Abstract
The role of PI-3K in leukocyte function has been studied extensively. However, the specific role of the p110γ isoform of PI- 3K in CD4 T lymphocyte function has yet to be defined explicitly. In this study, we report that although p110γ does not regulate antigen-dependent CD4 T cell activation and proliferation, it plays a crucial role in regulating CD4 effector T cell migration. Naïve p110γ−/− CD4 lymphocytes are phenotypically identical to their wild-type (WT) counterparts and do not exhibit any defects in TCR-mediated calcium mobilization or Erk activation. In addition, p110γ-deficient CD4 OT.II T cells become activated and proliferate comparably with WT cells in response to antigen in vivo. Interestingly, however, antigen-experienced, p110γ-deficient CD4 OT.II lymphocytes exhibit dramatic defects in their ability to traffic to peripheral inflammatory sites in vivo. Although antigen-activated, p110γ-deficient CD4 T cells express P-selectin ligand, β2 integrin, β1 integrin, CCR4, CXCR5, and CCR7 comparably with WT cells, they exhibit impaired F-actin polarization and migration in response to stimulation ex vivo with the CCR4 ligand CCL22. These findings suggest that p110γ regulates the migration of antigen-experienced effector CD4 T lymphocytes into inflammatory sites during adaptive immune responses in vivo.
Keywords: inflammation, chemokines, leukocyte, activation, trafficking
INTRODUCTION
The PI-3K family of enzymes has been studied extensively in multiple cellular systems and participates in a variety of signal transduction pathways that regulate multiple cellular functions, including growth, metabolism, survival, proliferation, differentiation, apoptosis, adhesion, and migration [1, 2]. Class 1A PI-3Ks consist of the p110α, p110β, and p110δ catalytic subunits that bind to the p85α regulatory subunit, which is regulated by tyrosine phosphorylation and is commonly downstream of growth or antigen receptors. Class 1B PI-3K is comprised solely of p110γ, which binds to the p101 regulatory subunit, regulated through its association with the βγ subunit of heterotrimeric G-proteins and activated downstream of seven-transmembrane G-protein-coupled receptors, including chemokine receptors [1, 3].
The contribution of PI-3K activity to the function of several leukocyte populations, such as neutrophils, mast cells, and monocytes, has been well documented [4,5,6,7,8,9]. However, the specific, individual contributions of the p110γ and -δ isoforms of PI-3K to naïve and effector T lymphocyte function have not been characterized completely. There are, at best, modest impairments in thymocyte development and lymphocyte maturation in p110γ−/− or p110δ−/− mice. In contrast, mice lacking both of these isoforms (p110γ/δ−/− or p110γknockout δD910A) display dramatic reductions in thymocyte development and reduced numbers of mature CD4 and CD8 T lymphocytes in the periphery [10, 11]. Naïve CD4 lymphocytes isolated from p110γ/δ-deficient mice have an activated phenotype and secrete increased levels of the Th2 cytokines IL-4 and IL-5 following TCR stimulation in vitro, suggesting that these two isoforms of PI-3K may also function in regulating some aspects of T cell activation [10, 11]. Studies using p110γ−/− and p110δ−/− mice have demonstrated that the p110δ isoform of PI-3K is the main isoform responsible for generating phosphatidylinositol-3,4,5-trisphosphate (PIP3) downstream of TCR engagement in mature lymphocytes [12]. In addition, the p110δ isoform of PI-3K has been shown to regulate PIP3 accumulation and localization at the immune synapse following TCR stimulation [13]. Kinase-inactive p110δ (p110δD910A) and p110δ−/− mice exhibit defects in antigen-specific CD4 T cell proliferation and differentiation in vitro and defective T-dependent antibody production in vivo but exhibit normal Erk activation and adhesion to integrin ligands in vitro [12,13,14,15]. Similarly, p110δ has also been shown to regulate antigen-dependent responses in mast cells [16]. p110γ−/− mice have been shown to exhibit decreased contact hypersensitivity and delayed-type hypersensitivity (DTH) reactions, presumably as a result of impaired migratory responses of p110γ−/− dendritic cells [17]. Impaired generation of T-dependent antibodies following immunization with T-dependent haptens has also been reported in p110γ−/− mice [7]. Furthermore, CD4 T lymphocytes, isolated from p110γ−/− mice, are reported to exhibit reduced proliferative responses following TCR stimulation in vitro [7]. However, the antigen-dependent responsiveness of p110γ-deficient CD4 T cells has yet to be described.
It has been demonstrated previously that p110γ PI-3K is a major regulator of neutrophil, monocyte, and mast cell migration, and p110δ plays a more dominant role in regulating B cell migration [4, 18,19,20,21]. Although p110γ PI-3K regulates PIP3 accumulation at the membrane downstream of CXCR4 stimulation, naive p110γ−/− T cells exhibit only modest reductions in CCR7- and CXCR4-mediated migration in vitro that are dependent in part on the chemokine being analyzed and the dose of chemokine tested [13]. Modest defects in p110γ−/− T cell homing to secondary lymphoid tissue have also been reported [19, 22]. The adaptor protein dedicator of cytokinesis 2 (DOCK2), which participates in downstream Rac-dependent pathways involved in actin remodeling and cell migration [1, 22, 23], has been proposed to be a major regulator of naive T cell migration via a mechanism that is independent of PI-3K. Recent studies have demonstrated that DOCK2, not p110γ, controls egress of T lymphocytes out of thymus and secondary lymphoid tissue, as well as T cell movement within peripheral lymph nodes (LN) [22, 23]. Although p110γ is not necessary for the basal migration of lymphocytes through secondary lymphoid tissues, the question of whether p110γ controls the movement of effector T cells into inflammatory sites in peripheral tissues remains to be addressed.
The loss of p110γ activity ameliorates the pathogenesis of several autoimmune diseases, using established animal models of collagen-induced arthritis and systemic lupus erythematosus [2, 24, 25]. Although these autoimmune diseases are dependent on the development of aberrant effector CD4 T cell responses, the specific effect of the loss of p110γ in CD4 T cells to the pathogenesis of these two autoimmune diseases is unclear. Moreover, the defects in T-dependent antibody production and DTH reactions, previously observed in p110γ-deficient mice, are also dependent on the proper initiation and execution of CD4 effector T cell functions [26,27,28]. Here, we sought to investigate the hypothesis that p110γ specifically regulates the migration of antigen-activated effector CD4 T cells into peripheral inflammatory sites. Our results demonstrate that although p110γ does not control initial antigen-dependent CD4 T cell activation, this isoform of PI-3K regulates the trafficking of antigen-experienced effector CD4 T cells into peripheral inflammatory sites through its regulation of F-actin polarization and migration downstream of inflammatory chemokine receptors.
MATERIALS AND METHODS
Mice
p110γ −/− mice were obtained from Dr. Charles Abrams (University of Pennsylvania, Philadelphia, PA, USA) [29]. OT.II transgenic mice were obtained from Dr. Marc Jenkins (University of Minnesota, Minneapolis, MN, USA). p110γ−/− were back-crossed onto the C57BL/6 and OT.II transgenic background for more than 10 generations and then bred to generate p110γ−/− OT.II transgenic Thy1.1+ mice. Thy1.2 congenic recipients were obtained from the National Cancer Institute (NCI; Bethesda, MD, USA) or Taconic (Hudson, NY, USA). All experiments involving the use of animals were completed under the approval of the University of Minnesota Institutional Animal Care and Use Committee.
