic cell numbers (AV+PI−) over the 24-h treatment period, suggesting that the effect of LL-37 (≤5 μM) is on apoptotic but not on live cells. In addition, our data from phase contrast microscopy, PI influx, CFSE release, LDH leakage, the phase contrast images, and EM analysis of 18 h-aged neutrophils support the conclusion that LL-37 functions are to induce the conversion of apoptotic neutrophil to secondary necrosis.
Our results also showed that Rhodamine B-labeled LL-37 bound equally well to AV− and AV+ neutrophils and that LL-37 treatment down-regulates surface expression of CXCR2 but not CXCR1 on live neutrophils. CXCR2 and CXCR1, which are highly expressed on neutrophils, are the receptors responsible for neutrophil chemotaxis and belong to the superfamily of G-protein-coupled receptors, whose signaling is mediated by their coupling to heterotrimeric G proteins, resulting in the exchange of GDP for GTP on the subunit of the G protein [38]. IL-8, the most potent of all of the human glutamic acid-leucine-arginine (ELR)-expressing CXC subfamily of chemokines and binds to both receptors with high-affinity, whereas most other ELR-expressing CXC chemokines, such as GROα, GROβ, GROγ, NAP-2, and ENA-78, bind with high-affinity to CXCR2 only. Down-regulation of neutrophil surface CXCR2 is expected to decrease the response to these chemoattractants and arrest the migration of neutrophils that have arrived at the site of inflammation. The release of IL-8 from LL-37-treated AV+ neutrophils is consistent with this idea, that the IL-8 will recruit further neutrophils to the site of inflammation, where their further responses will depend on the local environment.
In conclusion, LL-37, the processed product of hCAP-18, induces secondary necrosis of AV+ neutrophils in vitro, thus suggesting a new role in the regulation of the lifespan and function of neutrophils.
Acknowledgments
This work was partially supported by National Institutes of Health grant CA84202. The authors thank Allen Mao for live-cell imaging, John Hardy for electron microscopy, and Bruce Kaplan for peptide synthesis.
References
- Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Rydell-Tormanen K, Uller L, Erjefalt J S. Neutrophil cannibalism—a back up when the macrophage clearance system is insufficient. Respir Res. 2006;7:143. doi: 10.1186/1465-9921-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medan D, Wang L, Yang X, Dokka S, Castranova V, Rojanasakul Y. Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. J Cell Physiol. 2002;191:320–326. doi: 10.1002/jcp.10105. [DOI] [PubMed] [Google Scholar]
- Do Vale A, Costa-Ramos C, Silva A, Silva D S, Gartner F, dos Santos N M, Silva M T. Systemic macrophage and neutrophil destruction by secondary necrosis induced by a bacterial exotoxin in a Gram-negative septicaemia. Cell Microbiol. 2007;9:988–1003. doi: 10.1111/j.1462-5822.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- Rydell-Tormanen K, Uller L, Erjefalt J S. Direct evidence of secondary necrosis of neutrophils during intense lung inflammation. Eur Respir J. 2006;28:268–274. doi: 10.1183/09031936.06.00126905. [DOI] [PubMed] [Google Scholar]
- Sorensen O, Arnljots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson G H, Gallo R L, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1681/01.asn.0000926856.92699.53. [DOI] [PubMed] [Google Scholar]
- Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Stahle-Backdahl M, Borregaard N, Egesten A. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun. 2000;68:4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O, Bratt T, Johnsen A H, Madsen M T, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J Biol Chem. 1999;274:22445–22451. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- Scott M G, Davidson D J, Gold M R, Bowdish D, Hancock R E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Yang D, Biragyn A, Hoover D M, Lubkowski J, Oppenheim J J. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra P S, Vogelmeier C, Gallo R L, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilborn J D, Nilsson M F, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson G H. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- De Yang , Chen Q, Schmidt A P, Anderson G M, Wang J M, Wooters J, Oppenheim J J, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Johansson J, Gudmundsson G H, Rottenberg M E, Berndt K D, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, Eklund A, Gudmundsson G H. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- Chang D H, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman R M, Dhodapkar M V. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–3052. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- Barlow P G, Li Y, Wilkinson T S, Bowdish D M, Lau Y E, Cosseau C, Haslett C, Simpson A J, Hancock R E, Davidson D J. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjabringa G S, Aarbiou J, Ninaber D K, Drijfhout J W, Sorensen O E, Borregaard N, Rabe K F, Hiemstra P S. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- Li X, Dancausse H, Grijalva I, Oliveira M, Levi A D. Labeling Schwann cells with CFSE—an in vitro and in vivo study. J Neurosci Methods. 2003;125:83–91. doi: 10.1016/s0165-0270(03)00044-x. [DOI] [PubMed] [Google Scholar]
- Khakh B S, North R A. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Schroder A K, von der Ohe M, Kolling U, Altstaedt J, Uciechowski P, Fleischer D, Dalhoff K, Ju X, Zenke M, Heussen N, Rink L. Polymorphonuclear leucocytes selectively produce anti-inflammatory interleukin-1 receptor antagonist and chemokines, but fail to produce pro-inflammatory mediators. Immunology. 2006;119:317–327. doi: 10.1111/j.1365-2567.2006.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y E, Bowdish D M, Cosseau C, Hancock R E, Davidson D J. Apoptosis of airway epithelial cells: human serum sensitive induction by the cathelicidin LL-37. Am J Respir Cell Mol Biol. 2006;34:399–409. doi: 10.1165/rcmb.2005-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian U H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Whyte M K, Meagher L C, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–5134. [PubMed] [Google Scholar]
- Arend W P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Ong P Y, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo R L, Leung D Y. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Putsep K, Carlsson G, Boman H G, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- Hase K, Eckmann L, Leopard J D, Varki N, Kagnoff M F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feniger-Barish R, Belkin D, Zaslaver A, Gal S, Dori M, Ran M, Ben-Baruch A. GCP-2-induced internalization of IL-8 receptors: hierarchical relationships between GCP-2 and other ELR(+)-CXC chemokines and mechanisms regulating CXCR2 internalization and recycling. Blood. 2000;95:1551–1559. [PubMed] [Google Scholar]