Abstract
CSF-1 is the major regulator of tissue macrophage development and function. A GM-CSF-dependent, CSF-1 receptor (CSF-1R)-deficient F4/80hiMac-1+Gr1–CD11c+ bone marrow macrophage (BMM) line (MacCsf1r−/−) was developed to study the roles of the eight intracellular CSF-1R tyrosines phosphorylated upon receptor activation. Retroviral expression of the wild-type CSF-1R rescued the CSF-1-induced survival, proliferation, differentiation, and morphological characteristics of primary BMM. Mutation of all eight tyrosines failed to rescue, whereas the individual Y → F mutants (544, 559, 697, 706, 721, 807, 921, 974) rescued these CSF-1-inducible phenotypes to varying degrees. The juxtamembrane domain Y559F and activation loop Y807F mutations severely compromised proliferation and differentiation, whereas Y706, Y721F, and Y974F mutations altered morphological responses, and Y706F increased differentiation. Despite their retention of significant in vitro tyrosine kinase activity, Y559F and Y807F mutants exhibited severely impaired in vivo receptor tyrosine phosphorylation, consistent with the existence of cellular mechanisms inhibiting CSF-1R tyrosine phosphorylation that are relieved by phosphorylation of these two sites. The MacCsf1r−/− macrophage line will facilitate genetic and proteomic approaches to CSF-1R structure/function studies in the major disease-related CSF-1R-expressing cell type.
Keywords: tyrosine kinase, tyrosine phosphorylation, hematopoietic growth factor, receptor tyrosine kinase mutations
INTRODUCTION
CSF-1 is the primary regulator of the development of tissue macrophages and osteoclasts and plays important roles in inflammatory disease and cancer (reviewed in refs. [1,2,3]). These cells express the CSF-1 receptor (CSF-1R), and mice deficient in CSF-1 or the CSF-1R are osteopetrotic as a result of a deficiency of osteoclasts and have decreased densities of macrophages in most tissues [4,5,6]. In addition, because of the scavenger and trophic roles of macrophages and osteoclasts, CSF-1/CSF-1R-deficient mice have several secondary, developmental abnormalities and are more resistant to certain chronic inflammatory disorders than wild-type (WT) mice [3]. CSF-1 regulates macrophage survival, proliferation, and differentiation [1]. All of the effects of CSF-1 are mediated by the CSF-1R, a member of the platelet-derived growth factor receptor family, which possesses an Ig-like extracellular domain, a transmembrane domain, an intracellular juxtamembrane domain, a split tyrosine kinase domain, and a C-terminal tail [6, 7]. Incubation of macrophages with CSF-1 leads to dimerization, tyrosine phosphorylation, and ubiquitination of the CSF-1R [8, 9]. As the exacerbation of chronic inflammatory disease and cancer by CSF-1 is mediated by its action on macrophages, study of CSF-1R signal transduction in macrophages is important for the identification of new therapeutic targets.
CSF-1R structure/function studies of the response to CSF-1 have been carried out by expressing mutant CSF-1R proteins in cells that do not endogenously express the receptor, such as fibroblasts or certain myeloid progenitor cell lines (reviewed in refs. [1, 10,11,12]), or by expressing chimeric receptors possessing a heterologous extracellular domain in CSF-1R-expressing myeloid cells [13]. These cells respond to CSF-1 by proliferating (fibroblasts) or by proliferating and differentiating (myeloid cells). In several cases, different outcomes for the same CSF-1R mutation have been observed in these systems, emphasizing the need to study CSF-1R structure/function in the appropriate cellular context [1, 10, 11]. In response to CSF-1, the mouse CSF-1R has been reported to be phosphorylated at six tyrosine residues, i.e., Y559, Y697, Y706, Y721, Y807 (reviewed in ref. [12]), and Y974 [14]. In addition, the activated, oncogenic form of the receptor encoded by v-fms has been reported to be phosphorylated at Y544 [15] and Y921 [16]. Phosphorylation of most of these tyrosines creates specific binding sites for known downstream signaling molecules that mediate a CSF-1 response, i.e., Y544, an unidentified, 55 kDa protein [15]; Y559, Src family kinases; Y697, growth factor receptor binding 2 (Grb2), monocytic adaptor (Mona), suppressor of cytokine sigaling 1 (Socs1); Y706, no reproducible binding; Y721, p85 PI-3K, phospholipase Cγ2, Socs1; Y807, activation loop, no reported binding protein [12]; Y921, Grb2 [16]; and Y974, Cbl [14]. These studies have demonstrated that specific phosphotyrosines in the activated CSF-1R can mediate particular downstream signaling pathways.
Here, we describe a novel bone marrow macrophage (BMM)-derived cell line system for the analysis of CSF-1R function, in which the receptor is expressed at normal levels and in which macrophages expressing the WT receptor exhibit the survival, proliferation, differentiation, and morphological responses of primary BMMs. We have used this line to investigate the role of the CSF-1R intracellular domain tyrosines in receptor tyrosine phosphorylation and activation and in CSF-1-regulated responses. We show that different CSF-1R intracellular domain tyrosines in the activated receptor differentially regulate CSF-1-stimulated macrophage survival, proliferation, differentiation, and morphological changes, as well as CSF-1R degradation. The results indicate that not only the juxtamembrane Y559, as shown previously [13, 17], but also the activation loop Y807 are important for CSF-1R tyrosine phosphorylation in vivo, that both mutations significantly inhibit CSF-1-induced proliferation and differentiation, and that mutations at other sites have significant effects on the morphological phenotypes of macrophages cultured in CSF-1.
MATERIALS AND METHODS
Reagents
The anti-mouse CSF-1R peptide (962GDIAQPLLQPNNYQF976) antiserum carboxyl terminal (CT) and the antiphospho-Y559 (α-pY559) CSF-1R peptide antibody (555EGNSpYTFIDPTQLPYNEK572) were raised in rabbits and affinity-purified against their corresponding peptides. α-pY100 was from Cell Signaling Technology (Beverly, MA, USA). Antibodies to mouse CD11c and Gr1 were from eBiosciences (San Diego, CA, USA) and BD PharMingen (San Diego, CA, USA), respectively. Rat mAb to F4/80 was a gift from Dr. David Hume (Roslin Institute, University of Edinburgh, UK). EZ-Link sulfo-NHS-LC-LC-biotin (Pierce, Rockford, IL, USA) was used to biotinylate the anti-CSF-1R mAb and the poly-Glu-Ala-Tyr (polyEAY; Sigma Chemical Co., St. Louis, MO, USA), according to the manufacturer’s instructions. Human recombinant CSF-1 was a gift from Chiron Corp. (Emeryville, CA, USA). Pure GM-CSF was a gift from Dr. Maureen Howard (DNAX, Palo Alto, CA, USA).
Serum-free GM-CSF-conditioned medium (GMCM)
MelrmGMCSF cells overexpressing GM-CSF (a gift from Dr. Nicos Nicola, Walter and Eliza Hall Institute, Parkville, Victoria, Australia) were maintained in α-MEM (Gibco, Gaithersburg, MD, USA) containing nucleotides, 2.2 g/l sodium bicarbonate, 0.1 g/l penicillin G, 0.1 g/l streptomycin sulfate, and 10% FCS (Gibco). For preparation of GMCM, cells were harvested by centrifugation, washed twice in low bicarbonate-containing α+-medium (α-MEM containing 0.22 g/l sodium bicarbonate, 1.39 g/l sodium chloride, 0.1 g/l penicillin G, 0.1 g/l streptomycin sulfate, and 5.95 g/l HEPES buffer, pH 7.2) without 10% FCS, and cultured in T75 flasks (Falcon, Sacramento, CA, USA, 107 cells per flask), each containing 30 ml α+-medium. Once cells commenced to die (as early as 4 days), the medium (GMCM) was collected, concentrated at least 30-fold by ultrafiltration through a 2000 Mr cut-off Amicon filter, sterile-filtered, titered for the concentration yielding maximum proliferation of macrophages (∼2% unconcentrated GMCM), aliquotted, and stored at –20°C.