Antibodies and reagents
The following antibodies and reagents were purchased from eBioscience (San Diego, CA, USA): streptavidin-PE, CD4-FITC, CD8-FITC, B220-FITC, Thy1.1-FITC, B220-PE-Cy5.5, mouse IgG-FITC, CD62L-allophycocyanin, CD62L-PeCy7, Thy1.1-PE, Thy1.1-allophycocyanin, CD29-PE, CD49d-PE, purified CD49d, biotinylated rat IgG, and the corresponding isotype control antibodies. The following antibodies and reagents were purchased from BD PharMingen (San Jose, CA, USA): purified CXCR5, recombinant murine (rm)CXCL13, purified P-selectin-Fc chimera, I-Ad-FITC, CD4-PE-Cy7, Vα2 TCR-PE, and CD4-Pacific Blue. CFSE, calcein-acetoxymethylester (AM), and indo-1-AM were purchased from Invitrogen (Carlsbad, CA, USA). Ionomycin, PKH-26 reference beads, IFA, CFA, probenecid, and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA), and PMA was purchased from LC Laboratories (Woburn, MA, USA). Anti-FITC microbeads, streptavidin microbeads, and LS columns were purchased from Miltenyi Biotec (Auburn, CA, USA). The peptide OVA323–339 (ISQAVHAAHAEINEAGR) has been reported previously [30] and was synthesized by Invitrogen. Purified anti-CCR4 (clone K14) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat anti-human IgG-PE and anti-goat IgG-PE were purchased from Jackson ImmunoResearch (West Grove, PA, USA). Mouse/human CCL19-Fc, followed by incubation with a biotinylated anti-human Fc secondary antibody and streptavidin-PE (eBioscience), was used to detect CCR7 expression. rmCCL21, rmCCL22, and rmCCL3 were purchased from Peprotech (Rocky Hill, NJ, USA). The anti-phospho-Erk (anti-p-Erk; rabbit monoclonal 197G2), anti-total ERK (rabbit polyclonal), anti-p-AKT (Ser 473, rabbit monoclonal 58711F), and anti-total AKT (rabbit polyclonal) antibodies used in Western blotting analysis were purchased from Cell Signaling (Danvers, MA, USA).
Phenotypic analysis of naïve lymphoyctes
Naïve lymphocytes were harvested from wild-type (WT) or p110γ−/− OT.II transgenic animals and analyzed for GFP, TCR (Vα2), CD62L, CD44, and CCR7 expression on the CD4 lymphocytes using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) and FlowJo analysis software (Tree Star Inc., Ashland, OR, USA).
AKT activation
For analysis of AKT activation by Western blotting, naïve WT or p110γ−/− T cells were purified from peripheral LN by negative selection (purity, >95%). The cells were washed twice in PBS. Purified cells (3×106) were left unstimulated or stimulated with 5 or 10 μg/ml rmCCL21 for 1 min at 37°C. The cells were immediately placed on ice and then pelleted. The cells were resuspended in 1× Nonidet P-40 lysis buffer (1% Triton, 50 mM Tris, 150 mM NaCl, 4 mM EDTA, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin) and incubated on ice for 30 min. Lysates were clarified by centrifugation, reduced, and resolved on a 4–12% Bis-Tris acrylamide gel (Invitrogen). Proteins were transferred to a PVDF membrane, and the membranes were subsequently blocked in PBS + 0.2% casein and then probed with anti-p-AKT (Ser473, rabbit mAb) or anti-total AKT (rabbit polyclonal), followed by goat anti-rabbit IgG-Alexa 680 secondary antibody (Invitrogen). Proteins were visualized by scanning membranes on an infrared scanner (Odyssey, LI-COR Biosciences, Lincoln, NE, USA).
Erk activation
Analysis of Erk activation by Western blotting was preformed as described for AKT above. Lymphocytes were stimulated with 20 μg/ml anti-CD3 + 2 μg/ml anti-CD28, and Erk activation was detected using anti-p-Erk (Tyr/Thr rabbit mAb) or anti-total Erk (rabbit polyclonal) and goat anti-rabbit IgG-Alexa 680.
Calcium flux assays
Peripheral lymphocytes from WT or p110γ−/− mice were resuspended at a concentration of 75 × 106 cells/ml in PBS + 2% calf serum (CS) and stained for CD8 and CD4 T cells, which were then resuspended in RPMI + 1% FBS to a concentration of 10 × 106 cells/ml and were loaded with 3 μg/ml 100 mM indo-1-AM and 100 mM probenecid for 30 min at 37°C in the dark. After the collection of a 30-s base line, the cells were stimulated with 1 μM ionomycin or 8.4 μg biotinylated anti-CD3 antibody + 8 μg streptavidin (to cross-link anti-CD3). Calcium flux was analyzed using an LSRII flow cytometer and FlowJo analysis software.
In vivo functional assays
Peripheral lymphocytes from WT or p110γ−/− OT.II transgenic Thy1.1+ mice were resuspended to a final concentration of 10 × 106 cells/ml in RPMI containing 2% FCS and labeled with CFSE. The percentage of CD4+ OT.II (Vα2+) transgenic lymphocytes in the bulk suspension was determined by flow cytometry prior to CFSE-labeling. CD4+OT.II Thy1.1+ lymphocytes (0.5–1.0×106) were adoptively transferred i.v. into Thy1.2 congenic recipients. Twenty-four hours later, recipient animals were challenged with 10 μg OVA323–339 peptide with 25 μg LPS i.v., emulsified 1:1 in 100 μl IFA i.p., emulsified 1:1 in 10 μl IFA in the ear pinna, or emulsified 1:1 in 100 μl CFA s.c. At various time-points following challenge, the draining LN (i.v.=all peripheral LN; s.c.=axial and cervical LN; ear=cervical LN; i.p.=pancreatic and mesenteric LN) and spleen were harvested and dispersed into HBSS containing 0.2% sodium azide and 2% FCS. The phenotype of the CD4+Thy1.1+ cells was determined by analyzing CFSE dilution and CD62L, CD44, α4 integrin, and fucosylated PSGL-1 expression. In animals challenged i.p., peritoneal cells were recovered by washing the peritoneum with 5 ml PBS/2% CS. In animals challenged in the ear, excised ears were digested in RPMI/2% FCS containing 400 U/ml collagenase D in a rocking 37°C incubator for 1 h. The phenotype of the adoptively transferred CD4+Thy1.1+ cells was determined as described above.
