Abstract
Background and Aims
Imbibition of Japanese soybean (Glycine max) cultivars was studied using micro-magnetic resonance imaging (MRI) in order to elucidate the mechanism of soaking injury and the protective role of the seed coat.
Methods
Time-lapse images during water uptake were acquired by the single-point imaging (SPI) method at 15-min intervals, for 20 h in the dry seed with seed coat, and for 2 h in seeds with the seed coat removed. The technique visualized water migration within the testa and demonstrated the distortion associated with cotyledon swelling during the very early stages of water uptake.
Key Results
Water soon appeared in the testa and went around the dorsal surface of the seed from near the raphe, then migrated to the hilum region. An obvious protrusion was noted when water reached the hypocotyl and the radicle, followed by swelling of the cotyledons. A convex area was observed around the raphe with the enlargement of the seed. Water was always incorporated into the cotyledons from the abaxial surfaces, leading to swelling and generating a large air space between the adaxial surfaces. Water uptake greatly slowed, and the internal structures, veins and oil-accumulating tissues in the cotyledons developed after the seed stopped expanding. When the testa was removed from the dry seeds before imbibition, the cotyledons were severely damaged within 1·5 h of water uptake.
Conclusions
The activation of the water channel seemed unnecessary for water entry into soybean seeds, and the testa rapidly swelled with steeping in water. However, the testa did not regulate the water incorporation in itself, but rather the rate at which water encountered the hypocotyl, the radicle, and the cotyledons through the inner layer of the seed coat, and thus prevented the destruction of the seed tissues at the beginning of imbibition.
Key words: Dry seeds, Glycine max, MRI, seed coat, soaking injury, soybean, testa, role of inner layer of seed coat, water uptake
INTRODUCTION
Soaking injury or soaking damage of leguminous seeds is a classical problem in agriculture and food processing. Seeds are stored dry and hence have very low levels of metabolism. During imbibition of water, they swell and metabolic activity increases. Hydration of tissue components during imbibition takes place in a controlled way so that the reconstruction of internal structures of the cells and organelles is not affected. However, if seeds are severely desiccated, leakage of stored materials and enzymes, colouring, cracking or absence of cotyledons, and overall damage to the hypocotyl may occur during germination (Pollock et al., 1969; Hobbs and Obendorf, 1972). This leads to a reduction in seed quality and poor field performance, or, in the food industry, the degradation of the product. Much work has focused on this subject since the 1970s, using various methods: tracer measurements using dyes, enzymology, microscopy, electron microscopy, scanning electron microscopy, sorption isotherm analysis and spectrometry. These studies found that severe drying of seeds or the removal of the seed coat before imbibition induces soaking damage (Larson, 1968; Powell and Matthews, 1978; Duke and Kakefuda, 1981). This damage takes place in the early stages of imbibition (Parrish and Leopold, 1977) not only on the cotyledons but also on the embryonic axis (Ashworth and Obendorf, 1980; McDonald et al., 1988b). Seeds do not, however, suffer from soaking effects when the water concentration in seeds is 12–17 % (Pollock et al., 1969; Hobbs and Obendorf, 1972; Ashworth and Obendorf, 1980; Ishida et al., 1988a). Alleviation of the effects of soaking injury as a result of the increase in the moisture content of seeds before imbibition is related to the reduced binding energy of water molecules and the appearance of respiratory activity (Vertucci and Leopold, 1984; Ishida et al., 1988b). The molecular-level mechanism relating to the soaking effect of excessively desiccated seeds remains unknown.
Magnetic resonance imaging (MRI) has been used in studies of water uptake for maize (Ruan et al., 1992), barley (Gruwel et al., 2002; Molina-Cano et al., 2002), wheat (Song et al., 1998; Gruwel et al., 2004), legumes (Heil et al., 1992; Pietrzak et al., 2002; Garnczarska et al., 2007), tobacco (Manz et al., 2005) and western white pine (Terskikh et al., 2005). MRI has been found useful for tracing water dynamics and, in a previous paper, water uptake for dry kidney beans and adzuki beans was traced by MRI (Kikuchi et al., 2006). Water was taken up into the seeds through the lens of the seed coat as the sole water channel, delivered first to the radicle through the testa, and then to the cotyledons, producing significant swelling. However, the process of water uptake for dry seeds exhibits diversity even within a single species, and the requirements to be fulfilled for sound germination appear to be variable for each variety or cultivar because the complex regulatory networks of germination have undergone a species- or ecotype-specific adaptation to the habitat (Finch-Savage and Leubner-Metzger, 2006). In the current investigation, the early stages of water uptake for dry Japanese soybean cultivars were investigated by fast time-lapse measurements with 15-min intervals by the single-point imaging (SPI) method. Water entry into the seed, rapid testa enlargement with steeping in water, and the delivery of water to the radicle and the hypocotyl followed by the swelling of the cotyledons were visualized. The properties of the testa were different from those of kidney beans and adzuki beans. The protective role of the inner layer of the seed coat of soybeans in water uptake was analysed in relation to soaking injury or soaking damage to the seeds.
MATERIALS AND METHODS
Plant material
Soybean seeds [Glycine max (L.) Merr.] of the Japanese cultivars ‘Fukuyutaka’ and ‘Kuromame’ harvested in 2006, and ‘Mikawashima’ harvested in 2005, and subsequently stored at 15 °C and approx. 70 % relative humidity, were used to acquire time-lapse images from the autumn of 2006 to the spring of 2007. The seeds of ‘Fukuyutaka’ and ‘Mikawashima’ have yellow testa, and ‘Kuromame’ has a black testa. The water content of the dry seeds was 13·0 ± 0·25 % and increased to 63·1 ± 0·31 % during imbibition (mean ± s.d.). A soybean was fixed to a wooden stick attached to the lid of a 15-mm sample tube (Fig. 1A) or on a small bed at the bottom of the tube (Fig. 1B) with a plastic binder. The sample tube was then filled with water and inserted into an MRI probe, and images were acquired continuously. The testa was carefully removed from several seeds of ‘Mikawashima’ using tweezers so as not to injure the embryos (cotyledons plus embryonic axis).