Site-directed mutagenesis and retroviral constructs
The retroviral vector plasmid murine stem cell virus-internal ribosomal entry site-green fluorescent protein (pMSCV-IRES-GFP) [18] and the ecotropic, replication-defective helper virus plasmid SV40-psi-ecotropic-murine leukemia virus (pSV-Ψ-MLV) [19] cDNAs were gifts of Drs. A. W. Nienhuis (St. Jude Children’s Research Hospital, Memphis, TN, USA) and O. N. Witte (University of California Los Angeles, Los Angeles, CA, USA), respectively. The SV-U19-5 retrovirus containing a variant of the SV40 Large T antigen [20] was a gift from Dr. P. S. Jat (Ludwig Institute for Cancer Research, London, UK). A pGEM-2 plasmid containing an EcoRI fragment, including the complete c-fms c-DNA (nucleotides 1-36656, accession number NM_007779) and the pZen113xNc-FMS Y559F/Y697F/Y706F/Y721F/Y807F/Y974F plasmid, in which six tyrosines are mutated to phenylalanine, were gifts from Dr. L. R. Rohrschneider (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). Site-directed mutagenesis was performed using a kit according to the manufacturer’s instructions (Stratagene, La Jolla, CA, USA). All introduced mutations were confirmed by sequencing. The coding regions of mouse WT or mutant CSF-1R cDNAs were inserted into the MSCV-IRES-GFP vector at the EcoRI or EcoRI/XhoI site upstream of the IRES driving expression of GFP.
Derivation of the cloned MacCsf1r–/– (M–/–) and MacCsf1r+/+ (M+/+) cell lines
BMMs from Csf1r−/− and Csf1r+/+ outbred mice [6] were prepared as described [21] with the following modifications: Cells were cultured in BMM medium (α-MEM, supplemented with 3.4 μl/l β-ME, 0.29 g/l glutamine, and 0.2 g/l asparagine), containing 15% FCS, containing IL-3 and 2% GMCM, and the adherent cells were harvested between Days 5 and 8 of culture. Csf1r−/− BMM and Csf1r+/+ BMM were immortalized by infection with the SV-U19-5 retrovirus [22]. Briefly, the medium in 100 mm dishes of subconfluent BMM was replaced with diluted SV-U19-5 viral supernatant in BMM medium containing 8 μg/ml polybrene and 2% GMCM and incubated overnight. The cells were then washed once with PBS, the medium replaced with BMM medium containing GM-CSF, and the cells cultured until almost confluent and then split 1:5 in the same medium containing 250 μg/ml G418 with medium changes every 4 days for ∼10 days. Independently arising clones of transformed M−/− and M+/+ macrophages were purified by single cell plating and culture in 96-well microplates (Falcon) in α-MEM containing 10% newborn calf serum (Invitrogen, Chicago, IL, USA; macrophage medium), 2% GMCM, and 50 μg/ml G418.
Retroviral transfection of M−/− cells
For MSCV retroviral infection, human kidney 293T cells, cultured in 100 mm culture dishes with DMEM containing 10% FCS, were transfected with the pMSCV-IRES-GFP (12 μg) and pSV-ψ-E-MLV (12 μg) DNAs using calcium phosphate precipitation. The medium was changed to fresh 293T culture medium 24 h post-transfection. At 48 h post-transfection, the retroviral supernatant was harvested and filtered through a 0.45-μm filter. Subconfluent cultures of M−/− cells in 100 mm plates were incubated with the fresh retroviral supernatants in the presence of 2% GMCM and 4 μg/ml polybrene for 24 h prior to replacing the medium with fresh 2% GMCM medium and culturing the cells for a further 4–7 days. Cultured cells were harvested by cell scraping and subjected to FACS for GFP+ cells, using a FACSVantage SE cellsorter (BD Biosciences, San Jose, CA, USA). The GFP+ cells (25–50% of total) were expanded by further culture in 2% GMCM medium and subjected to Western blot analysis for CSF-1R expression. Generally, GFP expression correlated with CSF-1R expression. Cell surface expression of the CSF-1R was determined by FACScan (Becton Dickinson, La Jolla, CA, USA) analysis using the anti-CSF-1R AFS98 mAb [23] (gift of Dr. S. Nishikawa, Riken Center for Developmental Biology, Kyoto, Japan). If fewer than 90% of the expanded cells were GFP+ or if the level of cell surface CSF-1R expression were higher or lower than the level of expression in M+/+ cells, cell populations were resorted for GFP+ cells into high, medium, and low GFP expressers, and the subpopulations tested to choose lines with CSF-1R expression levels approximating those of M+/+ cells. Further selections were made on the basis of cell surface CSF-1R expression. Cells cultured in GM-CSF or CSF-1 for 3 months maintained stable CSF-1R expression, and cells thawed for experiments were passaged for no longer than 2 months.
Cell proliferation assays
The 4′,6′-diamidino-2-phenyl-indole dihydrochloride (DAPI) DNA staining method was modified from the QuantosTM cell proliferation assay kit (Stratagene). Cells previously cultured in GMCM medium were seeded at 5 × 103 cells per well in macrophage medium containing 36 ng/ml CSF-1 in a 48-well plate (Corning Costar, Boston, MA, USA) with at least three wells per point. The medium was changed at Days 3 and 5 and subsequently daily. For each day of the growth curve, one plate was cooled to –80°C for 15 min, the contents thawed at room temperature, and 200 μl 1 μg/μl DAPI (Invitrogen) in staining buffer [100 mM Tris, pH 7.4, 150 mM CaCl2, 0.5 mM MgCl2, 0.1% Nonidet-P-40 (NP-40)] was added to each well prior to incubation of the plate (1 h, 20°C) and reading of the fluorescence in wells using a microplate reading fluorometer (FLUOstar Optima, BMG Labtechnologies, Offenburg, Germany) with filters appropriate for 355 nm excitation and 460 nm emission. Data are expressed as the average fluorescence after subtraction of the average blank (no cell) value.
Cell survival assays
Cells were cultured in 2% GMCM, harvested by scraping, washed twice in macrophage medium, and plated in six-well plates [1.25×105 viable (trypan blue-excluding) cells in 2.5 ml medium containing 600 pg/ml CSF-1 per well]. CSF-1 (4 μl, 1500 pg/well) was added daily to maintain the survival-inducing concentration [24]. At Days 1–4, cells were scraped, collected, and washed twice with PBS containing 1% BSA (Sigma Chemical Co.), the cell pellet was suspended in 500 μl PBS containing 1% BSA and mixed, and the cell pellets from 200 μl aliquots were separately incubated (20 min, room temperature) with 500 μl propidium iodide (PI) staining fluid (PBS containing 1% BSA, 0.2 mg/ml RNase A) or PI staining fluid containing 0.1% Triton X-100 for analysis of viable cells (PI exclusion) and total cell numbers, respectively, by fluorescence activated cell scanning (FACScan).
Macrophage differentiation assay
For cell-surface Mac-1 expression assays, cells were cultured in 2% GMCM to semi-confluence, prior to incubation in 36 ng/ml CSF-1 or 2% GMCM for a further 3 days. The cells were harvested, washed once with ice-cold PBS, and incubated with PE-conjugated Mac-1 antibody (BD PharMingen) or control unrelated antibody in assay buffer (1% BSA in PBS) at 4°C for 20 min. Cells were washed twice with assay buffer and subjected to FACScan to monitor Mac-1 expression. The relative expression level of Mac-1 was determined as the difference between the geometric means of fluorescence densities for Mac-1 and the control mAb and expressed as a percentage of the difference for cells expressing the WT CSF-1R.
Cell morphology
For morphological assessment, individual cell lines were cultured in 36 ng/ml CSF-1 for at least 1 week prior to replating on fibronectin-coated coverslips. Cells were grown to 60–70% confluence in the continuous presence of CSF-1 over 2 days and then fixed in 3.7% formaldehyde for 10 min. After rinsing in TBS, the cells were photographed on an Olympus CK 2 inverted microscope.