Ex vivo transmigration assays
Peripheral lymphocytes harvested from WT or p110γ−/− OT.II transgenic Thy1.1+ mice were labeled with CFSE as described above. OT.II lymphocytes (1.0×106) were adoptively transferred into Thy1.2+ recipients. Twenty-four hours later, recipient animals were challenged with 100 μg OVA323–339 peptide, emulsified 1:1 in 100 μl CFA s.c. At various time-points following challenge, the draining LN were harvested, and chemokine receptor expression on the adoptively transferred CD4+Thy1.1+ cells was determined. Bulk lymphocytes (4×106) from the draining LN of challenged mice were added to the upper chamber of a 5-μM Transwell plate and were allowed to migrate toward 10 nM rmCCL21, 10 nM rmCXCL13, or 10 nM rmCCL22 in the lower chamber at 37°C, 5% CO2, for 3 h. Migrated cells in the lower chamber were harvested and stained for CD4+Thy1.1+ lymphocytes. Migration was quantified using PKH reference beads during flow cytometry analysis.
Ex vivo adhesion assays
WT (1.5×106) or p110γ−/− CD4+OT.II Thy1.1+ lymphocytes were adoptively transferred into Thy1.2+ recipients. Twenty-four hours later, recipient animals were challenged with 100 μg OVA323–339 peptide, emulsified 1:1 in 100 μl CFA s.c. On Day 7 postchallenge, the draining LN were harvested. The phenotype of the adoptively transferred CD4+Thy1.1+ cells was determined by analyzing CCR4, CCR7, PSGL-1, β1 integrin, and β2 integrin expression. The adoptively transferred donors were purified from the bulk suspension via negative selection. Total cells (0.8–5×105) were added to each well of a 96-well plate that had been coated previously with 0.6 μg/ml rmVCAM-1-Fc or 6 μg/ml rmICAM-Fc (R&D Systems Inc., Minneapolis, MN, USA) overnight. The plates were washed twice and coated with 1 μg/ml rmCCL22 for 1 h at 37°C. The plates were washed and then blocked with 2.5% BSA for 1 h at 37°C. The plates were quick-spun to allow the cells to settle to the bottom of the wells and were incubated at 37°C for 5 min. The plates were washed, and the number of adherent CD4+Thy1.1+ cells was analyzed by flow cytometry.
Ex vivo F-actin polarization in CD4 effector T cells
WT (2×106) or p110γ−/− CD4+OT.II Thy1.1+ lymphocytes were adoptively transferred into Thy1.2+ recipients. Twenty-four hours later, recipient animals were challenged with 100 μg OVA323–339 peptide, emulsified 1:1 in 100 μl CFA s.c. On Day 7 postchallenge, the draining LN were harvested. CCR7 and CCR4 expression on the adoptively transferred CD4+Thy1.1+ cells was analyzed as described above. The adoptively transferred Thy1.1+ donors were purified from the bulk suspension via sorting on a FACSAria cell sorter (purity, >99% Thy1.1+). The purified Thy1.1+ cells were then resuspended to a concentration of 1 × 106cells/ml in RPMI + 0.5% BSA and allowed to adhere to poly-L-lysine-coated slides for 2 h at 37°C. The media were aspirated, and the cells were then stimulated with media alone, 1 μg/ml rmCCL21, or 1 μg/ml rmCCL22 for 10 min at 37°C. The cells were washed with PBS, permeabilized, and stained with phalloidin-Alexa 594 (Invitrogen). F-actin polarization (phalloidin staining) was analyzed by confocal microscopy on an Olympus FluoView 1000 confocal microscope using Laser Sharp 3.0 software (Bio-Rad, Hercules, CA, USA). Confocal images were blinded and scored for polarization by three independent investigators.
Statistical analysis
The presented results are represented as the mean ± sem. Statistical significance between experimental conditions was determined using a Students’s t-test. P < 0.05 was considered significant; *, P < 0.05; **, P< 0.005.
RESULTS
Phenotypic analysis of naïve, p110γ-deficient CD4 T lymphocytes
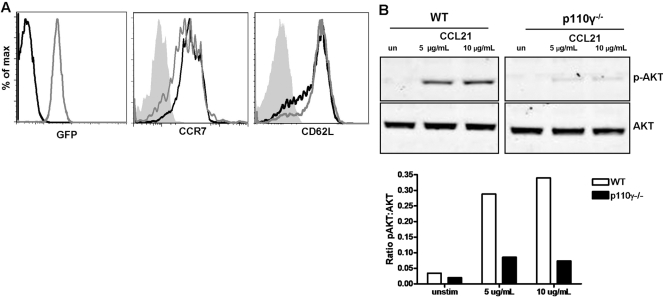
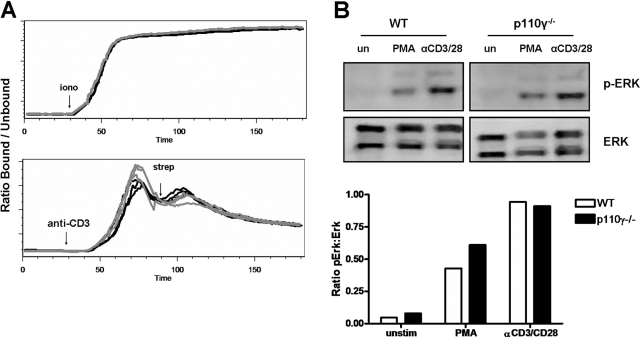
The p110γ-deficient mice used in these studies were derived through the insertion of GFP in-frame in the p110γ gene, such that GFP is expressed in lieu of p110γ [29]. Therefore, the expression of GFP in p110γ-deficient cells was monitored in all of the experiments reported in this study to ensure the effective loss of p110γ expression (Fig. 1A). In addition, the phenotype of naïve peripheral lymphocytes isolated from these p110γ−/− and control WT mice was analyzed by flow cytometry. As has been previously reported in other strains of p110γ-deficient mice [7], we did not observe any difference in the numbers of CD4 T cells in the peripheral LN (data not shown) or in the phenotype of naïve, peripheral CD4 T cells as assessed by CCR7, CD62L, CD44, or TCR expression (Fig. 1A, and data not shown). Activation of AKT or protein kinase B is a proximal event downstream of PI-3K activation [1]. Therefore, the loss of p110γ function in these lymphocytes was also confirmed through the analysis of AKT activation following chemokine receptor stimulation. AKT phosphorylation, following stimulation with the CCR7 ligand CCL21, was dramatically impaired in naïve, p110γ-deficient T cells (Fig. 1B). Furthermore, we did not observe any deficit in signal transduction pathways downstream of TCR ligation, as calcium responses (Fig. 2A) and Erk activation (Fig. 2B) progressed normally following anti-CD3 stimulation in p110γ-deficient lymphocytes. These observations further demonstrate that the loss of the p110γ isoform of PI-3K does not affect the phenotype of naïve CD4 T cells nor does it impact TCR-dependent signaling events in vitro.
Fig. 1.
Phenotypic analysis of naïve, p110γ-deficient CD4 lymphocytes. (A) Naïve, p110γ-deficient lymphocytes were analyzed for GFP, CCR7, and CD62L expression by flow cytometry. Shaded histogram indicates isotype control staining, black line indicates WT, and gray line indicates p110γ−/− CD4 T cells. (B) AKT phosphorylation in naive T cells following stimulation with 5 or 10 μg/ml rmCCL21 for 1 min was analyzed by Western blotting. Densitometric analysis was preformed digitally using the Odyssey imaging system and is presented as the ratio of p-AKT to total AKT in each sample.