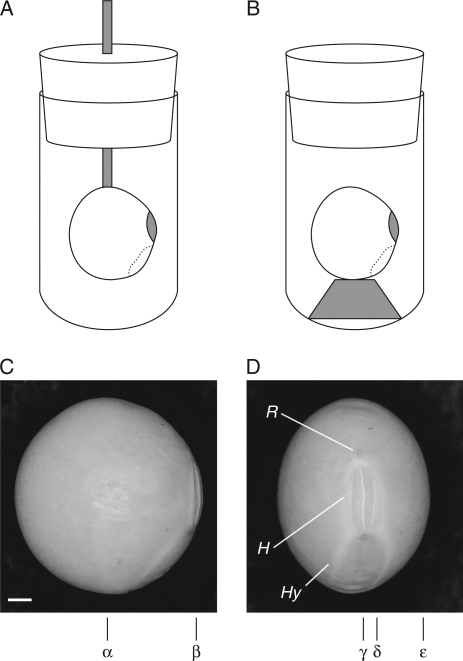
Fig. 1.
Soybean set in a sample tube for the acquisition of MR images. Two methods were employed: (A) a soybean was fixed by a plastic binder to a wooden stick attached to the lid of a 15-mm test tube, and (B) a soybean was fixed by a plastic binder to a small bed placed at the bottom of the test tube. The positions of sliced sections of images are indicated in (C) and (D): sections are at α in Figs 2, 4 and 9, and at γ in Figs 3, 6A–F and 7A–G; the sections used for MIP images are from δ to ε in Figs 6 G–L and 7H–N, and from α to β in Fig. 8. Abbreviations: R, raphe; H, hilum; Hy, hypocotyle–radicle axis. Scale bar in (C) = 1 mm.
Measurement of MR images
An NMR spectrometer (DRX 300, Bruker, Karlsruhe, Germany) and an imaging accessory were used for the measurements. Three-dimensional images were acquired by single-point imaging (SPI) using the protocol for the system. The field of view (FOV) was 20 × 20 × 20 mm with a 64 × 32 × 32 matrix. Dephase time was set at 0·1 ms, and repetition time (TR) at 5 ms. The method highlighted absorbed water, restricted in motion by suppressing the water signal out of the seed with high mobility, using a short TR (Kikuchi et al., 2006). This process took approx. 5 min per image. Images were acquired at 15-min intervals for 20 h from 5 min after imbibition of water for the intact seeds. Measurements were stopped after 2 h when they were taken during the imbibition of embryos (seed minus testa), since the cotyledons showed extensive damage by this stage. Measured data sets were Fourier transformed, transferred to a micro-computer, converted to data format and zero-filled; then images were created in a 64 × 64 × 64 or a 128 × 128 × 128 matrix. The resulting spatial resolution was 313 µm or 156 µm. More than ten replicate measurements were conducted for intact seeds for all three cultivars using two different fixing methods, and duplicate measurements were conducted for the embryo measurements using ‘Mikawashima’. Figure 1C (α, β) and 1D (γ–ε) depict digitally sliced sections of images.
Image data were further processed using the ImageJ program [a public-domain Java image processing program (ver. 1·33 equipped with plugins) available on the Internet at http://rsb.info.nih.gov/ij/], Scion Image program [public-domain NIH image program (National Institute of Health, USA) translated for Windows operating systems by Scion Co., Frederick, MD, USA; available on the Internet at http://www.scioncorp.com/], and NMRv2b [rendering software (MR Technology Inc., Tsukuba, Japan)]. The processes involved in the imbibition were quantitatively analysed using the Scion Image program and the KaleidaGraph program (Synergy Software, PA, USA).
RESULTS
Incorporation of water into soybeans with swelling of the testa and elongation of the cotyledons
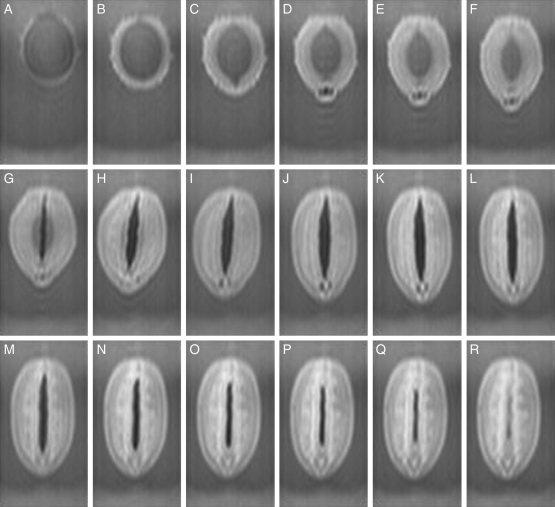
Figure 2 depicts the changes in the images during imbibition of a soybean seed at a median longitudinal section normal to the raphe–antiraphe of the seed (Fig. 1C, α section) at 5 min, and at 60-min intervals for 17 h. A seed of ‘Mikawashima’, harvested in 2005, was examined using the method indicated in Fig. 1A. The first image indicated a weak water signal outside the testa at 5 min after imbibition (Fig. 2A), and the signals intensified around the seed with increasing thickness of the testa. Water was incorporated into the cotyledons from their abaxial surfaces but not through their adaxial surfaces (Fig. 2D–G). These results differed from those with kidney beans and adzuki beans, which exhibited lag times before the appearance of the water signal and water incorporation from the adaxial surfaces into the cotyledons (Kikuchi et al., 2006). A protrusion was observed at 3 h in the radicle pocket with small, non-hydrated areas (Fig. 2D), and the seed elongated slowly for 5 h. As water almost filled the seed, an air space formed between the cotyledons at 6 h (Fig. 2G), and the seed then clearly increased in length. The hypocotyl was recognized at 8 h (Fig. 2I). Signals of the adaxial epidermal tissues and the internal surface of the cotyledons intensified after 13 h (Fig. 2 N), accompanied by a narrowing of the air space between the cotyledons. The signals of the adaxial epidermal tissues and the internal structures of the cotyledons intensified afterwards.