CSF-1 stimulation, immunoprecipitation, and Western blotting
Single Y → F mutant CSF-1R-expressing cells were cultured in CSF-1 and M−/−.CSF-1R Y eight F (M−/−.YEF) cells in GMCM. Subconfluent (∼60%) 100 mm dish cultures of cells were starved of growth factor for 16 h to up-regulate CSF-1R expression and then incubated with CSF-1 (360 ng/ml) at 37°C or 4°C. Following incubation, the cells were rinsed in ice-cold PBS, scraped into 200 μl lysis buffer (1% NP-40, 10 mM Tris–HCl, 50 mM NaCl, 30 mM Na4P2O7, 50 mM NaF, 100 μM Na3VO4, 5 μM ZnCl2, 1 mM benzamidine, 10 μg/ml leupeptin, and 10 μg/ml aprotinin, pH 7.2) at 4°C, vortexed, and centrifuged at 13,000 g for 30 min. The NP-40 lysates were incubated with 5 μg of a 1:1 mixture of two affinity-purified goat anti-mouse CSF-1R cytoplasmic domain peptide antibodies [25] and 40 μl 50% suspension of protein A-Sepharose 4B beads (Zymed, San Francisco, CA, USA) overnight at 4°C and then centrifuged at 13,000 g at 4°C for 30 s. The supernatant was removed, and the beads were washed five times with wash buffer (lysis buffer containing 0.5% NP-40 without leupeptin and aprotinin) at 4°C and once in double-distilled water, and the proteins were eluted with 10 μl 3× SDS sample buffer at 65°C for 10 min. Protein determinations, gradient (7.5–17.5% acrylamide) SDS-PAGE, and Western blots were performed as described previously [26]. For the detection of CSF-1R expression by Western blotting, cells were solubilized in SDS sample buffer as described previously, and 50 μg each SDS cell lysate was separated by 7% SDS-PAGE [27]. Equal protein loading was confirmed by blotting with anti-actin antibody (Abcam, Cambridge, UK). Blotted membranes were incubated with HRP substrate (Milipore Corp., Billerica, MA, USA), and the chemiluminescent signals were recorded by ImageReader LAS-3000 (Fuji Film, Tokyo, Japan) and analyzed with the software ImageGauge from Fuji Film.
In vitro receptor autophosphorylation and kinase assays
Cells were cultured in the absence of CSF-1 for 16 h, lysed in radioimmunoprecipitation assay (RIPA) buffer (lysis buffer, in which NP-40 is replaced with 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS), the lysate protein concentration adjusted to 2.0 mg/ml and 250 μg, and 500 μg of each lysate in duplicate subjected to immunoprecipitation with 5 μg anti-mouse CSF-1R antipeptide antibody (CT) or IgG and 20 μl packed protein A-Sepharose beads (4 h, 4°C). The immunoprecipitates were washed four times with RIPA buffer and then once with kinase assay buffer (100 mM HEPES, 3 mM MnCl2, 20 mM MgCl2, 50 μM Na3VO4, pH 7.0) prior to addition of 20 μl assay mixture (kinase buffer containing 10 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mM benzamindine, 0.5% BSA, 0.1% NP-40, 5 mM ATP, 0.2 mg/ml biotinylated polyEAY) to the beads and were incubated at room temperature for 15 min. Following incubation, the beads were centrifuged (4°C, 13,000 rpm, 30 s), and 10 μl supernatant was placed in 1.5 ml microfuge tubes containing 50 μl TBS, containing 0.05% NP-40 (TBSN) for antiphosphotyrosine (α-pTyr) ELISA. For determination of CSF-1R protein and tyrosine autophosphorylation, the beads were washed twice with RIPA buffer and then processed and subjected to 7.5–17.5% gradient SDS-PAGE as described for CSF-1R immunoprecipitates. For the α-pTyr ELISA, Avidin (Sigma Chemical Co.)-coated, 96-well plates were first blocked with 5% BSA (1 h, room temperature) and washed five times with TBSN prior to addition of the diluted supernatant (50 μl) and incubation (1 h, room temperature) with continuous shaking. Following incubation, each well was washed five times with TBSN and then incubated (with shaking) sequentially in 50 μl 10 μg/ml α-pTyr antibody (PY-20, Upstate Biotechnology, Lake Placid, NY, USA; 1 h, room temperature) and 50 μl one in 3000 dilution of anti-mouse IgG-HRP (GE Healthcare Ltd., UK; 30 min, room temperature), both diluted in TBSN containing 5% BSA and washing five times with TBSN between incubations. Finally, wells were incubated with 50 μl HRP substrate solution (3,3′,5,5′-tetramethyl-benzidine, Sigma Chemical Co.) for 5 min at room temperature, the reaction was stopped by addition of 10 μl 3 M H2SO4, and OD was read at 450 nm in a 96-well plate reader. Different assays always contained a M−/−.CSF-1R WT (M−/−.WT) lysate control, and results of different assays were normalized by comparison with the activity of stable aliquots of the BAC1.2F5 macrophage [28] lysate stored at –80°C.
RESULTS
Characteristics of M+/+ and M–/– macrophage cell lines
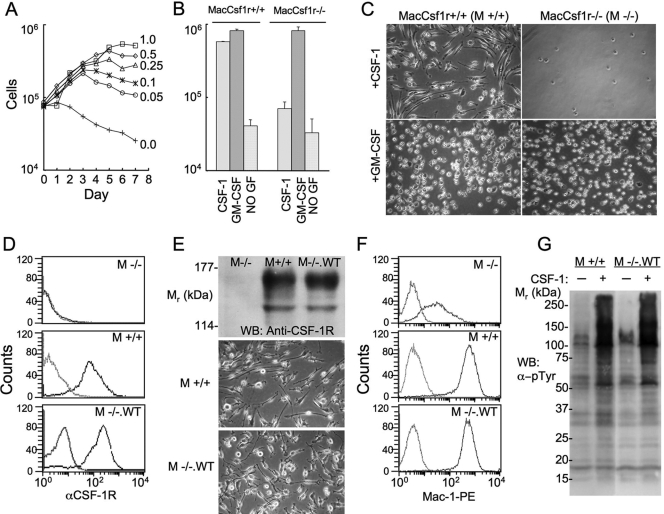
CSF-1R-deficient M−/− and CSF-1R-expressing M+/+ cell lines were prepared from Csf1r−/− and Csf1r+/+ BMM as described in Materials and Methods. M−/− and M+/+ cells exhibited a normal, proliferative response to GM-CSF and died in the absence of growth factor (Fig. 1, A and B). In contrast to M+/+ cells, which exhibited a normal macrophage proliferative response to CSF-1, M−/− cells died in CSF-1 (Fig. 1B). The morphology of M+/+ and M−/− cells cultured in GM-CSF was similar, and they were much rounder and less well-spread than the elongated M+/+ cells grown in the presence of CSF-1 (Fig. 1C). Retroviral expression of the WT CSF-1R in M−/− cells at levels normally found in BMM and in M+/+ cells (Fig. 1, D and E) conferred a proliferative response to CSF-1 and an elongated, mature, cellular morphology in the presence of CSF-1 that mimicked the behavior of M+/+ cells (Fig. 1E) and BMM (data not shown). These M−/−.WT cells grown in GM-CSF, when transferred to CSF-1 for 3 days, exhibited a dramatic increase in the expression of the Mac-1 macrophage differentiation marker, up to the level of its expression in M+/+ macrophages (Fig. 1F). In addition, M−/−.WT cells exhibited a CSF-1R tyrosine phosphorylation response that was indistinguishable from the response of M+/+ cells (Fig. 1G). These results demonstrate that M−/− cells are appropriate for structure-function studies of the CSF-1R in the regulation of macrophage survival, proliferation, and differentiation.
Fig. 1.