Fig. 2.
TCR-mediated activation of p110γ-deficient CD4 lymphocytes. (A) Calcium flux in naïve, p110γ-deficient CD4 T cells following stimulation with anti-CD3 antibody or ionomycin (iono; as a positive control) was analyzed by flow cytometry. Black lines represent WT, and gray lines represent p110γ−/− CD4 T cells. Individual lines represent analysis of individual samples. Data are representative of at least three independent experiments in which n ≥ 3. strep, Streptavidin. (B) Erk phosphorylation in naive T cells following stimulation with 50 ng PMA or 20 μg/ml anti-CD3 + 2 μg/ml anti-CD28 antibody for 5 min was analyzed by Western blotting. Densitometric analysis was preformed digitally using the Odyssey imaging system and is presented as the ratio of p-Erk to total Erk in each sample. Similar results were observed when Erk activation was analyzed by flow cytometry (data not shown).
Antigen-dependent CD4 lymphocyte activation is unaffected by the loss of p110γ
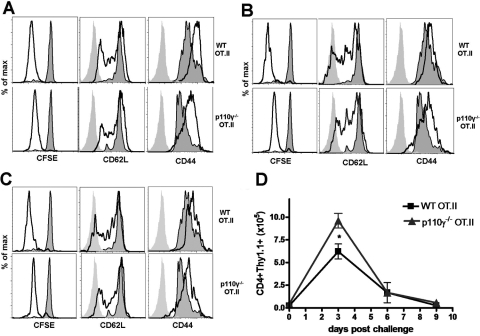
We next examined whether p110γ regulated antigen-dependent CD4 T cell activation and function during immune responses in vivo. To test the ability of p110γ-deficient CD4 T cells to respond to an immunogenic stimulus in vivo, we generated p110γ−/− OT.II TCR transgenic mice in which the CD4 T cells recognize a defined peptide fragment of chicken OVA. CFSE-labeled WT or p110γ−/− OT.II transgenic lymphocytes (1×106 per recipient) were adoptively transferred into normal Thy1.2 congenic recipients and subsequently challenged i.v. with 10 μg OVA in conjunction with 25 μg LPS as an adjuvant. Peripheral LN were harvested at various time-points following antigen challenge, and the proliferation (CFSE dilution) and activation status (CD62L and CD44 expression) of the adoptively transferred donors were analyzed by flow cytometry. Interestingly, we discovered that the loss of p110γ does not impair antigen-dependent CD4 T cell activation or proliferation in vivo (Fig. 3). The p110γ-deficient OT.II transgenic donors did not exhibit any defects in their ability to down-regulate L-selectin (CD62L) or up-regulate CD44 expression throughout the time course of the response (Fig. 3 A–C). In addition, the p110γ−/− OT.II donors expanded with similar kinetics as their WT OT.II counterparts, in which the response peaked on Day 3 and was resolved on Day 9 following OVA challenge (Fig. 3D). The difference in the extent to which the p110γ −/− donor cells dilute CFSE in comparison with WT is attributed to the endogenous GFP expression in the p110γ−/− cells (see Fig. 1A). These results demonstrate that p110γ does not regulate the initial events downstream of antigen-dependent CD4 T cell activation.
Fig. 3.
Antigen-dependent CD4 T cell activation is unaffected by the loss of p110γ. The phenotype and proliferation of WT and p110γ−/− OT.II transgenic donor lymphocytes on Day 3 (A), Day 6 (B), and Day 9 (C) following i.v. OVA/LPS challenge were determined by flow cytometry. Light-gray histogram indicates isotype control staining, dark-gray, shaded histogram indicates adoptively transferred, unchallenged donor cells, and black line indicates adoptively transferred, OVA-challenged CD4+Thy1.1+ donor cells. (D) The total number of WT or p110γ−/− OT.II donor cells (CD4+Thy1.1+) in the peripheral LN of recipient mice was quantified using flow cytometry. Data are representative of three independent experiments in which n ≥ 3. *P < 0.05.
Loss of p110γ impairs the migration of antigen-activated CD4 lymphocytes back to sites of initial antigen encounter
We hypothesized that p110γ may specifically regulate the trafficking of newly activated, effector CD4 T cells into peripheral inflammatory sites. To test our hypothesis, it was necessary to deposit antigen in a site where it could not freely diffuse, allowing us to accurately track the insinuation of the immune response in the draining LN as well as the migration of those newly activated lymphocytes back to the site of antigen deposition. We chose to deposit 10 μg OVA323–339 antigen emulsified in IFA in the ear pinna of congenic recipients that received 1 × 106 CFSE-labeled WT or p110γ−/− OT.II lymphocytes 24 h earlier. It is well established that lymphocytes differentially regulate the expression of adhesion molecules, such as L-selectin, PSGL-1, and integrins, to leave secondary lymphoid tissue and gain access to peripheral inflammatory sites [31,32,33,34,35,36]. Therefore, at various time-points following antigen challenge, the draining LN and challenged ear were harvested, and the proliferation (CFSE dilution) and phenotype [CD62L, PSGL-1, and α4 integrin (CD49d)] of the adoptively transferred donor cells were analyzed by flow cytometry.
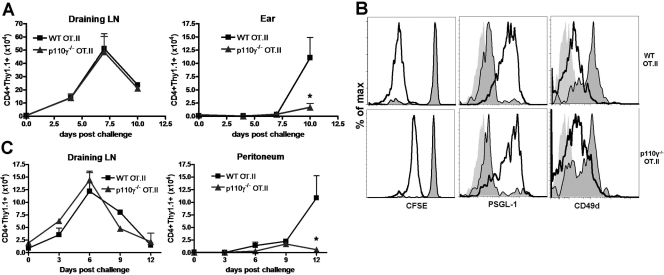
In line with our previous observations, there was no difference in the activation status or proliferation of p110γ −/− OT.II transgenic donors in the draining LN on Day 7 postantigen challenge, when the response peaks compared with their WT counterparts (Fig. 4A, and data not shown). Interestingly, we observed a dramatic reduction in the number of p110γ−/− OT.II transgenic donors that had migrated to the inflamed ear on Day 10 following antigen challenge, the peak of effector CD4 T cell accumulation in the ear (Fig. 4A). However, the phenotype of the few p110γ−/− OT.II donor cells that were able to migrate into challenged ears was similar to WT OT.II transgenic donors in that they were CFSEdilute, PSGL-1hi, CD49dlow, and CD62Llow (Fig. 4B, and data not shown). This defect in migratory capacity of p110γ−/− OT.II lymphocytes could not be attributed to differences in the endogenous inflammatory environment in the ears of the recipient mice, as the number of endogenous CD4 lymphocytes (CD4+Thy1.1–) isolated from the challenged ears throughout the time course was equivalent between recipients that had received WT or p110γ−/− OT.II donor lymphocytes (data not shown). In addition, these observations were not dependent on the particular site of initial antigen challenge, as we also observed a significant reduction in the ability of p110γ−/− OT.II donor lymphocytes to migrate into the peritoneum on Day 10 following an i.p. challenge with OVA/IFA (Fig. 4C). These results further suggest that p110γ may specifically regulate the migration of effector CD4 T cells, rather than the initial events involved in T cell activation and priming.