Fig. 2.
Changes in a soybean during imbibition at a median longitudinal section (Fig. 1C, α) normal to the raphe–antiraphe of the longer axis. A soybean (‘Mikawashima’) was fixed by the method indicated in Fig. 1A. Images were acquired continuously for 20 h at 15-min intervals after 5 min of imbibition, and those presented here are at 60-min intervals from 5 min of imbibition for a period of 17 h, as follows: (A) 5 min, (B) 1 h 5 min, (C) 2 h 5 min, (D) 3 h 5 min, (E) 4 h 5 min, (F) 5 h 5 min, (G) 6 h 5 min, (H) 7 h 5 min, (I) 8 h 5 min, (J) 9 h 5 min, (K) 10 h 5 min, (L) 11 h 5 min, (M) 12 h 5 min, (N) 13 h 5 min, (O) 14 h 5 min, (P) 15 h 5 min, (Q) 16 h 5 min, and (R) 17 h 5 min. Highlighted signals represent water taken up.
Figure 3 shows the changes in the images during imbibition of a seed at a median longitudinal section parallel to the raphe–antiraphe of the seed (Fig. 1D, γ section) at 5 min, and at 60-min intervals for 11 h. A seed of ‘Fukuyutaka’, harvested in 2006, was examined using the method indicated in Fig. 1A. The sliced section was positioned at the centre of the two cotyledons where the air space formed from 6 to 16 h in Fig. 2G–Q. Weak water signals appeared around the seed 5 min after imbibition (Fig. 3A). The signals intensified and circled the seed within 1 h (Fig. 3B); the radicle was distinguished at 2 h (Fig. 3C), and a convex expansion was visible at the corner of the seed on the ventral and the opposite side of the hilum from the radicle after 3 h (Fig. 3D–F). No strong signal was detected in the area of the cotyledons up to 5 h, due to the air space that formed between the cotyledons; this air space narrowed and had disappeared at 10 h as a result of being replaced by water (Fig. 3K). No obvious increase in the size of the seed was observed afterwards. The rate of water uptake was considerably faster than that of ‘Mikawashima’ (Fig. 2).
Fig. 3.
Changes in a soybean during imbibition at a longitudinal section (Fig. 1D, γ) parallel to the raphe–antiraphe of the longer axis in the centre between the cotyledons. A soybean (‘Fukuyutaka’) was fixed by the method indicated in Fig. 1A. Images were acquired continuously for 20 h at 15-min intervals after 5 min of imbibition, and those presented here are at 60-min intervals from 5 min of imbibition for a period of 11 h as follows: (A) 5 min, (B) 1 h 5 min, (C) 2 h 5 min, (D) 3 h 5 min, (E) 4 h 5 min, (F) 5 h 5 min, (G) 6 h 5 min, (H) 7 h 5 min, (I) 8 h 5 min, (J) 9 h 5 min, (K) 10 h 5 min, and (L) 11 h 5 min. Highlighted signals represent water taken up.
The faster hydration of the radicle, including the hypocotyl, and formation of air space between the cotyledons in ‘Fukuyutaka’ (Fig. 3) were confirmed (Fig. 4) with the use of images at a median longitudinal section normal to the raphe–antiraphe of the seed (Fig. 1C, α section). The same image data as in Fig. 3 were processed with a 30-min separation from 35 min to 3 h 35 min of imbibition. Following the hydration of the testa, a strong water signal was detected in the radicle, associated with a small space inside at 1 h 5 min (Fig. 4B), which corresponded to the narrow dark area along with the radicle pocket observed from 2 h to 5 h (Fig. 3C–F). The radicle swelled and protruded with time. Water was simultaneously incorporated from the abaxial surface of the cotyledons to form a long vertical air space between the cotyledons (Fig. 4B–F), which corresponded to the dark central area surrounded by highlighted testa in Fig. 3B–F. The seed elongated after most parts of the cotyledons were hydrated (Fig. 4F, G), as indicated in Fig. 2H–K.
Fig. 4.
Changes in a soybean during imbibition at a longitudinal section (Fig. 1C, α) normal to the raphe–antiraphe of the longer axis in the initial stages of imbibition. The same three-dimensional image data as presented in Fig. 3 were processed to indicate the protrusion of the radicle. The images are presented at 30-min intervals from 35 min to 3 h 35 min of imbibition: (A) 35 min, (B) 1 h 5 min, (C) 1 h 35 min, (D) 2 h 5 min, (E) 2 h 35 min, (F) 3 h 5 min, and (G) 3 h 35 min.
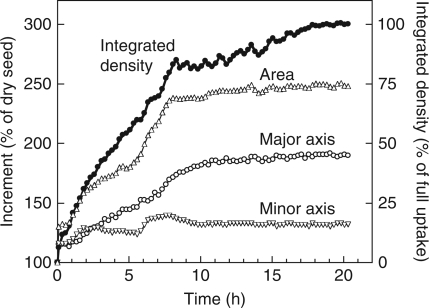
Figure 5 indicates the amount of water taken up, the increases in seed size (area), and the elongation of the longer and shorter axes as calculated using the original series of images in Fig. 2. The inside dark area of weak water signals in the first image at 5 min (Fig. 2A) was assumed to be the seed before imbibition (V0 in Fig. 5 caption). The seed size abruptly increased by up to 130 % with steeping in water and, after a pause for 30 min, rapidly increased for 3 h when the protrusion of the radicle was observed in Fig. 2D. The testa might be responsible for the enlargement of the seed size during that time. The rate of increase slightly slowed until 6 h, corresponding to Fig. 2G, then the seed size steeply increased again and reached a plateau before 10 h. This increase was ascribed to the enlargement of the cotyledons (Fig. 2H–K). The increment of the longer axis exhibited a change similar to that of the seed size (Fig. 5). The shorter axis abruptly increased by 10 % with steeping, and expanded for 3 h up to 125 %. The second increase took place from 6 to 7 h, but was followed by a decrease. The total expansion of the shorter axis was confined to 30 %. Water uptake proceeded linearly for 8 h, when the size of the seed reached almost maximum, then the rate of water uptake greatly declined.