The M−/− cell line and rescue of CSF-1-regulated responses by retroviral transduction of the CSF-1R. (A) Growth of M−/− cells in different concentrations of GM-CSF. (B) Growth of M−/− (MacCsf1r−/−) and M+/+ (MacCsf1r+/+) macrophages for 7 days with the indicated growth factors (GF; starting cell number as in A). (C) Phase contrast photomicrographs of M+/+ and M−/− macrophages cultured in GM-CSF and CSF-1. (D) FACScan analysis of the cell surface CSF-1R on growth factor-starved M−/−, M+/+, and M−/−.WT macrophages. (E) CSF-1R expression by Western blot (WB) and phase contrast morphology of M−/−, M+/+, and M−/−.WT macrophages. (F) FACScan analysis of the expression of the macrophage marker Mac-1 following a 3-day incubation with GM-CSF (top panel) or CSF-1 (middle and bottom panels). (G) α-pTyr Western blot of lysates of growth factor-starved M+/+ and M−/−.WT macrophages incubated with (+) or without (–) CSF-1 for 1 min at 37°C.
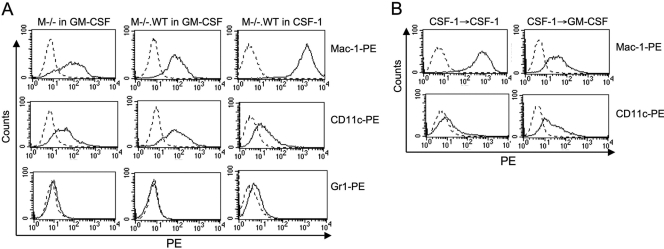
As the culture of bone marrow cells with GM-CSF has been described as a method to generate dendritic cells (DC) [29, 30], we carried out a phenotypic analysis of the M−/− cells cultured in GMCM and of M−/−.WT cells cultured in GMCM or CSF-1. Irrespective of whether they were cultured in GMCM or CSF-1, both cell types failed to express CD4 or CD8 but expressed high levels of the pan-macrophage marker F4/80, which is a marker of blood monocytes and tissue macrophages [31,32,33,34] (data not shown). M−/− or M−/−.WT cells cultured in GMCM expressed equivalent levels of the macrophage integrin marker Mac-1, and consistent with the results in Figure 1F, expression was elevated ∼18-fold on M−/−.WT cells cultured in CSF-1 (Fig. 2A). Expression of the granulocyte marker Gr1 could not be detected on M−/− or M−/−.WT cells cultured in GMCM, but low levels of expression were observed on M−/−.WT cells cultured with CSF-1. These observations are consistent with a monocyte/macrophage character (F4/80hiMac-1+Gr1–) of M−/− or M−/−.WT cells cultured in GMCM and a significant increase in the differentiated macrophage phenotype (F4/80hiMac-1hiGr1lo) when M−/−.WT cells were cultured in CSF-1. Interestingly, M−/− or M−/−.WT cells cultured in GMCM were positive for the DC marker CD11c, but CD11c expression decreased significantly when M−/−.WT cells were cultured in CSF-1. To determine whether the increase in the differentiated macrophage phenotype in M−/−.WT macrophages upon culture with CSF-1 was reversible, we incubated M−/−.WT cells with CSF-1 for 1 week, removed the CSF-1, and incubated them with GMCM or again with CSF-1 for another week, prior to examining their expression of Mac-1, F4/80, and CD11c (Fig. 2B). Cells cultured with CSF-1 for 2 weeks (Fig. 2B) had the same mature macrophage phenotype as cells cultured for 1 week (Fig. 2A). However, cells cultured for 1 week with CSF-1 (Mac-1hiGr1loCD11clo phenotype), when transferred to GMCM for 1 week, reverted to the Mac-1+Gr1–CD11c+ phenotype of M−/−.WT cells cultured in GMCM. These results demonstrate that M−/− and M−/−.WT cells grown in GM-CSF exhibit a monocyte/macrophage phenotype with some DC character and that culture with CSF-1 leads to loss of DC marker expression and the development of a highly differentiated macrophage phenotype. However, growth factor-induced differentiation with GM-CSF or CSF-1 is reversible. Transfer of the cells from GM-CSF to CSF-1 confers the differentiated macrophage phenotype, whereas their transfer from CSF-1 to GM-CSF confers the monocyte/macrophage/DC character phenotype.
Fig. 2.
Increased expression of Mac-1 and decreased expression of CD11c upon culture of M−/−.WT cells with CSF-1 are reversible upon culture with GM-CSF. (A) M−/− cells were cultured with GMCM and M−/−.WT cells with GMCM or CSF-1 for 1 week prior to FACScan analysis of their expression of Mac-1, Gr1, and CD11c. (B) M−/−.WT cells were cultured for 1 week with CSF-1, the medium changed to fresh CSF-1 or GMCM, and the cells cultured for an additional week, prior to analysis of their expression of Mac-1 and CD11c.
Role of individual tyrosines in CSF-1R-regulated macrophage proliferation
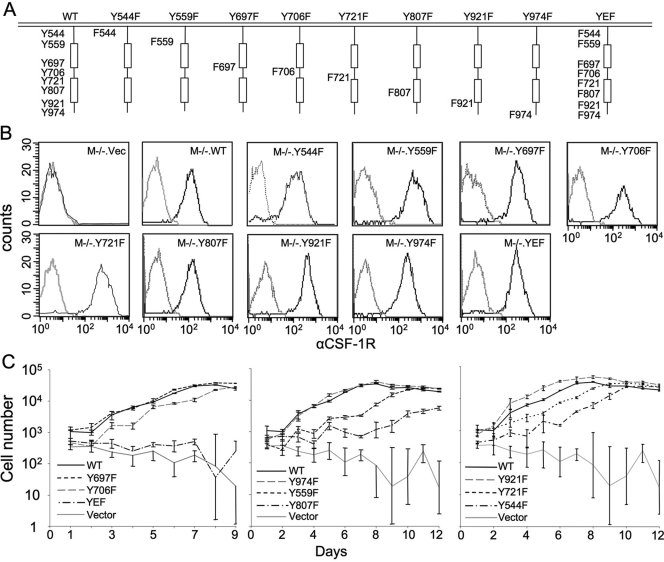
In studies of the activated CSF-1R, eight intracellular domain tyrosines have been reported to be phosphorylated [12, 14,15,16]. To examine the roles of individual CSF-1R tyrosines in CSF-1-regulated macrophage survival, proliferation, and differentiation, we created eight M−/− cell lines expressing CSF-1Rs bearing unique Y → F mutations and one M−/−.YEF, in which all eight tyrosines were mutated to phenylalanine (Fig. 3A). Following up-regulation after culture in GMCM, each of these cell lines expressed approximately WT levels of the CSF-1R on SDS-PAGE and Western blotting of whole cell lysates (data not shown) and WT levels of cell surface CSF-1R (Fig. 3B). M−/−.YEF and M−/−.vec cells failed to proliferate in CSF-1 (Fig. 3C and Table 1). Among the single tyrosine mutants, the most severe effects on proliferation rate were observed with the activation loop mutation Y807F and the juxtamembrane domain mutations Y559F and to a lesser extent, Y544F (Fig. 3C and Table 1). The doubling time for M−/−.WT cells was 26.4 h, whereas doubling times of the Y807F and Y559F mutations were increased significantly to 78.2 h and 45.2 h, respectively (Table 1). The doubling times of M−/−.Y706F, M−/−.Y721F, M−/−.Y697F, M−/−.Y921F, and M−/−.Y974F lines were not significantly different from those of M−/−.WT cells (Table 1).
Fig. 3.
Cell surface expression of the CSF-1R and CSF-1-stimulated cell proliferation of M−/− cells expressing CSF-1R Y → F mutations of the known tyrosine phosphorylation sites. (A) The Y → F mutant CSF-1Rs. (B) FACScan analysis of the cell surface CSF-1R on growth factor-starved cells. (C) Proliferation of the M−/−.CSF1R Y → F mutant and control [M−/−.WT and M−/−.vector (M−/−.vec.)] cells.
TABLE 1.