Fig. 4.
p110γ controls the migration of effector CD4 lymphocytes into peripheral inflammatory sites. (A) Proliferation of WT and p110γ−/− OT.II (CD4+Thy1.1+) adoptively transferred donors in the draining LN and accumulation of these donor lymphocytes in the ear at various time-points following OVA challenge in the ear were quantified by flow cytometry. Data are representative of three independent experiments in which n ≥ 3. *, P < 0.05. (B) Phenotype of adoptively transferred WT and p110γ−/− OT.II donors in challenged ears on Day 10. Light-gray histogram indicates isotype control staining, dark-gray, shaded histogram indicates expression levels on untransferred, unchallenged donors for reference, and black line indicates adoptively transferred, OVA-challenged CD4+Thy1.1+ cells. CFSE dilution is representative of three independent experiments in which n ≥ 3, and phenotypic analysis of PSGL-1 and CD49d expression represent a single, independent experiment in which n = 4. (C) Proliferation of WT and p110γ−/− OT.II (CD4+Thy1.1+) adoptively transferred donors in the draining LN and accumulation of these donor lymphocytes in the peritoneum at various time-points following i.p. OVA challenge were quantified by flow cytometry. Data are representative of at least four independent experiments in which n ≥ 3. *, P < 0.05.
The p110γ isoform of PI-3K controls chemokine receptor-mediated migration of effector CD4 lymphocytes
For newly activated CD4 T cells to appropriately traffic to peripheral inflammatory sites, they must be able to respond to numerous inflammatory signals. First, the antigen-experienced effector CD4 T cells must be able to appropriately interpret signals from chemokines and integrin ligands presented on inflamed endothelial venules that would allow them to adhere and extravasate into the surrounding tissue. Second, the cell must be able to migrate along a gradient of inflammatory chemokines produced in the inflamed tissue site. Therefore, to better understand the mechanism underlying the observed defect in migration of p110γ −/− OT.II effector CD4 T cells into peripheral inflammatory sites, we chose to further investigate the ability of these effectors to respond to inflammatory chemokines ex vivo.
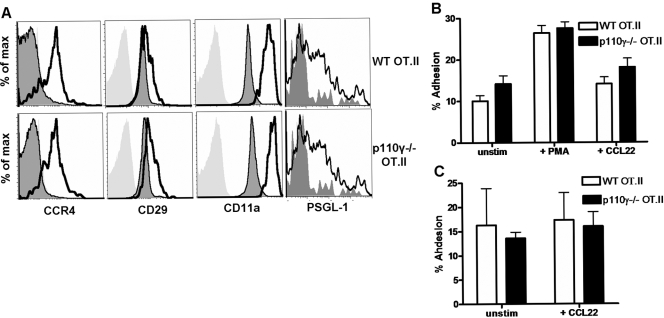
It has been proposed that the interaction between CCR4+ effector T cells and CCR4 ligands (CCL17 and CCL22) on inflamed, cutaneous venules signals the activation of β1 and β2 integrins on the T cell and is necessary for the migration of activated lymphocytes into peripheral cutaneous sites [33, 34, 37, 38]. Given that we primarily used s.c. routes of antigen delivery in our experimental system, we investigated whether defects in CCR4-induced adhesion and migration contributed to the migratory defects observed in p110γ−/− OT.II effector CD4 T cells in vivo. WT or p110γ−/− OT.II lymphocytes were adoptively transferred into WT congenic recipients and challenged s.c. 24 h later with 100 μg OVA emulsified in CFA. The phenotype and responsiveness of the adoptively transferred WT or p110γ−/− OT.II donors in the draining LN were analyzed at various time-points following challenge. We empirically determined that proliferation and chemokine receptor expression and responsiveness peaked on Day 7 following s.c. antigen challenge (data not shown). Therefore, we chose to focus on this time-point for further analysis. Antigen-challenged donor CD4 T cells were purified from the draining LN via positive selection, and their ability to adhere to the β1 integrin ligand VCAM-1 and the β2 integrin ligand ICAM-1 following CCL22 (CCR4 ligand) stimulation ex vivo was analyzed. WT and p110γ−/− OT.II effector T cells showed enhanced expression of the β1 integrin subunit (CD29), the β2 integrin subunit (CD11a), and the selectin ligand PSGL-1 when compared with unchallenged, donor T cells (Fig. 5A). In addition, we did not observe any difference in CCR4 expression levels between WT and p110γ−/− OT.II effector T cells (Fig. 5A). Antigen-experienced effector p110γ−/− OT.II effector T cells adhered to ICAM-1 and VCAM-1 comparably with WT OT.II effectors (Fig. 5, B and C). Although stimulation with CCL22 slightly enhanced the adhesion of WT and p110γ−/− OT.II effectors to ICAM-1, this enhancement was not statistically significant (Fig. 5B). Stimulation with CCL22 did not enhance the adhesion of WT or p110γ−/− OT.II effector T cells to VCAM-1 (Fig. 5C). These results suggest that defects in the migration of p110γ-/ effector CD4 T cells into inflammatory sites are not associated with defects in integrin-dependent adhesion.
Fig. 5.
Adhesion of antigen-activated CD4 lymphocytes is not regulated by p110γ. (A) The phenotype of antigen-activated WT and p110γ−/− OT.II (CD4+Thy1.1+) donor lymphocytes was analyzed by flow cytometry. Light-gray histogram indicates isotype control staining, dark-gray, shaded histogram indicates adoptively transferred, unchallenged donors, and black line indicates adoptively transferred, OVA-challenged donors. Data are representative of at least four independent experiments for CCR4, CD29, and PSGL-1 expression and of a single independent experiment for CD11a expression. (B) The adhesion of adoptively transferred, OVA-challenged WT and p110γ−/− OT.II donor cells isolated from the draining LN of recipient mice 7 days post-s.c. OVA challenge to 6 μg/ml-immobilized rmICAM-1 (unstimulated) in the presence of 50 ng/ml PMA (+PMA) or 1 μg/ml rmCCL22 (+CCL22) was analyzed. Data represent the average percent cell adhesion in a single independent experiment in which n ≥ 5. (C) The adhesion of adoptively transferred, OVA-challenged WT and p110γ−/− OT.II donor cells isolated from the draining LN of recipient mice 7 days post-s.c. OVA challenge to 0.6 μg/ml-immobilized rmVCAM-1 (unstimulated) in the presence or absence of rmCCL22 (+CCL22) was analyzed. The average background level of adhesion (adhesion to BSA alone) in the adhesion assays depicted in B and C was ∼5%. Data represent the average percent cell adhesion pooled between two independent experiments in which n ≥ 3.