Fig. 5.
Time course of water uptake, increase of size, and increments of the major and minor axes of soybean. Measurements were carried out on the same section as in Fig. 2. Water amount (density) is indicated by the integrated signal intensity, and the increase of size is indicated by the area of the bean. The increments (%) of area, the major axis and the minor axis are given by [(Vt – V0)/V0] × 100, where Vt is the value at time t and V0 is the value at time 0.
Water entry into the seed and migration in the testa
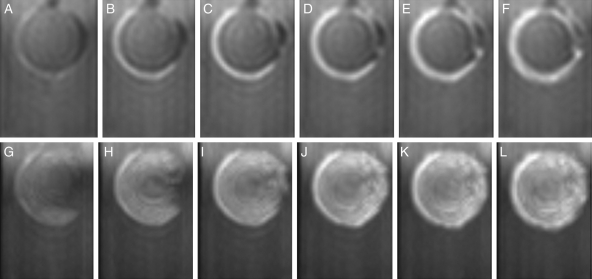
Figure 6A–F presents the changes in the images at a median longitudinal section parallel to the raphe–antiraphe of the seeds (Fig. 1D, γ section), and Fig. 6G–L depicts the maximum intensity projection (MIP) images through half of a seed (Fig. 1D, positions from δ to ε) with 15-min intervals after 5 min of imbibition to 1 h 20 min. The original series of the same results as shown in Fig. 2 using ‘Mikawashima’ were processed. Water signals rounded the dorsal surface at 20 min (Fig. 6B), then extended to the ventral surface at 35 min, and passed under the hilum to the radicle after 50 min of imbibition in the sectional images (Fig. 6C–F). The MIP images demonstrated that a hemispherical spread of water formed on the dorsal side of the seeds within 20 min (Fig. 6H), and widened around the hilum and the radicle with time (Fig. 6I–L).
Fig. 6.
Water entry and migration in the testa in the initial stages of imbibition for soybean (‘Mikawashima’). Changes in a soybean at a longitudinal section parallel to the raphe–antiraphe are indicated; the same three-dimensional image data as in Fig. 2 were processed. Top: the sliced images of γ section (Fig. 1D); bottom: MIP images from δ to ε sections (Fig. 1D). (A, G) 5 min, (B, H) 20 min, (C, I) 35 min, (D, J) 50 min, (E, K) 1 h 5 min, and (F, L) 1 h 20 min.
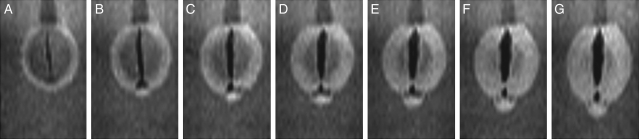
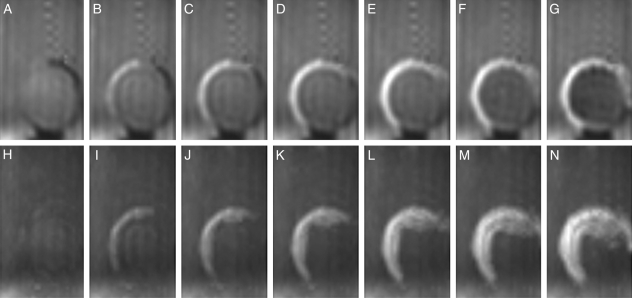
‘Kuromame’ seeds, which have thick black testae, took up water more slowly than the other cultivars. The seed was fixed on a small bed at the bottom of the sample tube (Fig. 1B), based on the results of preliminary measurements, in order to best display the processes of water entry. Figure 7A–G shows the changes in the images at a median longitudinal section parallel to the raphe–antiraphe of the seed (Fig. 1D, γ section), and Fig. 7H–N presents the MIP image through half of a seed (Fig. 1D, positions from δ to ε) for the initial 1 h 35 min of imbibition. Water signals first rounded the testa from near the raphe to the dorsal surface (Fig. 7B), then inversely migrated to the radicle, passing the hilum in the sectional images (Fig. 7C–G). The MIP images indicated that water signals spread opposite to the hilum (Fig. 7I, J), followed by a reverse spreading to the hilum (Fig. 7K, L), and later extended on the side surface of the seed (Fig. 7M, N).
Fig. 7.
Water entry and migration in the testa in the initial stages of imbibition for a soybean (‘Kuromame’). A soybean was fixed by the method indicated in Fig. 1B and images were acquired continuously for 20 h at 15-min intervals after 5 min of imbibition. Changes of soybeans at longitudinal sections parallel to the raphe–antiraphe are shown. Top: images from the γ section (Fig. 1D); bottom: MIP images from δ to ε (Fig. 1D). (A, H) 5 min, (B, I) 20 min, (C, J) 35 min, (D, K) 50 min, (E, L) 1 h 5 min, (F, M) 1 h 20 min, and (G, N) 1 h 35 min.
Figure 8 illustrates the changes of MIP images through half of a ‘Kuromame’ seed from normal to the raphe–antiraphe direction (Fig. 1C, sections from α to β) from 1 h 5 min to 4 h 20 min in order to indicate the migration of water around the hilum. Water signals appeared at a position opposite the radicle beyond the hilum (Fig. 8A), widened on both sides of the seed, and passed around the hilar region adjacent to the hilum towards the radicle (Fig. 8C–E). The radicle and the hypocotyl were hydrated before the seed exhibited obvious elongation (Fig. 8H–J). During this period, the hilum was not hydrated. In the case of ‘Kuromame’, it took 3 h, a rather long period compared with the other cultivars, before water covered all areas of the seed surface. Water penetrated into the testa from a position close to the raphe (Figs 7 and 8), but the exact channel of the entrance was not determined, since water did not stay at a definite place long enough to be absorbed.