Summary of Proliferation and Differentiation Characteristics of M–/– Macrophages Expressing Y → F Mutant CSF-1Rs
| Cell line | Doubling time (h) | % M–/–.WT expression of Mac-1 |
|---|---|---|
| M–/–.WT | 26.4 ± 1.9 | 100 ± 15 |
| M–/–.vec | NA | 12 ± 4a,b |
| M–/–.YEF | NA | 23 ± 7a,b |
| M–/–.Y544F | 35.1 ± 1.7a | 67 ± 7a |
| M–/–.Y559F | 45.2 ± 2.0a | 31 ± 3a |
| M–/–.Y697F | 28.6 ± 1.4 | 66 ± 14 |
| M–/–.Y706F | 29.8 ± 2.6 | 154 ± 7a |
| M–/–.Y721F | 29.4 ± 1.8 | 67 ± 9 |
| M–/–.Y807F | 78.2 ± 3.3a | 23 ± 13a |
| M–/–.Y921F | 24.0 ± 1.9 | 51 ± 10a |
| M–/–.Y974F | 27.0 ± 2.1 | 67 ± 12 |
Results of multiple growth curves and differentiation assays (n=3) for each cell line.
Significantly different from M–/–.WT macrophages (P<0.05; Student’s t-test).
Results for residual, viable cells. NA, not applicable, cells fail to survive in CSF-1.
Role of individual tyrosines in CSF-1R-regulated macrophage survival
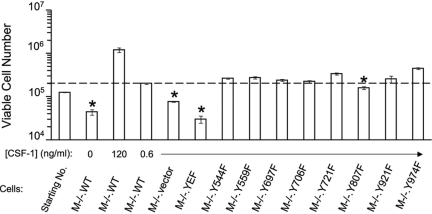
As changes in cell number result from a balance between cell survival and cell death, we determined the effect of the Y → F mutations on survival by incubating cells of each line for 4 days with daily medium changes in 600 pg/ml CSF-1, a concentration that induces survival of WT macrophages without significant proliferation [24] (Fig. 4). Compared with M−/−.WT cells cultured in 600 pg/ml CSF-1, the number of M−/−.YEF and M−/−.vec cells decreased in number to levels approximating those of M−/−.WT cells cultured without CSF-1. With the exception of the slightly compromised survival of M−/−.Y807F cells, each of the single Y → F mutant, CSF-1R-expressing cells survived to the same degree as cells expressing the WT CSF-1R.
Fig. 4.
Viability of M−/−.CSF-1R Y → F macrophages. Cells of the indicated lines were seeded at the indicated viable cell concentration. Following incubation with CSF-1 at the indicated concentrations for 4 days, the viable cell number was determined by PI exclusion. *, Significantly decreased compared with M−/−.WT cells cultured in 0.6 ng/ml CSF-1; P < 0.05, Student’s t-test (means±sd, n=3).
Role of individual tyrosines in CSF-1R-regulated macrophage morphology
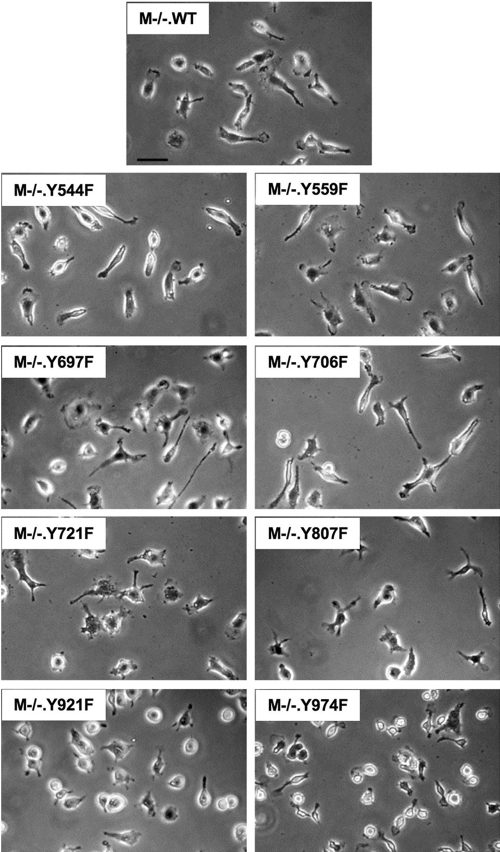
Compared with their morphology when cultured in GM-CSF, BMM became significantly more elongated and adherent when grown in the presence of CSF-1, a phenotype preserved in M+/+ and M−/−.WT macrophages (Fig. 1). Studies about M−/−.WT macrophages revealed that exposure to 36 ng/ml CSF-1 was required for 7 days for the cells to adopt the elongated, well-spread morphology, typical of macrophages grown continuously in CSF-1. To examine the morphology of macrophages in M−/− cell lines expressing CSF-1Rs bearing unique Y → F mutations, the cells were cultured in GMCM, the medium was replaced with CSF-1 medium, and the cells were cultured for 1 week further in the presence of CSF-1. Thus, these studies could not be carried out with M−/−.YEF macrophages, which did not survive in CSF-1 (Figs. 3 and 4). Morphological examination of the macrophage lines expressing different Y → F point mutations in the CSF-1R indicated that phosphorylation of individual tyrosines controls important aspects of macrophage morphology (Fig. 5). In particular, M−/−.Y721F and M−/−.Y974F cells exhibited a loss of elongated morphology, and M−/−.Y706F cells showed increased elongation [e.g., elongation ratios (maximum length/maximum width) for WT, 2.18±0.11; Y706F, 3.38±0.825; and Y721F, 1.59±0.05 (±sem; P<0.001)]. In addition, although M−/−.Y721F cells were well-spread, M−/−.Y974F cells spread poorly (Fig. 5, and data not shown). Although subtle morphological alterations cannot be ruled out by phase contrast studies, the phenylalanine mutations in the remaining tyrosines did not appear to cause major effects on macrophage morphology. These results demonstrate that specific Y → F CSF-1R mutations affect CSF-1-stimulated macrophage spreading and/or elongated morphology.
Fig. 5.
Morphology of M−/−.CSF-1R Y → F macrophages. Cells of the indicated lines were initially cultured in GMCM. The medium was replaced with CSF-1-containing medium, and the cells were cultured for 1 week prior to replating on fibronectin-coated coverslips for 2 days. Cells were fixed in 3.7% formaldehyde before phase-contrast photography. Original Bar = 20 μm.
Role of individual tyrosines in CSF-1R-regulated macrophage differentiation
The Y → F CSF-1R mutant cell lines were also examined for their capacity to express the macrophage differentiation marker, Mac-1, following a 3-day incubation with CSF-1, as shown for M−/−.WT cells in Figure 1F. M−/−.vec and M−/−.YEF cells cultured with CSF-1, respectively, exhibited only 12% and 23% of the Mac-1 expression of M−/−.WT cells. Among the other Y → F mutants, the Y559F and Y807F mutations lowered CSF-1-regulated Mac-1 expression substantially (31% and 23% of WT, respectively). Other mutations with major effects were Y921F (51%) and Y706F, which resulted in a substantial increase in CSF-1-induced expression of Mac-1 (154%) that was correlated with its elongated morphology (Table 1). The Y544F, Y697F, Y721F, and Y974F mutations only slightly suppressed the differentiation response (∼67% of WT). These results indicate that specific Y → F CSF-1R mutations can positively and negatively affect CSF-1 maintenance of the macrophage-differentiated phenotype and indicate important roles of Y559 and Y807 in this regulation. Overall, the broad variation of the morphologies and Mac-1 expression levels among the cells expressing individual CSF-1R Y → F point mutations indicates that these individual tyrosines control important aspects of morphology and differentiation of macrophages.