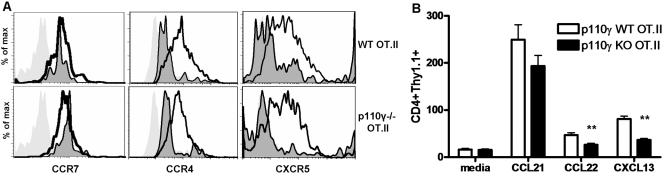
Next, we directly tested the ability of p110γ−/− OT.II effectors to migrate in response to CCR4 ligands and other inflammatory chemokines ex vivo. Antigen-experienced effector OT.II cells were generated as described above. We did not detect any differences in the expression levels of the chemokine receptors CCR7, CXCR5, or CCR4 on the antigen-experienced WT or p110γ−/− OT.II effector T cells on Day 7 following s.c. OVA challenge (Fig. 6A). Interestingly, however, we observed a significant reduction in the ability of effector p110γ−/− OT.II donor lymphocytes to migrate in response to the CCR4 ligand CCL22 as well as the CXCR5 ligand CXCL13 in ex vivo transmigration assays (Fig. 6B). We also observed a reduction in the responsiveness of these p110γ−/− effector CD4 lymphocytes to stimulation with the CCR1 and CCR5 ligand CCL3, but this defect was not statistically significant (data not shown). These results suggest that an impaired migratory responsiveness to “inflammatory” chemokines may be responsible for the reduced migration of p110γ-deficient effector CD4 T cells into peripheral inflammatory sites in vivo.
Fig. 6.
p110γ controls chemokine-mediated migration of effector CD4 lymphocytes. (A) The phenotype of antigen-activated WT and p110γ−/− OT.II (CD4+Thy1.1+) donor lymphocytes isolated from the draining LN of recipient mice 7 days post-s.c. OVA challenge was analyzed by flow cytometry. Light-gray histogram indicates isotype-control staining, dark-gray, shaded histogram indicates adoptively transferred, unchallenged donors, and black line indicates adoptively transferred, OVA-challenged donors. Data are representative of at least four independent experiments. (B) The migration of adoptively transferred, OVA-challenged WT and p110γ−/− OT.II donor T cells isolated from the draining LN of recipient mice on Day 7 post-OVA challenge to 10 nM rmCCL21 (CCR7 ligand), CCL22 (CCR4 ligand), or CXCL13 (CXCL13 ligand) was determined using ex vivo transmigration assays and analyzed by flow cytometry. Data from four independent experiments, in which n ≥ 2, were pooled together. **, P < 0.005.
The p110γ isoform of PI-3K regulates F-actin polarization downstream of inflammatory chemokine receptors
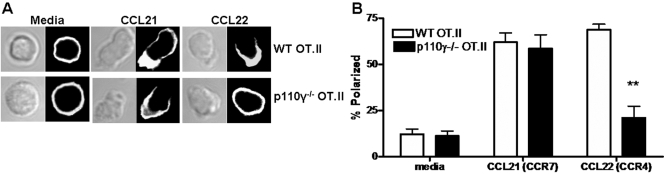
Cells migrating along a chemotactic gradient must be able to appropriately polarize their cytoskeletal machinery toward the leading edge of the cell [39, 40]. Accumulation of the cytoskeletal protein F-actin at the leading edge of the cell is one hallmark of cell polarization [39,40,41]. As the p110γ isoform of PI-3K has been shown to regulate F-actin polarization in neutrophils [41], we next investigated whether these p110γ-deficient effectors would also display defects in their ability to polarize F-actin effectively following CCR4 stimulation. Antigen-experienced effector cells were generated in vivo as described above. On Day 7 postantigen challenge, WT and p110γ−/− OT.II effector CD4+Thy1.1+ donors were purified from the draining LN by FACS. These purified effectors (>99% Thy1.1+) were analyzed for chemokine receptor expression and their ability to polarize F-actin following stimulation with the CCR7 ligand CCL21 or the CCR4 ligand CCL22. We observed a significant defect in the ability of effector p110γ−/− OT.II cells to polarize F-actin following CCR4 stimulation (Fig. 7, A and B). This observed defect is not attributable to differences in CCR4 expression levels between WT and p110γ−/− effectors (Fig. 6A). However, p110γ−/− OT.II effectors did not display any defect in their ability to polarize F-actin in response to stimulation with the CCR7 ligand CCL21 (Fig. 7, A and B). These results demonstrate that the p110γ isoform of PI-3K regulates F-actin polarization downstream of inflammatory chemokine receptors in effector CD4 T cells.
Fig. 7.
p110γ PI-3K regulates CCR4-dependent F-actin polymerization. (A) Confocal images of purified WT (upper panel) or p110γ−/− (lower panel) effector CD4 T cells stimulated with media alone, 1 μg/ml rmCCL21, or 1 μg/ml rmCCL22 for 20 min and then stained with phalloidin-Alexa 594. Images are representative of more than 45 images analyzed per treatment group in two independent experiments. (B) Blinded analysis of F-actin polarization in confocal images in A. Over 45 images per treatment group per experiment were blinded and scored for polarization by three independent investigators. The results from the blinded analysis were pooled and graphed as percent polarization ± sem. **, P < 0.005.
DISCUSSION
In these studies, we demonstrate that p110γ−/− CD4 T cells do not exhibit defects in their ability to respond to TCR stimulation in vitro. p110γ-deficient CD4 T cells were able to mobilize calcium and activate Erk equivalently following TCR stimulation. Although p110γ−/− T cells have been reported to have defects in proliferation in response to anti-CD3 antibody stimulation in vitro [7], we did not observe any defects in the activation phenotype or proliferative capacity of p110γ−/− OT.II transgenic T cells following antigen challenge in vivo. In addition, the kinetics of the response and phenotype of antigen-experienced p110γ−/− OT.II lymphocytes following systemic antigen challenge were similar to their WT counterparts. This further suggests that the newly activated, p110γ-deficient CD4 lymphocytes do not have any reduction in their capacity to respond to antigenic signals in vivo. These results support other studies suggesting that p110δ, not p110γ, is the predominant PI-3K isoform that is activated downstream of the TCR in T cells [12,13,14, 42]. One recent study reported a defect in anti-CD3-induced proliferation and ERK activation in vitro in p110γ-deficient T cells that could not be rescued with exogenous CD28 or IL-2 stimulation [43]. The basis for the discrepancy between this recent report and our own studies, as well as others [7, 12,13,14,15, 42], is currently unclear. However, despite this discrepancy in T cell responses in vitro, our studies have shown that p110γ does not play a major role in the antigen-dependent activation and clonal expansion of CD4 or CD8 naïve T cells in vivo [42].
In the current paradigm of T cell activation and effector differentiation, CD4 T cells increase CXCR5 expression early after initial activation, allowing a subset of these T cells to migrate to the B cell follicle to lend cytokine help to antigen-specific B cells [28]. Another subset of newly activated T cells increases their expression of inflammatory chemokine receptors (e.g., CXCR3, CCR4, and CCR5) and commiserates with the site and nature of initial antigen encounter by the APC [28, 44,45,46]. To gain access into inflamed peripheral tissue sites, activated T cells rely on complex interactions among selectin ligands, integrins, and chemokine receptors with selectins, chemokines, and integrin ligands expressed on the inflamed vascular endothelium. Skin-homing effector CD4 T cells preferentially increase their expression of CCR4 and PSGL-1 [33, 37, 38]. It has been suggested previously that the interaction between PSGL-1 on the activated T cell and P-selectin on the inflamed endothelium is necessary for the rolling of effector T cells on inflamed cutaneous venules. This rolling allows CCR4+ effector T cells to bind CCR4 ligands (CCL22, CCL17) expressed on the endothelial surface, leading to the enhancement of β1 and β2 integrin-mediated adhesion to the inflamed cutaneous venule. This allows the cells to subsequently extravasate into the surrounding tissue, perform their effector function, and help resolve the inflammatory response [34, 36].