Fig. 8.
Water migration from the entrance to the radicle along the hilum, following rapid water entry into the dorsal testa in the initial stages of imbibition. The same three-dimensional image data as presented in Fig. 7 were processed. The MIP images from α to β sections in Fig. 1C are presented from 1 h 5 min to 4 h 20 min of imbibition. (A) 1 h 5 min, (B) 1 h 20 min, (C) 1 h 35 min, (D) 1 h 50 min, (E) 2 h 5 min, (F) 2 h 20 min, (G) 2 h 35 min, (H) 2 h 50 min, (I) 3 h 5 min, (J) 3 h 20 min, (K) 3 h 35 min, (L) 3 h 50 min, (M) 4 h 5 min, and (N) 4 h 20 min.
Role of the testa in preventing soaking damage
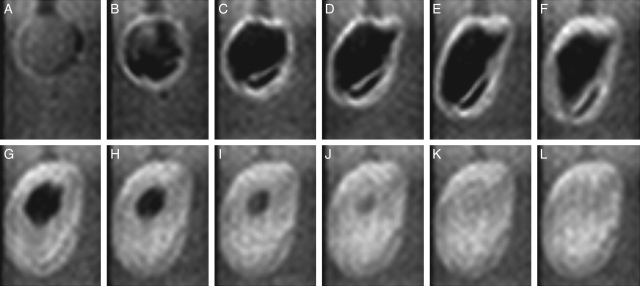
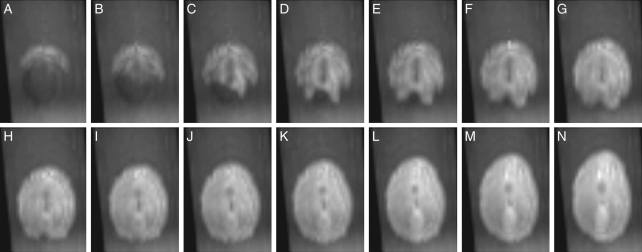
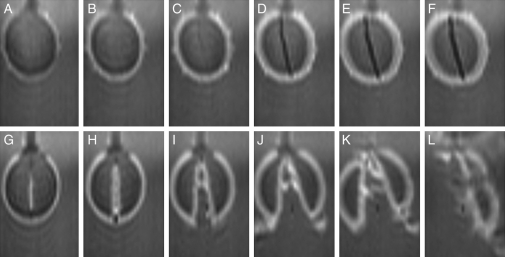
The testa was removed from dry seed of ‘Mikawashima’ and the resultant embryo (seed minus testa) was soaked in water. Changes in the images at a median longitudinal section normal to the raphe–antiraphe (Fig. 1C, α section) are presented from 5 min after the start of imbibition with 15-min intervals to 1 h 20 min for an intact seed in Fig. 9A–F, and for the embryo in Fig. 9G–L. Changes for the intact seed were similar to those in Fig. 2: i.e. a weak water signal was detected in the first image; the signals subsequently intensified with thickening of the testa (Fig. 9A–C); and a vertical air space was formed between the cotyledons (Fig. 9D–F). The measurements were continued for 20 h for intact seeds. In contrast, in the embryos strong water signals were already around the embryo and on the adaxial surface of the cotyledons in the first image (Fig. 9G) and grew stronger. The cotyledons opened up at 35 min (Fig. 9I); the left cotyledon was separated from right after 1 h 5 min (Fig. 9K), and the right cotyledon broke into pieces at 1 h 20 min (Fig. 9L).
Fig. 9.
Changes in soybeans with and without testa in the initial stages of imbibition. Seeds (‘Mikawashima’) were used. Top: a seed with testa; bottom: a peeled seed. Images at a longitudinal section (Fig. 1C, α) normal to the raphe–antiraphe of the longer axis are presented. Images were acquired continuously for 20 h for the intact soybean (top) and for approx. 2 h for the peeled soybean (bottom) at 15-min intervals after 5 min of imbibition. Changes in images are indicated from 5 min of imbibition: (A, G) 5 min, (B, H) 20 min, (C, I) 35 min, (D, J) 50 min, (E, K) 1 h 5 min, and (F, L) 1 h 20 min.
DISCUSSION
The early stages of water uptake by dry soybeans were traced with time-lapse images at short intervals. The process of water uptake seemed essentially similar to that reported for kidney beans and adzuki beans (Kikuchi et al., 2006): water first wetted the testa, hydrated the radicle, and then the cotyledons elongated. A notable difference from kidney beans and adzuki beans was that water signals were soon detected around the seed during imbibition (Figs 2–4, 6 and 9A–F) and the seed size increased by 30 % (Fig. 5) within 20 min for ordinary cultivars with yellow testae. This was similar to the finding that air-dried seeds rapidly took up water for 10 min (Parrish and Leopold, 1977). Since the outermost cuticle of the seed is hydrophobic (Saio et al., 1973; Ma et al., 2004; Shao et al., 2007), the non-uniform appearance of water signals on the surface of soybeans at 5 min of imbibition (Figs 3A, 6A and 7A) could be attributed to wetting inside the testa. No lag-time for water entry was associated with the activation of the water channel.