In vitro tyrosine phosphorylation and kinase activity of mutant CSF-1R immunoprecipitates
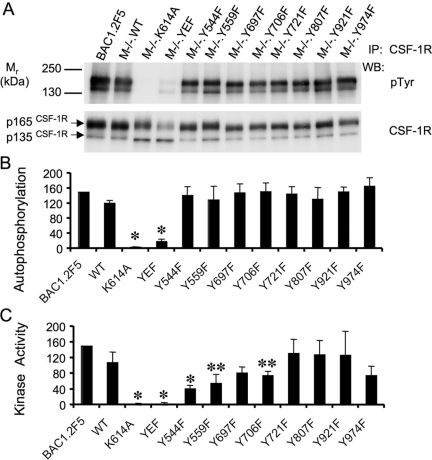
Phosphorylation of specific CSF-1R tyrosine residues could regulate receptor kinase activity and thereby regulate receptor autophosphorylation and the tyrosine phosphorylation of CSF-1R substrates. In vitro, the purified CSF-1R exhibits autophosphorylation and kinase activity on exogenous substrates in the absence of CSF-1 with slight enhancement in the presence of CSF-1 [35]. As similar results were obtained with immunoprecipitates of the CSF-1R from M−/−.WT cells (data not shown), to determine the intrinsic kinase activities of the mutant receptors, we examined the in vitro activities of CSF-1R immunoprecipitates in the absence of CSF-1. CSF-1R immunoprecipates from unstimulated cells were incubated with ATP in the presence of the exogenous substrate polyEAY under standard conditions in which the degree of tyrosine phosphorylation of substrate was linearly related to the amount of CSF-1R immunoprecipitate added (Supplementary Fig. 1). In addition, immediately following removal of the supernatant for determination of phosphorylated polyEAY, the CSF-1R immunoprecipitates were recovered and subjected to SDS-PAGE for analysis of CSF-1R autophosphorylation. The control kinase-dead CSF-1R, K614A, failed to exhibit tyrosine phosphorylation (Fig. 6, A and B) or kinase activity (Fig. 6C), reflecting the absence of a significant contaminating tyrosine kinase in the immunoprecipitates and validating the CSF-1R phosphorylation observed in this assay as autophosphorylation. As expected, autophosphorylation of CSF-1R.YEF was barely detectable. Furthermore, this receptor was devoid of detectable kinase activity (Fig. 6C), suggesting that CSF-1R tyrosine phosphorylation is required for activation of CSF-1R kinase activity. Examination of the individual Y → F receptor mutants revealed that their overall CSF-1R autophosphoryation did not significantly differ from levels determined for the WT receptor (Fig. 6B), indicating that none of the tyrosines was detectably, dominantly tyrosine-phosphorylated or required for autophosphorylation in vitro. In contrast, analysis of the kinase activity of the individual Y → F mutant receptors revealed that mutations of the juxtamembrane tyrosines (Y544 and Y559) and Y706F (to a lesser degree) reduced CSF-1R kinase activity (Fig. 6C). As the Y544F mutation was shown to compromise Y559 phosphorylation (data not shown), this discrepancy between the autophosphorylation and kinase activity data suggested that the tyrosine phosphorylation of the Y559F receptor might be kinetically limited. Indeed, examination of the kinetics of tyrosine phosphorylation of the Y559F and WT receptors indicated that the initial rate of tyrosine phosphorylation of Y559F was approximately one-half that of the WT receptor (Supplementary Fig. 2). Taken together, these in vitro studies indicate that the CSF-1R has abundant tyrosine kinase activity in vitro in the absence of ligand and that CSF-1R tyrosine phosphorylation appears to be important in activation of the kinase. In addition, although the juxtamembrane domain Ys are required for full WT kinase activity, Y559F still retains ∼50% of the WT kinase activity for polyEAY and autophosphorylation.
Fig. 6.
In vitro autophosphorylation and tyrosine kinase activity of CSF-1R Y → F mutant receptors. (A) Analysis of autophosphorylation of the CSF-1R in receptor immunoprecipitates (IP) from kinase assays by Western blotting with α-pTyr and α-CSF-1R antibodies. (B) Autophosphorylation of WT and mutant CSF-1Rs, normalized with respect to CSF-1R protein and the specific CSF-1R autophosphorylation in immunoprecipitates from BAC1.2F5 macrophages. *, Significantly different from WT (P<0.05, Student’s t-test, n=3–5). (C) Kinase activity of WT and mutant CSF-1Rs for a polyEAY substrate normalized with respect to CSF-1R protein and the specific activity of CSF-1R immunoprecipates from BAC1.2F5 cells. *, **, Significantly different from WT (*, P<0.001; **, P<0.05, Student’s t-test, n=3–5). Procedures are described in detail in Materials and Methods.
CSF-1-induced CSF-1R tyrosine phosphorylation in vivo in M−/− cells expressing the mutant CSF-1Rs
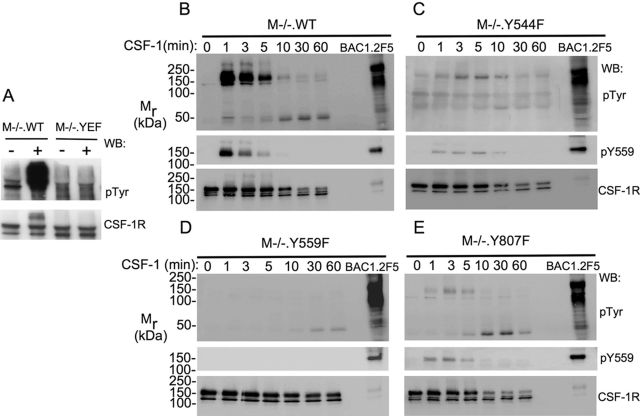
The foregoing in vitro studies of the kinase activity and autophosphorylation of mutant CSF-1Rs provided important information about their capacity to be activated. We next determined how the receptors were tyrosine-phosphorylated in the context of the cell, using optimal conditions for CSF-1 stimulation of CSF-1R tyrosine phosphorylation. Consistent with the in vitro results, receptor tyrosine phosphorylation in M−/−.YEF macrophages in response to CSF-1 could not be detected in vivo, compared with the strong CSF-1R tyrosine phosphorylation in M−/−.WT cells (Fig. 7A). To determine which sites were important for CSF-1R tyrosine phosphorylation, M−/− cells expressing individual Y → F mutant receptors were stimulated with CSF-1 for 2 min at 37°C, and the relative CSF-1R tyrosine phosphorylation per unit CSF-1R protein was determined as a percentage of the relative CSF-1R tyrosine phosphorylation for WT cells. The results indicated that the phosphorylation of Y706F, Y921F, and Y974F mutant receptors was indistinguishable from WT and that as expected, the phosphorylation of mutants of the major phosphorylation sites (Y697F and Y721F) was reduced by ∼50%. However, tyrosine phosphorylation of the juxtamembrane domain Y544F and Y559F mutations and the activation loop Y807F mutation was severely compromised (data not shown). CSF-1 stimulation time courses for M−/− cells expressing these latter mutations revealed that maximum tyrosine phosphorylation of Y544F and Y807F receptors was reduced to ∼45% and ∼20% of WT, respectively, and prolonged and tyrosine phosphorylation of Y559F was barely detectable (Fig. 7B–E and data not shown). Consistent with other reports [13, 17], the rate of Y559F receptor degradation was also decreased, whereas the rate of Y544F and Y807F degradation was indistinguishable from the WT rate. Of interest in this respect, Y559 phosphorylation was observed in the Y554F and Y807F mutant receptors (Fig. 7, C and E). Ignoring the results for Y544F, the phosphorylation of which has only been reported in the oncogenic form of the CSF-1R [15], these in vitro and in vivo studies suggest that there are mechanisms within the macrophage that negatively regulate CSF-1R phosphorylation in the absence of ligand and that CSF-1R phosphorylation of Y559 and Y807 plays important roles in overcoming these inhibitory mechanisms, as well as in activating the receptor and in permitting its full tyrosine phosphorylation. In addition, they indicate that phosphorylation at Y559 is necessary for CSF-1-induced CSF-1R degradation at WT rates in macrophages.
Fig. 7.