Our studies have shown that p110γ is not required for the activation-dependent changes in expression of chemokine receptors (CCR7, CXCR5, CCR4), integrins (β1 and β2), and PSGL-1 following s.c. antigen challenge in vivo. It is also important to note that changes in L-selectin expression following antigen challenge in vivo were not altered by the loss of p110γ, as recent work has revealed a role for p110δ in controlling L-selectin and CCR7 expression following TCR stimulation [47]. This provides further support for our hypothesis that p110γ does not participate in the initial priming or differentiation events necessary for effective antigen-dependent CD4 T cell activation during immune responses in vivo.
Although the initial phase of T cell activation was unaffected, there was a significant reduction in the number of antigen-experienced p110γ−/− OT.II donor T cells in the inflamed ear. This defect in CD4 effector T cell migration is not site-specific, as we also observed a significant reduction in the ability of antigen-activated, p110γ−/−deficient effectors to traffic into an inflamed peritoneum following an i.p. antigen challenge. Adhesion of effector CD4 T cells to the inflamed vascular endothelium is an important step in the migration of activated T cells into peripheral tissues sites. We observed that expression of the β1 and β2 integrin subunits increased on WT and p110γ-deficient CD4 effector T cells on Day 7 following s.c. antigen challenge. However, p110γ-deficient CD4 effector T cells were not impaired in their ability to bind to VCAM-1 or ICAM-1 in ex vivo adhesion assays. In vitro studies using naïve p110γ- or p110δ-deficient leukocytes have also not implicated these isoforms of PI-3K in regulating adhesion to integrin ligands [7, 14]. In addition, naïve p65PI-3K transgenic p110γ−/− T cells, which express a constitutively active form of Class 1A PI-3K and display an activated phenotype, do not rely on p110γ expression to migrate into noninflamed peripheral tissues [48]. Therefore, it is perhaps not surprising that effector p110γ−/− OT.II lymphocytes did not exhibit defects in their basal adhesion to VCAM-1 or ICAM-1 ex vivo. We also did not observe changes in adhesion of these antigen-experienced effector CD4 T cells to VAM-1 with the addition of CCL22. However, we did observe a small increase in the adhesion of effector T cells to ICAM-1 in the presence of CCL22. The concentration of immobilized integrin ligand used in these assays may already be high enough to overcome dependence on chemokine-mediated, inside-out integrin activation in these effector T cells. In addition, activated effector CD4 T cells may exhibit a higher basal state of integrin functional activity that is not appreciably augmented by chemokine stimulation. It is also possible that the other CCR4 ligand CCL17 may be a more potent agonist for adhesion than CCL22.
Although adhesion of CD4 effector T cells to integrin ligands was not affected by the loss of p110γ, we observed significant defects in the migration of antigen-experienced p110γ−/− OT.II T cells to the CCR4 ligand CCL22. In addition, we have demonstrated that p110γ PI-3K regulates F-actin polarization downstream of CCR4 stimulation. These results suggest that p110γ is critical for generating a polarized cell morphology critical for effector T cell migration in response to chemokines. Importantly, the loss of p110γ in effector CD4 T cells did not impact CCR7-dependent, F-actin polarization or migration, demonstrating that these observed defects are a result of the loss of p110γ PI-3K and not a result of a global, functional defect in p110γ-deficient cells. We have also demonstrated that p110γ−/− effector T cells exhibit severe impairments in their ability to access the inflamed ear as well as the inflamed peritoneum in vivo. These observations suggest that p110γ may not be acting solely downstream of CCR4, as CCR9-dependent interactions with its ligand CCL25 are thought to control effector T cell homing to intestinal sites [31, 32]. Moreover, we also observed a significant reduction in the ability of p110γ−/− CD4 effector T cells to migrate in response to the CXCR5 ligand CXCL13 and the CCR1/CCR5 ligand CCL3 (data not shown). The observed defects in CXCR5-dependent migration in p110γ-deficient effectors suggest that these effectors may be limited in their ability to migrate toward the B cell follicle and may provide further insights into the defects previously reported in T-dependent antibody production in p110γ−/− mice [7]. Therefore, we propose that p110γ is likely functioning downstream of multiple inflammatory chemokine receptors that are up-regulated following antigen-dependent CD4 T cell activation and functions to fine-tune the migratory capacity of effector CD4 T cells.
The findings presented here demonstrate that p110γ PI-3K regulates chemokine-dependent migration of antigen-experienced effector CD4 T cells into peripheral inflammatory sites via regulation of F-actin polarization downstream of inflammatory chemokine receptors. In addition, these results provide further insights into the previously reported deficiencies in T-dependent antibody production, as well as the reduced ability to mount DTH reactions previously observed in p110γ-deficient mice [7, 17]. The ability of p110γ to specifically regulate effector CD4 T cell migration, without impacting initial T cell activation or priming, makes it an attractive target for therapeutic intervention in multiple allergic and autoimmune diseases [2, 4, 20, 24, 25]. Careful manipulation of p110γ activity may allow for the inhibition of migration of effector T cells into inflammatory sites, which could ameliorate disease pathogenesis without drastically immunocompromising the patient.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grant AI064271 and the Harry Kay Chair in Biomedical Research at the University of Minnesota (Y. S.), NIH Training Grants T32DE007288 (M. S. T.), T32CA009138 (J. S. M.), and T32AI07313 (C. C. D.), and an American Heart Association predoctoral fellowship (A. L. M.). We thank S. Highfill, M. Schwartz, and R. Srivastava for their valuable technical assistance and the assistance of the Flow Cytometry Core of the Masonic Cancer Center at the University of Minnesota, a comprehensive cancer center designated by the NCI (supported in part by NIH grant P30 CA77598). M. S. T. developed, performed, and analyzed the majority of the experiments presented in this manuscript and wrote the manuscript. C. C. D. performed and analyzed ex vivo adhesion assay experiments. J. S. M. performed the confocal analysis of F-actin polarization in p110γ-deficient effector lymphocytes. A. L. M. performed the CCR7 phenotyping of naïve p110γ-deficient lymphocytes. Y. S. conceptualized and developed the experiments, analyzed and interpreted data, and edited the manuscript. The authors have no competing financial interests to declare.