Water signals first surrounded the dorsal surface of the seed (Figs 6B, H and 7B, I), agreeing with previous observations at low magnification using digital camera (Meyer et al., 2007) and with scanning electron microscope studies (McDonald et al., 1988a), and indicating that the first part of the seed coat to wrinkle is on the side opposite the hilum. Water is often considered to be taken up from the hilum side because imbibition is facilitated when the hilum region is exposed to water. Ordinary Japanese cultivars incorporated water too fast to be able to determine distinctively the water entrance and the paths of water transport inside the testa (Figs 3 and 6). Only the results using ‘Kuromame’ (Fig. 7) with a thick black testa, in which water uptake was considerably slower than the other cultivars, demonstrated that water entered the seed near the raphe and quickly proceeded to the area opposite the hilum in the testa (Fig. 7A–G), slightly spreading to the side (Fig. 7H–N) probably through the vascular system, as reported for Albizia lophantha (Dell, 1980). Part of the water migrated inversely after a short delay, framing the hilum towards the radicle (Fig. 8). Although the water channel could not be located precisely due to the fact that no water stayed on the testa before absorption (Figs. 7 and 8), it is plausible that water was taken up into the seed from a specific place on the convex area (Fig. 3D–F) close to the raphe (Fig. 7B, I) in the hilum region (McDonald et al., 1988a) as a sole water channel. The natural entrance of water into the seed is considered to be a strophiolar plug (Dell, 1980) or lens (Manning and Van Staden, 1987; Van Staden et al., 1989). As water reached the radicle, an obvious protrusion of the embryonic axis with a large swelling capacity (McDonald et al., 1988b) appeared, followed by elongation of the cotyledons (Figs. 2, 4 and 8), similar to previous findings with kidney beans and adzuki beans (Kikuchi et al., 2006).
The pathway of water uptake by dry seeds of other species has also been studied by using MRI. Water is taken up through two pathways in corn seeds: one is from the tip cap, through the cross- and tube-cells of the pericarp layers, and into the endosperm; and the other is through the germ and into the interior of the endosperm (Ruan et al., 1992). For barley seeds, water enters the kernel through the kernel surfaces and into the endosperm (McEntyre et al., 1998), or initially enters the embryo followed by the ingress of water into the endosperm from the scutellum (Molina-Cano et al., 2002). In western white pine, water penetrates through the seed coat and megagametophyte and then hydrates the embryo (Terskikh et al., 2005). The micropylar seed end is the major entry point of water in tobacco seeds (Manz et al., 2005), whilst the micropyle and the hilum regions are noted as entry points for pinto beans (Heil et al., 1992) and soybeans (Pietrzak et al., 2002). Thus water enters seed through various paths depending upon individual species and varieties or cultivars.
Cracks in the seed coat may also be a route of water entry. Ma et al. (2004) noted that many small cracks in the covering cuticle control the permeability of the seed coat to water, this being the case for pea seeds (Powell and Matthews, 1980). In the current investigation, permeation of water from irregular cracks did not seem to be the normal method for imbibition by Japanese cultivars of soybean.
In contrast to kidney beans and adzuki beans (Kikuchi et al., 2006), water inflow into the cotyledons of soybean took place from the abaxial surface to the adaxial surface, and a large air space developed between the adaxial surface of the two cotyledons (Figs 2 and 4). The greater swelling of the more-hydrated abaxial surfaces compared with the adaxial surfaces led to the formation of the space by physical stress (Hobbs and Obendorf, 1972; Duke and Kakefuda, 1981). This space may serve as a trap for desorbed gases from the solid surface during replacement with water (Parrish and Leopold, 1977). The air space between the adaxial surfaces was maintained until the cotyledons expanded fully (Figs. 2 and 3). However, in the embryos (seed minus testa), water intruded between the cotyledons, and the cotyledons were disrupted in a very short period of time (Fig. 9G–L; Duke and Kakefuda, 1981). The removal of the testa was also found to enhance the leakage of solutes and to induce the rupture of cotyledonary cells of the abaxial surface of pea seeds directly in contact with water (Larson, 1968; Powell and Matthews, 1978). This pointed to the role of the testa in preventing soaking injury by regulating water uptake. In the absence of the testa, the non-regulated water uptake by the hypocotyl, radicle and cotyledons induces mechanical damage caused by the stress of uneven swelling of the tissues.
Imbibition of water, which triggers the active metabolism of dry seeds, is frequently associated with soaking injury to legumes. The damage to tissues is severe in seeds with initial water concentrations under 10 %. However, when the seed moisture content before imbibition is 12–17 % there is an alleviation of damage, associated with a decline in the leakage of solutes, the appearance of respiratory activity, and clear phosphate signals in 31P-NMR spectra (Pollock, 1969; Pollock et al., 1969; Hobbs and Obendorf, 1972; Ashworth and Obendorf, 1980; Vertucci and Leopold, 1984; Ishida et al., 1988a, b). This indicates that membrane functions are restored, even though the activities of respiration and metabolism are restricted. Sorption isotherm and 1H-NMR studies have demonstrated that water molecules are semi-bound and that mobile water necessary for metabolism is deficient for moisture contents between 12–24 %. Therefore, the hydration of seeds in this moisture range is through physical processes regulated by the free energy difference (Vertucci and Leopold, 1984; Ishida et al., 1988b), as discussed for pea seeds using the kinetics of solute leakage (Powell and Matthews, 1981). This corresponds to the period before water signals covered the cotyledons at 6 h of imbibition (Fig. 2G), when the radicle protruded (Fig. 2D–F) and the increase rate in size was retarded after the steep rise of testa hydration (Fig. 5). Soaking damage is seldom observed in seeds with an initial moisture content above 20 %, where respiration and metabolic activity rapidly increase with the increase of moisture content (Vertucci and Leopold, 1984; Ishida et al., 1988b).
Low-molecular-weight solutes can diffuse out of the seed before the dry membranes are hydrated, due to the absence of sufficient water to maintain the hydrophilic/hydrophobic orientation of the lipid membranes (Simon, 1974). When the dry membrane is hydrated slowly, the hydrophilic components of the membrane hydrate first, resulting in rearrangement of the membrane components to form a functional bilayer membrane with selective permeability (Singer and Nicolson, 1972; Saiz and Klein, 2002; Bemporad et al., 2005). In contrast, if large amounts of water flow in rapidly, excess water molecules, not used for the hydration of dry membrane materials, pass through non-functional membranes. This water encounters accumulated macromolecules in cells, such as starches and proteins in the embryonic axis and cotyledons, that have a large swelling capacity with hydration (Ashworth and Obendorf, 1980; McDonald et al., 1988b). This induces soaking injury, due to the rupture of the irregular membrane configurations in the dry seeds (Larson, 1968; Powell and Matthew, 1981).