Dramatically reduced tyrosine phosphorylation of the CSF-1R in CSF-1R immunoprecipitates of M−/−.YEF, M−/−.Y544F, M−/−.Y559F, and M−/−.Y807F macrophages following stimulation with CSF-1 in vivo. (A) Lack of detectable in vivo tyrosine phosphorylation of the CSF-1R in CSF-1R immunoprecipates from M−/−.YEF macrophages stimulated with CSF-1 for 2 h at 4°C. (B–E) Kinetics of CSF-1-stimulated CSF-1R and Y559 tyrosine phosphorylation in M−/−.WT (B), M−/−.Y544F (C), M−/−.Y559F (D), and M−/−.Y807F (E) macrophages at 37°C. A stable cell lysate of CSF-1-stimulated BAC1.2F5 macrophages was used to normalize the exposure times. Note that despite the relatively higher exposure of the pTyr Western blot in D, tyrosine phosphorylation of the CSF-1R in M−/−.Y559F macrophages is not apparent, except for a small amount of the ∼50-kDa species of degraded CSF-1R, which appears with slower kinetics than in M−/−.WT cells. Y559 tyrosine phosphorylation (middle panels) was detected in M−/−.Y544F and M−/−.Y807F macrophages. Note: repeat experiments (B–E) yielded higher CSF-1R tyrosine phosphorylation for M−/−.Y544F and M−/−.Y807F than shown here (see text).
DISCUSSION
We have described a novel, CSF-1R-deficient macrophage line that can be used to examine the structure-function relationships of the CSF-1R in the context of the macrophage, the predominant CSF-1-regulated cell type. The M−/− macrophage cell line allows analysis of CSF-1R-regulated pathways controlling survival, proliferation, differentiation, morphology, motility, and function. Our macrophage proliferation results, which demonstrate that Y559 and Y807 are important for proliferation, generally agree with those of Takeshita et al. [13], who examined the same responses to erythropoietin in erythropoietin receptor-CSF-1R chimera-expressing primary macrophages. This agreement with their studies of primary macrophages validates the use of the M−/− macrophage line, compared with other cell line systems involving CSF-1R-transfected fibroblasts or myeloid cells (reviewed in refs. [10, 11]). The advantage of the M−/− system over the system of Takeshita et al. [13] is that it allows structure-function analysis of the full-length receptor in the correct cellular context and provides sufficient cell numbers for the use of proteomic approaches.
Our marker studies have shown that M−/−.WT macrophages grown in GM-CSF adopt a monocyte/macrophage phenotype with some DC character, whereas culture in CSF-1, within 3 days (Fig. 1F), confers the differentiated macrophage phenotype. Both phenotypes are reversible. For the experiments on CSF-1-stimulated proliferation (Fig. 3 and Table 1), survival (Fig. 4), and differentiation (Table 1), the GM-CSF medium was changed to CSF-1 medium. In the case of these longer-term assays, the cells rapidly adopt the differentiated macrophage phenotype. For the cell morphology experiments, cells were cultured for at least 1 week after changing the medium from GM-CSF to CSF-1. Most importantly, for the short-term CSF-1 stimulation experiments (data not shown and Fig. 7), cells from all of the single Y → F mutant lines were cultured in CSF-1 (despite the slow growth rate of some) and then starved of CSF-1 prior to CSF-1 stimulation. Thus, the vast majority of the experiments we describe has been carried out in the context of the differentiated macrophage.
A survey of individual mutations of tyrosines known to be phosphorylated in the activated CSF-1R indicates that individual mutations differentially contribute to macrophage responses to CSF-1. However, two tyrosine phosphorylation sites, the juxtamembrane Y559 and activation loop Y807, are important for macrophage proliferation and differentiation, as Y → F mutations at these sites reduced these responses considerably. Compared with the other Y → F mutants, other than Y544F (which also reduced Y559 phosphorylation), mutation of either of these sites severely reduced CSF-1-stimulated CSF-1R phosphorylation in vivo. These findings, in agreement with the work of others [10, 12, 13, 17], indicate that Y559 and Y807 play a central role in major responses to CSF-1, consistent with their possible role in CSF-1R activation.
As well as signaling to macrophage survival, proliferation, and differentiation, the CSF-1R regulates many aspects of macrophage morphology, acutely and over the longer term [1]. Macrophages grown in CSF-1 reduce their adhesion structure numbers and round up upon overnight removal of CSF-1. When subsequently stimulated with CSF-1, the cells ruffle and spread within minutes of growth factor addition and show peak focal complex formation by 15–20 min [36, 37]. In addition to these rapid changes seen upon CSF-1 stimulation, there are much slower changes effected by CSF-1 when macrophages grown previously in GM-CSF are exposed to CSF-1 for the first time. Following the change of growth factor from GM-CSF to CSF-1, it takes 1 week for the rounded up, poorly spread cells to fully adopt the elongated, well-spread morphology, typical of macrophages grown in CSF-1. It is likely that this is a result of the increase in expression of markers of macrophage differentiation, such as the integrin Mac-1, which plays important roles in macrophage adhesion and motility. As expected, M−/−.WT cells express normal levels of Mac-1 and adopt an elongated morphology within 1 week in CSF-1. Phase contrast morphological screening of cells expressing each of the individual Y → F mutations demonstrated clear shape differences in at least three cell lines after prolonged exposure to CSF-1. M−/−.Y706F cells appeared more elongated, cells expressing the Y721F mutant were less elongated than M−/−.WT cells, and the Y974F mutants were poorly spread. Interestingly, the elongated, morphologically differentiated phenotype of Y706F macrophages reflected its significantly elevated Mac-1 expression. These results indicate important roles for Y706, Y721, and Y974 in regulating morphological responses of macrophages to CSF-1.
Our studies about the capacity of the CSF-1R mutants to phosphorylate a broad specificity substrate, polyEAY, have demonstrated that activation fails to occur when all eight sites are mutated to phenylalanine, suggesting that kinase activity is dependent on receptor tyrosine phosphorylation. Among the eight tyrosines examined, the juxtamembrane domain tyrosines Y544 and Y559 were shown to be critically required for the WT kinase activity, possessing ∼50% of WT kinase activity for polyEAY and autophosphorylation (Fig. 6). Relevant to this observation, a juxtamembrane peptide containing Ys 567 and 569 of the stem cell factor receptor (SCFR) corresponding to Y559, was shown to inhibit kinase activation in coincubations with the SCFR kinase domain, and this inhibition was reduced when the peptide was phosphorylated, indicating a role for the juxtamembrane domain Ys in the in vitro activation of the SCFR [38]. Our finding that the activation loop Y807 is not required for in vitro kinase activity is consistent with the demonstration that the equivalent mutation in the human CSF-1R also had full WT activity [39] but differs from the results of others showing that Y807F CSF-1R immunoprecipitates had ∼60% of the kinase activity of WT receptors [40]. Compared with both of these studies, our assays were carried out under conditions in which we have demonstrated linear kinetics and concentration dependence (Supplementary Fig. 1).
In contrast to these in vitro findings, we found that the receptor phosphorylation of the Y559F and Y807F mutants was reduced severely in vivo (Fig. 7). For Y559F, this reduction of in vivo phosphorylation was much more marked than expected from the ∼50% of WT kinase activity observed in vitro. For Y807F, possessing full WT kinase activity in vitro, this reduction was surprisingly drastic. These CSF-1R phosphorylation results indicate that there are active mechanisms inhibiting receptor tyrosine phosphorylation in vivo and that phosphorylation of the juxtamembrane Y559 and of the activation loop Y807 plays a role in relieving these inhibitory effects during the in vivo activation of the WT CSF-1R. These observations are consistent with reports of similar roles of the juxtamembrane and activation loop tyrosines in other class III receptor tyrosine kinases [41].
The present study demonstrates that the M−/− system can be used to address a number of questions concerning receptor structure and function, including CSF-1R activation and regulation initiated by CSF-1 and novel CSF-1R ligands [42], as well as the mechanisms underlying CSF-1-regulated functions such as adherence, motility, and differentiation. The injection of CSF-1R mutant macrophages, coupled with intravital imaging, can be used to study the role of particular CSF-1R phosphotyrosyl signaling pathways in different tissue microenvironments, such as tumors [43]. In addition, our confirmation of the central role of Y559 and Y807 in the regulation of macrophage survival, proliferation, and differentiation demands that their role in CSF-1R activation be focused on in future studies. On the other hand, the demonstration that Y706, Y721, and Y974 play important roles in morphological responses to CSF-1 provides new avenues for investigation of their roles in the regulation of macrophage adherence and motility using this system.