References
- Ward S G. T lymphocytes on the move: chemokines, PI 3-kinase and beyond. Trends Immunol. 2006;27:80–87. doi: 10.1016/j.it.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Rommel C, Camps M, Ji H. PI3K δ and PI3K γ: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- Brock C, Schaefer M, Reusch H P, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. Roles of G βγ in membrane recruitment and activation of p110 γ/p101 phosphoinositide 3-kinase γ. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann M P, Bjorklof K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Phosphoinositide 3-kinase γ: a key modulator in inflammation and allergy. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- Fukao T, Terauchi Y, Kadowaki T, Koyasu S. Role of phosphoinositide 3-kinase signaling in mast cells: new insights from knockout mouse studies. J Mol Med. 2003;81:524–535. doi: 10.1007/s00109-003-0475-2. [DOI] [PubMed] [Google Scholar]
- Maus U A, Backi M, Winter C, Srivastava M, Schwarz M K, Ruckle T, Paton J C, Briles D, Mack M, Welte T, Maus R, Bohle R M, Seeger W, Rommel C, Hirsch E, Lohmeyer J, Preissner K T. Importance of phosphoinositide 3-kinase γ in the host defense against pneumococcal infection. Am J Respir Crit Care Med. 2007;175:958–966. doi: 10.1164/rccm.200610-1533OC. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones R G, Oliveira-dos-Santos A J, Stanford W L, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak T W, Ohashi P S, Suzuki A, Penninger J M. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Weiss-Haljiti C, Pasquali C, Ji H, Gillieron C, Chabert C, Curchod M L, Hirsch E, Ridley A J, van Huijsduijnen R H, Camps M, Rommel C. Involvement of phosphoinositide 3-kinase γ, Rac, and PAK signaling in chemokine-induced macrophage migration. J Biol Chem. 2004;279:43273–43284. doi: 10.1074/jbc.M402924200. [DOI] [PubMed] [Google Scholar]
- Smith D F, Deem T L, Bruce A C, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase γ is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006;80:1491–1499. doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- Webb L M, Vigorito E, Wymann M P, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110γ and p110δ catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- Ji H, Rintelen F, Waltzinger C, Bertschy Meier D, Bilancio A, Pearce W, Hirsch E, Wymann M P, Ruckle T, Camps M, Vanhaesebroeck B, Okkenhaug K, Rommel C. Inactivation of PI3Kγ and PI3Kδ distorts T-cell development and causes multiple organ inflammation. Blood. 2007;110:2940–2947. doi: 10.1182/blood-2007-04-086751. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Patton D T, Bilancio A, Garcon F, Rowan W C, Vanhaesebroeck B. The p110δ isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- Garcon F, Patton D T, Emery J L, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek S E, Salpekar A, Waterfield M D, Smith A J, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signaling: a distinctive role for the p110δ isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K, Camps M, Pearce W P, Ji H, Ruckle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B. Isoform-specific functions of phosphoinositide 3-kinases: p110δ but not p110γ promotes optimal allergic responses in vivo. J Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, Bernasconi S, Sironi M, Santoro A, Garlanda C, Facchetti F, Mymann M P, Vedcchi A, Hirsch E, Mantovani A, Sozzani S. Defective dendritic cell migration and activation of adaptive immunity in PI3Kγ-deficient mice. EMBO J. 2004;23:3505–3515. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel K M, McHeyzer-Williams L J, Ngo V N, McHeyzer-Williams M G, Cyster J G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif K, Okkenhaug K, Sasaki T, Penninger J M, Vanhaesebroeck B, Cyster J G. Cutting edge: differential roles for phosphoinositide 3-kinases, p110γ and p110δ, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev V L, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann M P. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Kuan Y H, Lin R H, Chen Y L, Tsao L T, Tzeng C C, Wang J P. Effective attenuation of acute lung injury in vivo and the formyl peptide-induced neutrophil activation in vitro by CYL-26z through the phosphoinositide 3-kinase γ pathway. Biochem Pharmacol. 2006;72:749–760. doi: 10.1016/j.bcp.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Lacalle R A, Montoya M C, Kunisaki Y, Megias D, Marques M, Carrera A C, Manes S, Fukui Y, Martinez A C, Stein J V. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity. 2004;21:429–441. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Mempel T R, Soriano S F, Mazo I, Wymann M P, Hirsch E, Martinez A C, Fukui Y, von Andrian U H, Stein J V. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte P A, Hirsch E, Wymann M P, Cirillo R, Schwarz M K, Rommel C. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Barber D F, Bartolome A, Hernandez C, Flores J M, Redondo C, Fernandez-Arias C, Camps M, Ruckle T, Schwarz M K, Rodriguez S, Martinez A C, Balomenos D, Rommel C, Carrera A C. PI3Kγ inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- Gaspal F M, Kim M Y, McConnell F M, Raykundalia C, Bekiaris V, Lane P J. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174:3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- Walker L S, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka A V, Wu D. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Murphy K M, Heimberger A B, Loh D Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Soler D, Humphreys T L, Spinola S M, Campbell J J. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- Reiss Y, Proudfoot A E, Power C A, Campbell J J, Butcher E C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D J, Butcher E C. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116:4695–4705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- Syrbe U, Hoffmann U, Schlawe K, Liesenfeld O, Erb K, Hamann A. Microenvironment-dependent requirement of STAT4 for the induction of P-selectin ligands and effector cytokines on CD4+ T cells in healthy and parasite-infected mice. J Immunol. 2006;177:7673–7679. doi: 10.4049/jimmunol.177.11.7673. [DOI] [PubMed] [Google Scholar]
- Lord G M, Rao R M, Choe H, Sullivan B M, Lichtman A H, Luscinskas F W, Glimcher L H. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J J, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew D P, Warnke R, Ruffing N, Kassam N, Wu L, Butcher E C. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Campbell J J, O'Connell D J, Wurbel M A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- Finkelstein L D, Schwartzberg P L. Tec kinases: shaping T-cell activation through actin. Trends Cell Biol. 2004;14:443–451. doi: 10.1016/j.tcb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang C K. Neutrophils lacking phosphoinositide 3-kinase γ show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci USA. 2002;99:3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A L, Schwartz M D, Jameson S C, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110γ isoform of phosphatidylinositol 3-kinase. J Immunol. 2008;180:2081–2088. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- Alcazar I, Marques M, Kumar A, Hirsch E, Wymann M, Carrera A C, Barber D F. Phosphoinositide 3-kinase γ participates in T cell receptor-induced T cell activation. J Exp Med. 2007;204:2977–2987. doi: 10.1084/jem.20070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D J, Debes G F, Johnston B, Wilson E, Butcher E C. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Kunkel E J, Campbell D J, Butcher E C. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- Kim C H, Nagata K, Butcher E C. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J Immunol. 2003;171:152–158. doi: 10.4049/jimmunol.171.1.152. [DOI] [PubMed] [Google Scholar]
- Sinclair L V, Finlay D, Feijoo C, Cornish G H, Gray A, Ager A, Okkenhaug K, Hagenbeek T J, Spits H, Cantrell D A. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D F, Bartolome A, Hernandez C, Flores J M, Fernandez-Arias C, Rodriguez-Borlado L, Hirsch E, Wymann M, Balomenos D, Carrera A C. Class IB-phosphatidylinositol 3-kinase (PI3K) deficiency ameliorates IA-PI3K-induced systemic lupus but not T cell invasion. J Immunol. 2006;176:589–593. doi: 10.4049/jimmunol.176.1.589. [DOI] [PubMed] [Google Scholar]