In this context, soaking injury occurs if the radicle, hypocotyl and cotyledons irregularly take up large amounts of water (Fig. 9G–L). The intact testa rapidly took up and held considerable amounts of water (Figs 2, 4 and 9A–F). The cotyledons elongated after the radicle protruded significantly (Figs 2 and 4) and hydrated (Fig. 8). During this period, hourglass cells around the hilum with a large water-holding capacity might be an effective buffer (McDonald et al., 1988a) for avoiding too-rapid wetting of the radicle and the hypocotyl (Fig. 8), and compensate for the lack of an activation lag of the water channel (Figs 2, 3, 6 and 7). It is noteworthy that water was always incorporated from the abaxial surfaces of the cotyledons (Figs 2 and 4) and was kept out of the air space formed between the adaxial surfaces of the cotyledons until termination of the steep increment of seed size (Figs 2, 3 and 5). Water never appeared on the adaxial surfaces until the cotyledons were fully hydrated, and therefore the incorporation of water into the radicle, hypocotyl and cotyledons was controlled. The inner layer of the seed coat, composed of a living cell layer of aleurone with a cuticle abutting the outer periclinal walls (Yaklich et al., 1992; Chamberlin et al., 1994; Ma et al., 2004), is considered to act as the barrier to free penetration of water inside (Matsui et al., 1996; Tian et al., 2005). The delivery of water to individual tissues is biologically regulated, probably by the embryo (Van Staden et al., 1989), as observed in western white pine seeds where, unlike soybean, the cotyledons are hydrated before the hypocotyl and the radicle (Terskikh et al., 2005). Similarly, gas must be exhausted by water passing through the barrier, which maintains the air space for a long period (Figs 2 and 3). The process must be physiological reactions so as to avoid disrupting the inner layer of the seed coat by rapid expansion of gases.
Slow and controlled hydration is essential as the first step in the reactivation of metabolic processes in the dry seed, leading to germination and growth. This physical process is driven by free energy differences of water binding with constituent molecules or atoms of membrane under a limited supply of water. We conclude that the intact testa of soybean seeds regulates water incorporation into the radicle, the hypocotyl and the cotyledons through its inner barrier in order to accomplish the regular reconstitution of the membrane organization of these tissues and thus prevent the destruction of the seed tissues at the beginning of imbibition. After the reconstruction of the membrane organization is accomplished, the regulation of water inflow by the inner layer of the seed coat is biologically controlled, which continues during enlargement and after the cotyledons stop expanding.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by National Food Research Institute, Japan.
LITERATURE CITED
- Ashworth EN, Obendorf RL. Imbibitional chilling injury in soybean axes: relationship to stelar lesions and seasonal environments. Agronomy Journal. 1980;72:923–928. [Google Scholar]
- Bemporad D, Luttmann C, Essex JW. Behaviour of small solutes and large drugs in a lipid bilayer from computer simulations. Biochimica et Biophysica Acta. 2005;1718:1–21. doi: 10.1016/j.bbamem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Chamberlin MA, Horner HT, Palmer RG. Early endosperm, embryo, and ovule development in Glycine max (L.) Merr. International Journal of Plant Science. 1994;155:421–436. [Google Scholar]
- Dell B. Structure and function of the strophiolar plug in seeds of Albizia lophantha. American Journal of Botany. 1980;67:556–563. [Google Scholar]
- Duke SH, Kakefuda G. Role of the testa in preventing cellular rupture during imbibition of legume seeds. Plant Physiology. 1981;67:449–456. doi: 10.1104/pp.67.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Garnczarska M, Zalewski T, Kempka M. Water uptake and distribution in germinating lupine seeds studied by magnetic resonance imaging and NMR spectroscopy. Physiologia Plantarum. 2007;130:23–32. [Google Scholar]
- Gruwel MLH, Yin XS, Edney MJ, Schroeder SW, MacGregor AW, Abrams S. Barley viability during storage: use of magnetic resonance as a potential tool to study viability loss. Journal of Agricultural and Food Chemistry. 2002;50:667–676. doi: 10.1021/jf0108617. [DOI] [PubMed] [Google Scholar]
- Gruwel MLH, Latta P, Volotovskyy V, Šramek M, Tomanek B. Magnetic resonance imaging of seeds by use of single point acquisition. Journal of Agricultural and Food Chemistry. 2004;52:4979–4983. doi: 10.1021/jf049078f. [DOI] [PubMed] [Google Scholar]
- Heil JR, McCarthy MJ, Özilgen M. Magnetic resonance imaging and modeling of water up-take into dry beans. Lebensmittel-Wissenschaft und-Technologie. 1992;25:280–285. [Google Scholar]
- Hobbs PR, Obendorf RL. Interaction of initial seed moisture and imbibitional temperature on germination and productivity of soybean. Crop Science. 1972;12:664–667. [Google Scholar]
- Ishida N, Kano H, Kobayashi T, Hamaguchi H, Yoshida T. The relationship between imbibitional damage and initial water content of soybeans. Agricultural and Biological Chemistry. 1988;a 52:2771–2775. [Google Scholar]
- Ishida N, Kano H, Kobayashi T, Yoshida T. Analysis of physical states of water in soybean seeds by NMR. Agricultural and Biological Chemistry. 1988;b 52:2777–2781. [Google Scholar]