Acknowledgments
This work was supported by National Institutes of Health Grants CA26504, PO1 CA 100324 (E. R. S.), and KO8 CA097348 (F. J. P.), the Albert Einstein College of Medicine Cancer Center Grant 5P30-CA13330, an American Society of Hematology Fellow Scholar Award (X-M. D.), and a Leukemia and Lymphoma Society Special Fellow Award (X-M. D.). We thank Dr. Larry R. Rohrschneider for advice and for providing us with several c-fms c-DNA constructs that were invaluable for these studies. We thank Dr. Jiangwei Li, Dr. Xiao-Hua Zong, and Henry Kurniawan for technical assistance and members of the Albert Einstein College of Medicine DNA Sequencing and Analytical Imaging and FACS facilities for assistance in different aspects of this work.
References
- Pixley F J, Stanley E R. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ross F P, Teitelbaum S L. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley E R. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Pollard J W, Stanley E R. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic. Adv Dev Biochem (Amsterdam, Netherlands) 1996;4:153–193. [Google Scholar]
- Cecchini M G, Dominguez M G, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard J W, Stanley E R. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Dai X M, Ryan G R, Hapel A J, Dominguez M G, Russell R G, Kapp S, Sylvestre V, Stanley E R. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Sherr C J, Rettenmier C W, Sacca R, Roussel M F, Look A T, Stanley E R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41:665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Li W, Stanley E R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991;10:277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P S W, Wang Y, Dominguez M G, Yeung Y-G, Murphy M A, Bowtell D D L, Stanley E R. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J A. CSF-1 signal transduction. J Leukoc Biol. 1997;62:145–155. doi: 10.1002/jlb.62.2.145. [DOI] [PubMed] [Google Scholar]
- Hamilton J A. The biochemistry of colony stimulating factor-1 action. Thomson A W, editor. San Diego, CA, USA: Academic; The Cytokine Handbook. (3rd ed) 1999:689–712. [Google Scholar]
- Bourette R P, Rohrschneider L R. Early events in M-CSF receptor signaling. Growth Factors. 2000;17:155–166. doi: 10.3109/08977190009001065. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Faccio R, Chappel J, Zheng L, Feng X, Weber J D, Teitelbaum S L, Ross F P. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem. 2007;282:18980–18990. doi: 10.1074/jbc.M610938200. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K, Burkhalter S, van der Geer P. C-Cbl binds the CSF-1 receptor at tyrosine 973, a novel phosphorylation site in the receptor’s carboxy-terminus. Oncogene. 2002;21:1079–1089. doi: 10.1038/sj.onc.1205166. [DOI] [PubMed] [Google Scholar]
- Joos H, Trouliaris S, Helftenbein G, Niemann H, Tamura T. Tyrosine phosphorylation of the juxtamembrane domain of the v-Fms oncogene product is required for its association with a 55-kDa protein. J Biol Chem. 1996;271:24476–24481. doi: 10.1074/jbc.271.40.24476. [DOI] [PubMed] [Google Scholar]
- Mancini A, Niedenthal R, Joos H, Koch A, Trouliaris S, Niemann H, Tamura T. Identification of a second Grb2 binding site in the v-Fms tyrosine kinase. Oncogene. 1997;15:1565–1572. doi: 10.1038/sj.onc.1201518. [DOI] [PubMed] [Google Scholar]
- Rohde C M, Schrum J, Lee A W. A juxtamembrane tyrosine in the colony stimulating factor-1 receptor regulates ligand-induced Src association, receptor kinase function, and down-regulation. J Biol Chem. 2004;279:43448–43461. doi: 10.1074/jbc.M314170200. [DOI] [PubMed] [Google Scholar]
- Persons D A, Allay J A, Allay E R, Ashmun R A, Orlic D, Jane S M, Cunningham J M, Nienhuis A W. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. [PubMed] [Google Scholar]
- Muller A J, Young J C, Pendergast A M, Pondel M, Landau N R, Littman D R, Witte O N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991;11:1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P S, Sharp P A. Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol. 1986;59:746–775. doi: 10.1128/jvi.59.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E R. Murine bone marrow-derived macrophages. Walker J M, Pollard J W, editors. Totowa, NJ, USA: Humana; Animal Cell Culture. 1998:301–304. [Google Scholar]
- Jat P S, Sharp P A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989;9:1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Nishikawa S, Ogawa M, Kataoka H, Ohno N, Izawa A, Hayashi S I, Nishikawa S I. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 1995;11:2469–2476. [PubMed] [Google Scholar]
- Tushinski R J, Oliver I T, Guilbert L J, Tynan P W, Warner J R, Stanley E R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yeung Y-G, Stanley E R. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J Cell Biochem. 1999;72:119–134. [PubMed] [Google Scholar]
- Yeung Y-G, Wang Y, Einstein D B, Lee P S W, Stanley E R. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J Biol Chem. 1998;273:17128–17137. doi: 10.1074/jbc.273.27.17128. [DOI] [PubMed] [Google Scholar]
- Berg K L, Siminovitch K A, Stanley E R. SHP-1 regulation of p62dok tyrosine phosphorylation in macrophages. J Biol Chem. 1999;274:35855–35865. doi: 10.1074/jbc.274.50.35855. [DOI] [PubMed] [Google Scholar]
- Morgan C, Pollard J W, Stanley E R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC12F5. J Cell Physiol. 1987;130:420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Lardon F, Snoeck H W, Berneman Z N, Van Tendeloo V F, Nijs G, Lenjou M, Henckaerts E, Boeckxtaens C J, Vandenabeele P, Kestens L L, Van Bockstaele D R, Vanham G L. Generation of dendritic cells from bone marrow progenitors using GM-CSF, TNF-α, and additional cytokines: antagonistic effects of IL-4 and IFN-γ and selective involvement of TNF-α receptor-1. Immunology. 1997;91:553–559. doi: 10.1046/j.1365-2567.1997.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J M, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Hume D A, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med. 1983;157:1704–1709. doi: 10.1084/jem.157.5.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D A, Loutit J F, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Dai X M, Zong X H, Sylvestre V, Stanley E R. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- Yeung Y-G, Jubinsky P T, Sengupta A, Yeung D C-Y, Stanley E R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci USA. 1987;84:1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock C A, Jones G E, Stanley E R, Pollard J W. Colony-stimulating factor-1 induces rapid behavioral responses in the mouse macrophage cell line, BAC1.2F5. J Cell Sci. 1989;93:447–456. doi: 10.1242/jcs.93.3.447. [DOI] [PubMed] [Google Scholar]
- Pixley F J, Lee P S, Condeelis J S, Stanley E R. Protein tyrosine phosphatase φ regulates paxillin tyrosine phosphorylation and mediates colony-stimulating factor 1-induced morphological changes in macrophages. Mol Cell Biol. 2001;21:1795–1809. doi: 10.1128/MCB.21.5.1795-1809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P M, Ilangumaran S, La Rose J, Chakrabartty A, Rottapel R. Autoinhibition of the kit receptor tyrosine kinase by the cytosolic juxtamembrane region. Mol Cell Biol. 2003;23:3067–3078. doi: 10.1128/MCB.23.9.3067-3078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M F, Shurtleff S A, Downing J R, Sherr C J. A point mutation at tyrosine-809 in the human colony-stimulating factor 1 receptor impairs mitogenesis without abrogating tyrosine kinase activity, association with phosphatidylinositol 3-kinase, or induction of c-fos and junB genes. Proc Natl Acad Sci USA. 1990;87:6738–6742. doi: 10.1073/pnas.87.17.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Geer P, Hunter T. Tyrosine 706 and 807 phosphorylation site mutants in the murine colony-stimulating factor-1 receptor are unaffected in their ability to bind or phosphorylate phosphatidylinositol-3 kinase but show differential defects in their ability to induce early response gene transcription. Mol Cell Biol. 1991;11:4698–4709. doi: 10.1128/mcb.11.9.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S R. Autoinhibitory mechanisms in receptor tyrosine kinases. Front Biosci. 2002;7:d330–d340. doi: 10.2741/A778. [DOI] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein S K, Williams L T. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Wyckoff J B, Wang Y, Lin E Y, Li J F, Goswami S, Stanley E R, Segall J E, Pollard J W, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]