- Kikuchi K, Koizumi M, Ishida N, Kano H. Water uptake by dry beans observed by micro-magnetic resonance imaging. Annals of Botany. 2006;98:545–553. doi: 10.1093/aob/mcl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson LA. The effect soaking pea seeds with or without seedcoats has on seedling growth. Plant Physiology. 1968;43:255–259. doi: 10.1104/pp.43.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Cholewa E, Mohamed T, Peterson CA, Gijzen M. Cracks in the palisade cuticle of soybean seed coats correlate with their permeability to water. Annals of Botany. 2004;94:213–228. doi: 10.1093/aob/mch133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JC, Van Staden J. Annals of Botany. Vol. 59. Leguminosae: Papilionoideae; 1987. The role of the lens in seed imbibition and seedling vigour of Sesbania punicea (Cav.) Benth; pp. 705–713. [Google Scholar]
- Manz B, Müller K, Kucera B, Volke F, Leubner-Metzger G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiology. 2005;138:1538–1551. doi: 10.1104/pp.105.061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Uenaka T, Toyosawa I, Fukuda M. Role of soybean aleurone layer in water uptake in seeds. Nippon Nogeikagaku Kaishi. 1996;70:663–669. (in Japanese with English abstract) [Google Scholar]
- McDonald MB, Jr, Vertucci CW, Roos EE. Seed coat regulation of soybean seed imbibition. Crop Science. 1988;a 28:987–992. [Google Scholar]
- McDonald MB, Jr, Vertucci CW, Roos EE. Soybean seed imbibition: water absorption by seed parts. Crop Science. 1988;b 28:993–997. [Google Scholar]
- McEntyre E, Ruan R, Fulcher RG. Comparison of water absorption patterns in two barley cultivars, using magnetic resonance imaging. Cereal Chemistry. 1998;75:792–795. [Google Scholar]
- Meyer CJ, Steudle E, Peterson CA. Patterns and kinetics of water uptake by soybean seeds. Journal of Experimental Botany. 2007;58:717–732. doi: 10.1093/jxb/erl244. [DOI] [PubMed] [Google Scholar]
- Molina-Cano JL, Sopena A, Polo JP, Bergareche C, Moralejo MA, Swanston JS, Glidewell SM. Relationships between barley hordeins and malting quality in a mutant of cv. Triumph. II. Genetic and environmental effects on water uptake. Journal of Cereal Science. 2002;36:39–50. [Google Scholar]
- Parrish DJ, Leopold AC. Transient changes during soybean imbibition. Plant Physiology. 1977;59:1111–1115. doi: 10.1104/pp.59.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak LN, Frégeau-Reid J, Chatson B, Blackwell B. Observations on water distribution in soybean seed during hydration processes using nuclear magnetic resonance imaging. Canadian Journal of Plant Science. 2002;82:513–519. [Google Scholar]
- Pollock BM. Imbibition temperature sensitivity of lima bean seeds controlled by initial seed moisture. Plant Physiology. 1969;44:907–911. doi: 10.1104/pp.44.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock BM, Roos EE, Manalo JR. Vigor of garden bean seeds and seedlings influenced by initial seed moisture, substrate oxygen, and imbibition temperature. Journal of the American Society for Horticultural Science. 1969;94:577–584. [Google Scholar]
- Powell AA, Matthews S. The damaging effect of water on dry pea embryos during imbibition. Journal of Experimental Botany. 1978;29:1215–1229. [Google Scholar]
- Powell AA, Matthews S. The significance of damage during imbibition to the field emergence of pea (Pisum sativum L.) seeds. Journal of Agricultural Science. 1980;95:35–38. [Google Scholar]
- Powell AA, Matthews S. A physical explanation for solute leakage from dry pea embryos during imbibition. Journal of Experimental Botany. 1981;32:1045–1050. [Google Scholar]
- Ruan R, Litchfield JB, Eckhoff SR. Simultaneous and nondestructive measurement of transient moisture profiles and structural changes in corn kernels during steeping using microscopic nuclear magnetic resonance imaging. Cereal Chemistry. 1992;69:600–606. [Google Scholar]
- Saio K, Arai K, Watanabe T. Fine structure of soybean seed coat and its changes on cooking. Cereal Science Today. 1973;18:197–201 & 205. [Google Scholar]
- Saiz L, Klein ML. Computer simulation studies of model biological membranes. Accounts of Chemical Research. 2002;35:482–489. doi: 10.1021/ar010167c. [DOI] [PubMed] [Google Scholar]
- Shao S, Meyer CJ, Ma F, Peterson CA, Bernards MA. The outermost cuticle of soybean seeds: chemical composition and function during imbibition. Journal of Experimental Botany. 2007;58:1071–1082. doi: 10.1093/jxb/erl268. [DOI] [PubMed] [Google Scholar]
- Simon EW. Phospholipids and plant membrane permeability. New Phytologist. 1974;73:377–420. [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Song HP, Delwiche SR, Line MJ. Moisture distribution in a mature soft wheat grain by three-dimensional magnetic resonance imaging. Journal of Cereal Science. 1998;27:191–197. [Google Scholar]
- Terskikh VV, Feurtado JA, Ren C, Abrams SR, Kermode AR. Water uptake and oil distribution during imbibition of seeds of western white pine (Pinus monticola Dougl. ex D. Don) monitored in vivo using magnetic resonance imaging. Planta. 2005;221:17–27. doi: 10.1007/s00425-004-1426-z. [DOI] [PubMed] [Google Scholar]
- Tian X-H, Nakamura T, Kokubun M. The role of seed structure and oxygen responsiveness in pre-germination flooding tolerance of soybean cultivars. Plant Production Science. 2005;8:157–165. [Google Scholar]
- Van Staden J, Manning JC, Kelly KM. Stirton CH, Zarucchi JL, editors. Legume seeds – the structure: function equation. Advances in legume biology. Monographs in Systematic Botany from the Missouri Botanical Garden. 1989;29:417–450. [Google Scholar]
- Vertucci CW, Leopold AC. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiology. 1984;75:114–117. doi: 10.1104/pp.75.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaklich RW, Vigil EL, Erbe EF, Wergin WP. The fine structure of aleurone cells in the soybean seed coat. Protoplasma. 1992;167:108–119. [Google Scholar]